Abstract
Purpose
To compare the area of the superficial foveal avascular zone (SFAZ) and deep foveal avascular zone (DFAZ) between patients with nanophthalmos and age matched controls.
Methods
This prospective and comparative study included 19 eyes from 11 patients with nanophthalmos (study group) and 19 eyes from 19 healthy subjects (control group). SFAZ and DFAZ were measured with optical coherence tomography angiography (OCT-A). All participants underwent a standardised ocular examination including best corrected visual acuity (BCVA), central macular thickness (CMT), subfoveal choroidal thickness (SFCT) anterior chamber depth (ACD), axial length (AL), and refractive error (RE) measurements.
Results
Mean SFAZ and DFAZ area in the nanophthalmic eyes and in the control eyes were 0.09 ± 0.12 mm2, 0.10 ± 0.10 mm2 and 0.37 ± 0.10 mm2, 0.37 ± 0.10 mm2 respectively (p < 0.001 and p < 0.001). Mean BCVA, RE, AL, ACD CMT, SFCT, were 0.40 ± 0.34 logMAR Unit, 10.0 ± 2.2 18.1 ± 1.5 mm, 2.15 ± 0.28 mm, 367.1 ± 87.4 µm, 489.2 ± 85.2 µm respectively, in nanophthalmic eyes and there was a statistically significant difference between groups (p < 0.001 for each). There were negative correlations for both SFAZ and DFAZ with RE (r = −0.733 and r = −0.758, p < 0.001), CMT (r = −0.823 and r = −0.82, p < 0.001), SFCT (r = −0.647 and r = −0.717 p < 0.001) for the entire study population. SFAZ and DFAZ area were significantly correlated with AL (r = 0.732 and r = 0.745, p < 0.001) and ACD (r = 0.614 and r = 0.654, p < 0.001). In study group, 5 eyes did not have neither SFAZ nor DFAZ, 3 eyes had only DFAZ and 1 eye had only SFAZ in the OCT-A images.
Conclusions
SFAZ and DFAZ area were significantly smaller in nanophthalmic eyes than control eyes.
Similar content being viewed by others
Introduction
Nanophthalmos is a rare bilateral congenital disorder in which the eye volume is reduced without other major ocular or systemic anomalies [1]. The nanopthalmic eye is characterized by hyperopia, narrow palpebral fissure, shallow anterior chamber (AC), high lens/eye volume ratio, short axial length (AL), thick sclera, and some ocular complications such as angle-closure glaucoma and uveal effusion [1,2,3]. Many posterior segment findings associated with nanophthalmos have also been described, including foveal hypoplasia, macular cysts and striae, pseudopapilloedema, pigmentary retinal dystrophy, and choroidal thickening [4,5,6].
The fovea is defined as the central point of visual acuity and is comprised only of photoreceptors (cones) with elongated outer segments underlying a capillary free zone, termed the “foveal avascular zone” (FAZ).
The human retina has three distinct vascular plexuses; the radial peripapillary capillary plexus, the superficial (inner) vascular plexus, and the deep (outer) vascular plexus. Within the radial peripapillary capillary plexus, the superficial (inner) vascular plexus, and the deep (outer) vascular plexus reside the nerve fiber layer, the ganglion cell layer (GCL) and the inner nuclear layer (INL), respectively [7]. In the foveal region, the superficial and deep vascular plexus create a special capillary-free region, the FAZ, by forming a ring of interconnecting capillaries at the margin of the fovea [8].
Several studies have described the FAZ size and shape in healthy populations [9,10,11]. Abnormal FAZ features of some congenital disease such as foveal hypoplasia ocular albinism and nanopthalmos have also been previously described [12,13,14].
Optical coherence tomography angiography (OCT-A) is a new, non-invasive imaging technique that uses motion control contrast imaging to obtain high resolution volumetric blood flow information and generate a useful angiographic image without the use of any injectable fluorescein dyes [15].
The aim of this study was to evaluate FAZ in patients with nanopthalmos via OCT-A. To the best of our knowledge, the current study is the first study to describe quantitative FAZ measurement in nanophthalmic eyes using by OCT-A.
