Abstract
The inherited disease community in Sri Lanka has been widely neglected. This article aimed to present accumulated knowledge in establishing a pro bono cost-effective national, island-wide, free-of-charge molecular diagnostic service, suggesting a model for other developing countries. The project provided 637 molecular diagnostic tests and reports free of charge to a nation with limited resources. We pioneered the implementation of mobile clinics and home visits, where the research team acted as barefoot doctors with the concept of the doctor and the researcher at the patient’s doorstep. Establishing pro bono, cost-effective molecular diagnostics is feasible in developing countries with limited resources and state funding through the effort of dedicated postgraduate students. This service could provide an accurate molecular diagnosis of Duchenne muscular dystrophy, Huntington’s disease, Spinocerebellar ataxia, and Spinal muscular atrophy, a diagnostic yield of 54% (343/637), of which 43% (147/343) of the patients identified as amenable for available gene therapies. Initiated human resource development by double doctoral degree opportunities with international collaborations. Established a neurobiobank and a national registry in Sri Lanka, a rich and unique repository, wealth creation for translational collaborative research and sharing of information in neurological diseases, as well as a lodestar for aspiring initiatives from other developing countries.
Similar content being viewed by others
Introduction
The inherited diseases community in Sri Lanka has been highly neglected, often being labeled as “incurable”. It is noteworthy that comparatively little attention seems to be given to patients with neurogenetic diseases. Neurogenetic testing is almost nonexistent in Sri Lankan government hospitals and is only available in a few private sector centers at exorbitant costs. Moreover, research in developing countries has been hampered by limited advanced clinical resources (lack of clinicians/experts having sound knowledge and experience in clinical genetics and lack of standardized clinical guidelines and specific clinical infrastructure) and far-to-approach sporadically localized genetic service centers [1,2,3]. This has resulted in needy patients undergoing unavoidable clinical investigations, leading to multiple indefinite diagnoses [4, 5].
Addressing these issues, the corresponding author pioneered in establishing a free-of-charge neuromolecular diagnostic service at a governmental institute, despite many challenges, efforts led to the successful molecular diagnosis of 343 out of 637 patients with rare diseases, representing a 54% confirmation rate as summarized in Table 1. We assess the demographic features, diagnostic yield through conventional PCR and Multiplex Ligation Dependent Probe Amplification (MLPA) analysis, a pro bono neurogenetic diagnostic service in national single center in Sri Lanka.
In Low Middle-Income Countries, a critical shortage of health-care workers such as medical geneticists and scientists is clearly evident due to the movement of skilled labor to developed countries [6]. Thereby, in literature to the best of our knowledge pro bono molecular genetic testing services were reported only in India (http://gomed.igib.in) and the West Indies [7]. In this context this article aimed to present accumulated knowledge in establishing a pro bono cost-effective national, island-wide, free-of-charge molecular diagnostic service, suggesting a model for other developing countries. It shows the diagnostic yield of selected rare neuromuscular, and trinucleotide repeat disorders in Sri Lanka, which were executed with minimal resources and cost, the uphill task of establishing a neurobiobank, and how investing in human resources is crucial for sustainability.
Materials and Methods
The establishment of pro bono molecular diagnostic model was based on following key strategic areas (Fig. 1),
The key strategic areas comprised of Infrastructure and human resource development achieved through international/ local funding and Double doctoral degree opportunities with international collaborations, Island wide Patient recruitment based on neurology clinic visits and pro bono mobile home visits followed by Molecular diagnostics.
Infrastructure development
Securing international/ local funding and donations for the basic molecular biology laboratory infrastructure development and human resource development
The corresponding author received a donation of equipment from the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institute of Health (NIH), USA in 2006 through the International Brain Research Organization Asia Pacific Regional Committee (IBRO-APRC). Additionally, equipment was donated by the Chinese Neuroscience Society (CNS) in 2014. This was a pivotal stepping stone for the corresponding author as the principal investigator (PI) in setting up the pro bono molecular diagnostic service at the Interdisciplinary Center for Innovation in Biotechnology and Neuroscience, University of Sri Jayewardenepura (USJ), Sri Lanka. As of 2021, this service is being supported by the General Sir John Kotelawala Defence University in Sri Lanka. The World Health Organization (WHO) in 2012 and the Muscular Dystrophy Association (MDA) in the USA in 2010 funded the project to ensure the sustainability of this approach. Research grants were received from the University of Sri Jayewardenepura, General Sir John Kotelawala Defence University, and Ministry of Primary Industries, Sri Lanka.
