Abstract
Design Randomised controlled trial.
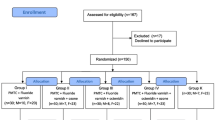
Study population Patients undergoing routine orthodontic treatment, aged 12 years and older, were randomly allocated to use either Clinpro 5000, Clinpro Tooth Crème or MI Paste Plus for 4 months.
Data Analysis Three-way analysis of variance (ANOVA) was used to analyse the Enamel Decalcification Index (EDI) scores of the maxillary and mandibular arches from the right first premolar to the left first premolar at 0.05 level of significance. Fisher-protected least significant difference intervals were used to compare mean EDI scores. Two operators scored photographs independently and their scores were compared by using the t-test; no statistically significant difference (P ≥.05) was found between the operators. Results of each product were analysed individually and amongst themselves. A multilevel mixed-effects Poisson regression was used to model factors predictive of EDI scores for individual teeth.
Results Of the 120 subjects enrolled, 100 (35 using Clinpro 5000, 32 using Clinpro Tooth Crème, and 33 using MI Paste Plus) completed the study. The data lends support for Clinpro 5000 providing superior protection against enamel decalcification when compared to Clinpro Crème, and mixed support when compared to MI Paste Plus.
Conclusions The use of Clinpro 5000, Clinpro Tooth Crème, and MI Paste Plus all have a reduction effect on white spot lesions when compared to studies reported previously. They found Clinpro 5000 to have a marginally better effect than the two other test pastes.
Similar content being viewed by others
A Commentary on
Kau C H, Wang J, Palombini A, Abou-Kheir N, Christou T. Effect of fluoride dentifrices on white spot lesions during orthodontic treatment: A randomized trial. Angle Orthod 2019; 89: 365-371.

GRADE rating
Commentary
White spot lesions (WSL) are a common side effect following fixed orthodontic appliance treatment.1 The reported prevalence ranges widely from 2% to 96% depending on the method and criteria for detection as well as patient compliance with preventative measures.2,3,4 Clinically detectable WSL's have been reported to occur as early as one month after the placement of orthodontic appliances.5 Whilst the processes that can lead to enamel demineralisation are well understood, methods to reduce or eliminate the degradation of enamel surfaces are still being developed.
This paper reports on a randomised controlled trial to determine the effect of Clinpro 5000, Clinpro Tooth Crème, and MI Paste Plus on the formation of white spot lesions in patients undergoing orthodontic treatment. MI Paste Plus, which contains casein phosphopeptide-amorphous calcium phosphate, has previously been shown to be effective in the reduction of WSLs,6 whereas the other two products are new anticavity sodium fluoride toothpastes which have only been reported to be useful in WSL reduction in case reports.
The trial was registered in the Registry of Clinical Trials run by the United States National Library of Medicine and the authors report that they followed the CONSORT checklist to ensure transparent and standardised reporting of the trial. The authors carried out a sample size calculation based on the results of a previous study with similar groups of subjects6 which was adequately powered to detect a difference of 1.0 unit change in the enamel decalcification index (EDI). This was a good use of effect size findings from the previous study to calculate sample size. The subjects were randomised by drawing individual slips of paper, however, there is no mention of further blinding of either the subjects or investigators. Their method of randomisation doesn't ensure distribution of patients at risk of enamel demineralisation across groups. It would have been better if they had considered stratified randomisation which would have taken into account amplification effects of fixed appliances on poor oral hygiene.
Of the 120 subjects recruited into the study, only 100 were recalled and analysed, giving a 20% dropout rate which is quite high. The drop outs are accounted for and no harms were reported, however, this could have skewed their results if all of these subjects dropped out due to oral hygiene reasons. The authors did not carry out an 'intention to treat analysis', which would have preserved the sample size and accounted for non-compliant subjects who may have dropped out from the study due to their response to treatment. With respect to the groups at the start of treatment, we are told no information on the patient demographics such as age, sex, dental history or social class, all which could have an effect on compliance, oral hygiene technique and susceptibility to WSL's. The authors did acknowledge that the baseline EDI scores were different across the groups, and their multilevel mixed-effects Poisson regression method, which is appropriate for discrete data, did at least attempt to control for this.
The statistical analysis was appropriate, with a three-way analysis of variance used to analyse the EDI scores, and Fisher-protected least significant differences intervals used to compare the mean EDI scores. Two operators scored the photographs independently and no statistical differences between operators was noted.
The results are clearly presented and the authors used a multilevel mixed-effects Poisson regression to model the data which is appropriate given the distribution of EDI scores. The results indicate that treatment effects did exist, above and beyond the other variables controlled for. The significant difference between Clinpro 5000 and Clinpro Tooth Crème was at the 95% level, however, when comparing Clinpro 5000 and MI Paste Plus the significance was only marginal, and at a lower significance level of 90%.