Methods
This observational, comparative, prospective study was carried out at the Health of Science University Beyoglu Eye Training and Research Hospital, Istanbul. The study was approved by Health of Science University Taksim Training and Research Hospital Medical Ethics Committee and performed in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient or from their parents for participants younger than 18 years old.
A total of 38 eyes of 30 participants were examined. The participants were divided into two groups: the study group included 19 eyes of 11 patients with nanophthalmos who have been followed up at our retina and glaucoma clinic, and the control group included 19 eyes of 19 gender- and age-matched healthy emmetropic cases. The diagnosis of nanopthalmos was based on a shorter than average AL (≤20 mm), a shallow AC (<3.0 mm), and moderate to severe hyperopia (>+3.5 diopters) [16]. The nanophthalmos patients in this study had no systemic disorders. All nanophthalmos patients were isolated type in the study. The control group was composed of age matched individuals with no known ocular diseases who admitted to our clinic for routine controls. The inclusion criteria for the normal group included: a best-corrected visual acuity (BCVA) of ≥16/20, a refractive error <3 diopters, normal slit-lamp and fundoscopy examinations, and no history of ocular or systemic disease.
Exclusion criteria included history of eye trauma, intraocular surgery, the use of medication either systemically or topically, optic neuropathy other than glaucoma or any retinal pathology other than specific retinal findings of nanophthalmos such as pseudopapilloedema, papillomacular fold, and leopard spots, conditions such as cataracts that cause insufficient imaging quality, and an inability to cooperate during OCT-A measurements and other examinations. Additionally, among the nanophthalmic eyes that presented with at least one of the following criteria were also excluded: an intraocular pressure (IOP) of >21 mmHg measured by Goldmann applanation tonometry at a minimum of 2 consecutive visits, or presence of cup-to-disc ratio >0.5 or cup-to-disc ratio asymmetry >0.2, or a definite glaucomatous visual field defect (paracentral scotoma, arcuate scotoma, nasal step, tubular vision, etc) on visual field test or any suspicious retinal nerve fiber thickness on optic nerve head OCT.
Examination procedures
Each participant underwent a comprehensive ophthalmological evaluation, including the measurement of best corrected visual acuity (BCVA) with a Snellen chart (converted to logMAR), measurement of intraocular pressure with a Goldmann applanation tonometer, slit-lamp biomicroscopy, and dilated fundus examination with a 90D lens. For all study participants, AL was measured with IOL Master optical biometry (IOL-Master, V.918471, model 1322-734; Carl Zeiss Meditec, La Jolla, California, USA). Anterior chamber depth (ACD, anteroposterior distance from the corneal epithelium to the lens surface) was measured via anterior segment OCT (Visante OCT 3.0 Model 1000, Carl Zeiss Meditec, Inc.). After pupil dilatation with tropicamide 1% (Alcon, Rødovre, Denmark) all OCT scans were obtained by the experienced operator (AD) with spectral domain OCT (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany). Central macular thickness (CMT) was measured automatically from the software of the system using the fast macular scan mode. The subfoveal choroid was imaged using the enhanced depth imaging (EDI) technique and subfoveal choroidal thickness (SFCT) was measured as previously [6].
All OCT-A scans also were obtained by the same experienced operator (AD) with the Spectralis OCT-2 prototype (Heidelberg Engineering, Heidelberg, Germany). The system uses 880 nm wavelengths Super Luminescence Diode (SLD) for tomography imaging and 85.000 A-scan per second scan rate. Each B-scan is currently up to 512 a-scans in high speed mode. It has 3.9 μm axial and 11 μm transverse resolutions. All macular OCT-A scans are available with a dual-beam active eye tracking system to eliminate eye movement artifacts and failures during the exam. The distances between B-scans are selected from 11 μm to the exact and real structures of the microvascular veins in all the segments without software corrections and approaches.