Patient recruitment
Island-wide pro bono mobile clinics in selected government hospitals and home visits in the Western, North-Western, North Central, Central, Southern Provinces and Northern Provinces. In the Northern Province, the Jaffna Teaching Hospital was the hub for patient recruitment. It is noteworthy that offering such medical genetic services to patients from Jaffna has been hindered for nearly 30 years because of the civil war.
Patient recruitment was based on island-wide neurology clinics in ten (10) government teaching hospitals from 2014–2020. Independent registries were maintained by the pro bono team and representative doctors at each neurology clinic.
Establishment of pro bono mobile home visits
During clinic visits, the visiting pro bono team observed that patient participation in the clinic for follow-up studies was hindered. This was due to:
-
I.
Patients’ hopelessness mindset, which was developed due to the labeling of these patients as “incurable” in the initial clinic visit and lack of proper counseling.
-
II.
High cost of travel to the hospital for disabled or wheelchair-bound patients.
-
III.
The poor mutual understanding and relationship between patients and the healthcare team in the clinic, creating a gap that made the patients and their family members reluctant to express their true experience of social, emotional, and financial hardships of living with an inherited disease.
This led to the establishment of pro bono mobile home visits by the visiting pro bono team, who acted as barefoot doctors with the concept of the doctor and the researcher at the patient’s doorstep.
The team comprised medical doctors, neuroscientists, postgraduate students, and technical assistants. Detailed clinical evaluations were performed, and the patients were directed to the nearest neurology clinic at a government hospital for further medical assistance. Moreover, frequent home visits helped develop a rapport with the pro bono team and the patient and their family members, which was beneficial in identifying medical, social, and financial problems. Patients who were identified with specific medical needs (psychiatric, cardiology, occupational therapy, physiotherapy, and counseling) were referred to relevant specialties. Financial and social issues were addressed and facilitated through relevant government departments, and progress was followed by the pro bono research team.
This approach met the ethical guidelines of the Sri Lankan Institutional Review Board and complied with the Declaration of Helsinki. Written informed consent was obtained from each proband where applicable. Informed consent was obtained from a proxy if the patient was unable to provide consent on their own.
Molecular diagnostic
This study utilized the molecular diagnostic approach established in-house following published papers as described in Wijekoon et al. 2023 [8]. A summary of this approach is as follows. The initial diagnostic test for detecting deletions and duplications followed a level one testing approach, utilizing Multiplex PCR (mPCR) for 20 exons covering proximal and distal hot-spot regions of the Duchenne Muscular Dystrophy (DMD) gene as described by Chamberlain et al. [9] and Beggs et al. [10] followed by the MLPA assay (MRC Holland SALSA MLPA Probe mixes P034 and P035) for all the clinically diagnosed dystrophinopathy patients. The diagnostic procedure was established utilizing the primary molecular diagnostic recommendations as outlined by Abbs et al. in 2010, as well as the revised edition by Fratter et al. in 2020, in alignment with the European Molecular Quality Genetics Network’s (EMQN) optimal practice guidelines for genetic testing in dystrophinopathies [11, 12]. In order to ascertain the impact of variations on the reading frame, the frame-shift checker available on the Leiden Muscular Dystrophy website (www.dmd.nl) was utilized to scrutinize all identified deletions and duplications.
Spinocerebellar Ataxia (SCA) genetic analysis was also based on the EMQN guidelines [13] where we have modified SCA testing priorities and considered testing for further genes on a case-by-case basis on the basis of local population disease frequencies, the geographic and ethnic origin of the patient and the information provided in the referral with regard to clinical presentation and the apparent mode of inheritance. Huntington’s Disease (HD) genetic analysis was also based on the EMQN guidelines [14].
This was funded by foreign and local donations and research grants to the PI, with inadequate support from the government, which was challenging. Molecular diagnostics and genomic data interpretation were performed by two PhD scholars in neuroscience (LG and NW, the co-primary authors of this manuscript).