It would be expected that a fluoride paste with 5000 ppm fluoride ion would perhaps perform better than a paste with 950 ppm fluoride ion, and also it is interesting that MI Paste Plus did not perform significantly worse in relation to the higher concentration fluoride paste. The results of Model 5 indicate the use of Clinpro Tooth Crème or MI Paste Plus as an alternative to Clinpro 5000, could over the course of the four month investigation period be associated with an increase of approximately 1.5 EDI point and 1 EDI point per tooth respectively. There is some ambiguity as to whether this is clinically relevant especially given the large confidence intervals and the limitations of some of the study designs. The authors could benefit from providing guidance as to how EDI scores should be interpreted as clinically relevant, whether this be through the use of thresholds or otherwise.
This clinical study had some limitations which were acknowledged by the authors. They encountered difficulties with controlling patient compliance, to overcome this they gave the patients brushing technique instructions, standardised the time and frequency for brushing, and used a brushing diary as a control for their actual compliance with the protocol. The authors, however, fail to acknowledge that diary studies can be unreliable and do not account for demand characteristics, ie where patients provide responses they believe are desired.7 Despite the authors efforts to ensure adequate oral hygiene throughout treatment, a considerable number of patients developed suboptimal oral hygiene levels with plaque accumulation and gingival inflammation.
A more serious limitation of this study was its duration of only four months. The prevalence of white spots in orthodontic treatment has been reported to reach 38% in six months, and increase slightly to 46% after 12 months.8 The longer orthodontic treatment is, the more likely WSLs will develop and also patients' oral hygiene may deteriorate with increasing treatment time. It would be beneficial to observe the effect of these fluoride pastes over more than just the early stage of orthodontic treatment, for example the entire duration of orthodontic treatment, which is usually 18-24 months.
The conclusion by the authors that the results of the study can be used by clinicians to decide the 'effectiveness of using fluoride dentifrice products to prevent white spot lesions in their orthodontic practice' would have benefitted from mentioning that it is with reference to the three tested fluoride dentifrice products specifically. The two new sodium fluoride pastes, Clinpro 5000 and Clinpro Tooth Crème, are both produced by 3M ESPE (St. Paul, Minn, USA), and the authors declared that the research was supported by an Industry Grant from 3M ESPE. It would be interesting if future studies investigated similar fluoride pastes from other brands to reduce potential biases.
In summary, this study was well designed but would have been strengthened by being of longer duration. It is perhaps not surprising that an increased fluoride concentration paste would result in lower levels of WSL formation, however, the results only give an indication that it is better and do not necessarily show that it is clinically significant. Further research over longer time periods would perhaps strengthen this work. However, it does give useful information to help clinicians decide whether to incorporate recommendations for the use of these new anticavity toothpastes, over and above their existing recommendations, into an oral hygiene regimen for their orthodontic patients.
References
Chapman J A, Roberts W E, Eckert G J, Kula K S, Gonzalez-Cabezas C. Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances. Am J Orthod Dentofacial Orthop 2010; 138: 188-194.
Gorelick L, Geiger A M, Gwinnett A J. Incidence of white spot formation after bonding and banding. Am J Orthod 1982; 81: 93-98.
Mitchell L. Decalcification during orthodontic treatment with fixed appliances--an overview. Br J Orthod 1992; 19: 199-205.
Tufekci E, Dixon J S, Gunsolley J C, Lindauer S J. Prevalence of white spot lesions during orthodontic treatment with fixed appliances. Angle Orthod 2011; 81: 206-210.
Melrose C A, Appleton J, Lovius B B. A scanning electron microscopic study of early enamel caries formed in vivo beneath orthodontic bands. Br J Orthod 1996; 23: 43-47.
Robertson M A, Kau C H, English J D, Lee R P, Powers J, Nguyen J T. MI Paste Plus to prevent demineralization in orthodontic patients: a prospective randomized controlled trial. Am J Orthod Dentofacial Orthop 2011; 140: 660-668.
McCambridge J, de Bruin M, Witton J. The effects of demand characteristics on research participant behaviours in non-laboratory settings: a systematic review. PLoS One 2012; 7: e39116.
Artun J, Brobakken B O. Prevalence of carious white spots after orthodontic treatment with multibonded appliances. Eur J Orthod 1986; 8: 229-234.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Smyth, R., Noar, J. Preventing white spot lesions with fluoride pastes. Evid Based Dent 20, 88–89 (2019). https://doi.org/10.1038/s41432-019-0041-6
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41432-019-0041-6