The scanning algorithm is as follows: Each B-scan section has been used for 4 frames and the system is following the fluctuation of the beam from each segment of the retina at every A-scan points using full-spectrum amplitude decorrelation technique. The foveola centered 20°x20° area was chosen on the macula for imaging the parafoveal region. OCT-A images contain horizontal 512 B-scans. The automatic real-time mode which increases the quality of each single B-scan and improves the signal-to-noise ratio was used during each image acquisition. Using confocal focus in small eye mode of Heidelberg OCT-A device provided better image quality in nanophthalmos patients who show a difficulty during image acquisition because of shorter axial length and higher hyperopia.
All retinal layers were automatically segmented by 6.8a Spectralis family software. Manual segmentation was used to confirm that the presence or absence of the FAZ was not due to segmentation error. The superficial vascular plexus en-face image was segmented with internal limiting membrane and inner plexiform layer, while the deep vascular plexus en-face image was segmented with inner plexiform layer and outer plexiform layer. Superficial vascular plexus FAZ (SFAZ) area and deep vascular plexus FAZ (DFAZ) area measurements were obtained by the same operators. For the measurement of the FAZ area, a 3 × 3 mm large, en-face image of the superficial retinal layer containing FAZ was selected and cropped. The SFAZ and DFAZ were manually outlined and measured by using the manufacturer’s software (Fig. 1).
En-face image of superficial foveal avascular zone (left) and cross sectional B- scan image (right) obtained with optical coherence tomography angiography. Foveal avascular zone area was markedly smaller in a patient with nanophthalmos (top) than in age-matched healthy control (bottom). SVP superficial vascular plexus, DVP deep vascular plexus
Outcome measures
SFAZ, DFAZ, AL, ACD, CMT, SFCT, BCVA, and refractive error (RE) were analyzed and compared between groups. The correlations of the measured parameters with SFAZ and DFAZ were investigated.
Statistical analysis
Snellen BCVA was converted into logarithm of the minimal angle of resolution (logMAR) for statistical analysis. Continuous variables are expressed as mean ± standard deviation (SD). Categorical variables are expressed as numbers (n) and percentages (%). The normality of continuous variables was assessed with Kolmogorov Smirnov test. Comparisons between groups were performed with Mann–Whitney U test. The Chi-square test was used to analyze qualitative independent data. Spearman test was used for correlation analysis. Analysis of covariance (ANCOVA) was used for multivariate analyses. SPSS 22.0 was used for all statistical analyses. p < 0.05 was accepted as statistically significant.
Results
The mean age was 18 ± 9.2 years in the study group and 17 ± 9.5 years in the control group (p = 0.84). There were 6 female and 5 male participants in the study group and 12 female and 7 male participants in the control group (p = 0.32). The demographic and clinical data of the groups are shown in Table 1.
The study group had lower BCVA, higher RE with hypermetropic deviation, higher CMT, higher SFCT, and shorter ACD-AL than the control groups expected (p < 0.05 for each comparison, Table 1).
In study group, 5 eyes did not have neither SFAZ nor DFAZ, 3 eyes had only DFAZ, and 1 eye had only SFAZ in the OCT-A images (Fig. 2). The mean SFAZ was 0.09 ± 0.12 mm2 and the mean DFAZ was 0.10 ± 0.10 mm2 in the study group. The mean SFAZ was 0.37 ± 0.10 mm2 and the mean DFAZ was 0.37 ± 0.10 mm2 in the control group. The mean SFAZ and DFAZ values were significantly smaller in study group than in control group (p < 0.001 and p < 0.001, respectively).
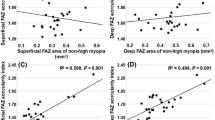
Both SFAZ and DFAZ were negatively correlated with SFCT, CMT, SE, and BCVA in the entire study population. The SFAZ and DFAZ were positively correlated with AL and ACD (Table 2).
When all of the studied parameters (AL, ACD, CMT, SFCT, and SE) that could affect SFAZ and DFAZ were eliminated in ANCOVA analyses, SFAZ and DFAZ were still found to be smaller in the study group (Table 3).
Discussion
Since the study of Novotny and Alvis in 1961, fluorescein angiography (FA) has been the mainstay technique for imaging the retinal vasculature [17]. However, FA can only achieve visualization of the superficial plexus. In contrast to FA, OCTA visualizes all three retinal vascular layers [7].
In this prospective comparative study we assessed the FAZ in patients with nanophthalmos and compared these findings with age-matched healthy individuals using OCT-A. It was more difficult to get high-quality images in patients with nanophthalmos. Because they had smaller eye, higher hyperopia, and crowded retina. To overcome these problems we chose “small eye mode” and we used “confocal focus” of Heidelberg OCT-A device. In the nanophthalmos group, 5 eyes did not have neither SFAZ nor DFAZ, 3 eyes had only DFAZ, and 1 eye had only SFAZ in OCT-A images. We also observed small or rudimentary SFAZ and DFAZ in nanopthalmos group (Fig. 1). These findings are in agreement with previous reports by Walsh et al [14]. They investigated FAZ situation in four patients with nanophthalmos using FA. They stated that FAZ was rudimentary, absent, or small in nanopthalmos. Our study differs from that of Walsh and colleagues in a number of ways; we evaluated SFAZ and DFAZ using by OCT-A, we reported quantitative FAZ measurement and its correlations in a larger sample size, and compared these results with normal subjects. The SFAZ and DFAZ were significantly smaller in the nanophthalmos group compared with healthy individuals.
SFAZ and DFAZ showed a positive correlation with AL and ACD and there was a negative correlation between both SFAZ and DFAZ and hyperopic RE in the current study. These findings are opposite to those of Wang et al. who performed a study in healthy individuals [18]. They reported that larger FAZ area significantly correlated with shorter AL. Additionally they did not find any correlation between FAZ area and ACD and hyperopic RE. The disparity between these findings may be caused by much shorter AL, shallow AC, and higher hyperopia in patients with nanophthalmos in our study. We found an inverse correlation between SFAZ and DFAZ with CMT, SFCT, and BCVA (LogMAR). A negative correlation was found between the CMT and FAZ in other studies [9,10,11].
The small size of the FAZ and its associations with AL, ACD, RE, CMT, and SFCT in nanophthalmos can be explained through foveal development and arrrest upon axial elongation of the globe. A developmental model of the primate fovea by Springer and Hendrickson suggested that the organization of the foveal layers is influenced by FAZ size and intraocular pressure. This model proposes that once the FAZ is formed at 24–25 week of gestation, the foveal pit progressively deepens under the effect of intraocular pressure. Then, with axial growth, there is migration of the inner retinal layers away from the foveal center, migration of the cone photoreceptors into the foveal center, and elongation of the photoreceptors [19,20,21].
In nanopthalmos this axial growth is restricted as a result of arrest in the development of the globe in all dimensions. For geometrical reasons, one might have expected that with decreasing axial length ACD decreases, CMT and SFCT increase, and SFAZ and DFAZ decrease. However, when we analyzed SFAZ and DFAZ between groups after eliminating AL, RE, CMT, SFCT, and ACD, the SFAZ and DFAZ area is still small in nanopthalmic eyes. In addition to elongation of the globe, intact FAZ is required to form the foveal morphology. Hence, one reason for the foveal hypoplasia seen in the nanophthalmos group may be that the foveal avascular zone is absent or small in these patients.
The current study has certain limitations. These include the manual measurement of FAZ area that might be subject to interobserver variability, difficulty in measuring the FAZ area in nanophthalmic eyes because of poorly defined borders when compared with controls, and there was no visible SFAZ and/or DFAZ in some eyes in study group.
In summary, SFAZ and DFAZ were significantly smaller in nanophthalmic eyes. Further advances in OCT and OCT-A devices may help to explain the pathogenesis of the nanophthalmos findings.
Summary
What was known before
-
In the litherature there are a few reports associated foveal avascular zone situation in patients with nanohthalmos. Flörescein angiography was used to show foveal avascular zone in these studies.
What this study adds
-
The current study is the first study to describe quantitative foveal avascular zone measurement in nanophthalmic eyes using by optical coherence tomography angiography.Our study differs from others in a number of ways; we evaluated süperficial and deep foveal avascular zone using by optical coherence tomography angiography, we reported quantitative foveal avascular zone measurement and its correlations in a larger sample size, and compared these results with normal subjects.
References
O’Grady RB. Nanophthalmos. Am J Ophthalmol. 1971;71:1251–3.
Kimbrough RL, Trempe CS, Brockhurst RJ, Simmons RJ. Angle-closure glaucoma in nanophthalmos. Am J Ophthalmol. 1979;88:572–9.
Brockhurst RJ. Cataract surgery in nanophthalmic eyes. Arch Ophthalmol. 1990;108:965–7.
Duke-Elder S. System of ophthalmology. vol. 3. St. Louis: CV Mosby; 1963. p. 488–95.
MacKay CJ, Shek MS, Carr RE, Yanuzzi LA, Gouras P. Retinal degeneration with nanophthalmos, cystic macular degeneration and angle closure glaucoma. A new recessive syndrome. Arch Ophthalmol. 1987;105:366–71.
Demircan A, Altan C, Osmanbaoglu OA, Celik U, Kara N, Demirok A. Subfoveal choroidal thickness measurements with enhanced depth imaging optical coherence tomography in patients with nanophthalmos. Br J Ophthalmol. 2014;98:345–9.
Spaide RF, Klancnik JM, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50.
Snodderly DM, Weinhaus RS, Choi JC. Neural-vascular relationships in central retina of macaque monkeys (Macaca fascicularis). J Neurosci. 1992;12:1169–93.
Tick S, Rossant F, Ghorbel I, Gaudric A, Sahel JA, Chaumet-Riffaud P, et al. Foveal shape and structure in a normal population. Invest Ophthalmol Vis Sci. 2011;52:5105–10.
Wu LZ, Huang ZS, Wu DZ, Chan E. Characteristics of the capillary-free zone in the normal human macula. Jpn J Ophthalmol. 1985;29:406–11.
Samara WA, Say EA, Khoo CT, Higgins TP, Magrath G, Ferenczy S, et al. Correlatıon of foveal avascular zone sıze wıth foveal morphology ın normal eyes usıng optıcal coherence tomography angıography. Retina. 2015;35:2188–95.
Bazvand F, Karkhaneh R, Roohipoor R, Rajabi MB, Ebrahimiadib N, Davoudi S. et al.Optical coherence tomography angiography in foveal hypoplasia.Ophthalmic Surg Lasers Imaging Retina. 2016;47:1127–31.
Dubis AM, Hansen BR, Cooper RF, Beringer J, Dubra A, Carroll J. Relationship between the foveal avascular zone and foveal pit morphology. Invest Ophthalmol Vis Sci. 2012;53:1628–36.
Walsh MK, Goldberg MF. Abnormal foveal avascular zone in nanophthalmos. Am J Ophthalmol. 2007;143:1067–8.
De Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous. 2015;1:5.
Singh OS, Simmons RJ, Brockhurst RJ, Trempe CL. Nanophthalmos: a perspective on identification and therapy. Ophthalmology. 1982;89:1006–12.
Novotny HR, Alvis DL. A method of photographing fluorescence in circulating blood in the human retina. Circulation. 1961;24:82–86.
Wang Q, Chan S, Yang JY, You B1, Wang YX, Jonas JB, et al. Vascular density in retina and choriocapillaris as measured by optical coherence tomography angiography. Am J Ophthalmol. 2016;168:95–109.
Springer AD, Hendrickson AE. Development of the primate area of high acuity. 1. Use of finite-element analysis models to identify mechanical variables affecting pit formation. Vis Neurosci. 2004;21:53–62.
Springer AD, Hendrickson AE. Development of the primate area of high acuity. 2. Quantitative morphological changes associated with retina and pars plana growth. Vis Neurosci. 2004;21:775–90.
Springer AD, Hendrickson AE. Development of the primate area of high acuity, 3: temporal relationships between pit formation, retinal elongation and cone packing. Vis Neurosci. 2005;2:171–85.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Demircan, A., Yesilkaya, C., Altan, C. et al. Foveal avascular zone area measurements with optical coherence tomography angiography in patients with nanophthalmos. Eye 33, 445–450 (2019). https://doi.org/10.1038/s41433-018-0236-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-018-0236-7