The accuracy, precision and validity of molecular diagnostics performed in our laboratory were confirmed by re-evaluating random samples of DMD, HD, SCA and SMA for genetic mutations following the same protocol at collaborative laboratories in India (Center for DNA Fingerprinting & Diagnostics, Institute of Genomics and Integrative Biology). Moreover, at the inception, the positive controls were provided by the Center for DNA Fingerprinting & Diagnostics, Institute of Genomics and Integrative Biology, India. The interpretation of genetic testing reports was based on the guidelines published in NCBI Gene reviews [15,16,17,18,19,20].
Human resource development
Human resource development by double doctoral degree opportunities with international collaborations and disseminating scientific knowledge by organizing international workshops, training schools and conferences.
Results
Molecular Diagnostics
A nationwide, free-of-charge, traditional molecular diagnostic service was offered to utilize basic equipment (i.e., a polymerase chain reaction [PCR] machine and gel documentation system). The project provided 637 molecular diagnostic tests (PCR-based methods) and reports free of charge as a service to the nation (Table 1).
With a diagnostic yield of 54% (343/637), our approach may accurately diagnose DMD, HD, SCA, and SMA patients using molecular means. Of the myopathy patients in this cohort, we could give molecular diagnostic for 55% (131/236). It’s interesting to note that 96% (131/136) of the patients in this cohort with deletion mutations could be diagnosed by mPCR. A group of patients with SCA type 1 in three villages in the southern part of Sri Lanka was identified (n = 29/61). 41 patients (DMD, 9; SMA, 2; SCA, 17; and HD, 13) in our cohort had afflicted siblings or relatives. Of the 343 genetically diagnosed patients, 147 (DMD, 83; SMA, 15; and HD, 49) qualified for gene therapy.
Cost effectiveness
The authors were successful in providing a low-cost molecular diagnostic approach, including the multiplex ligation probe amplification test and PCR-based molecular diagnostics, with 27–54 USD spent on the chemicals and consumables per test. The cost inclusive of utility and labor is 42-69 USD. However, it is noteworthy that the costs associated with labor and utilities were covered by the institute. This cost was cheaper than the costs reported in the literature for regional countries (inclusive of utility and labor); in Pakistan, the cost of a PCR test for 22 exons of the DMD gene is approximately 333 USD [21], and in India, the same test costs approximately 52 USD [22]. Moreover, in India, genetic testing for HD and SCA panels (types 1, 2, and 3) costs 35 USD and 79 USD, respectively.
Benefit to the patient
With our efforts, the patients and their families benefited from a definitive diagnosis achieved through a free of charge molecular diagnostic report, resulting in better disease management and allowing them to understand the implications. The patients were referred for formal physical, speech, nutritional, and rehabilitative therapy and counseling under the supervision of the respective consultants. Moreover, patient’s specific medical needs and financial and social issues were addressed by referring to relevant medical specialties and relevant government departments.
Human resource development
Testing genetic diseases, conducting research, and training personnel who can make genetic testing more widely available to the public and serve the needs of the inherited disease community in Sri Lanka are crucial. In this regard, the author successfully initiated:
-
a.
Double doctorate opportunities between Maastricht University in the Netherlands and Sri Lanka; the first double doctorate was awarded in 2020, with the corresponding author as the PI. Two more PhDs are registered for double doctorate opportunities with Maastricht University, which will result in two neuroscientists with international training capable of continuing molecular diagnostic services.
-
b.
Six international neuroscience and molecular biology workshops and postgraduate training through exchange programs with support from the IBRO and the International Society for Neurochemistry. The Center for DNA Fingerprinting and Diagnostics, India; CSIR Institute of Genomics and Integrative Biology, India; Alzheimer’s Association, USA; The Movement Disorders Society; and the National Institute of Advanced Industrial Science and Technology, Japan provided training.
More than 15 Medical Graduates were recruited for this ongoing research during the study period and were trained in neurogenetics and clinical neuroscience.
Discussion
To the best of our knowledge, our service stands out due to its unique approach of providing doctors and researchers at the doorstep, in contrast to the reported pro-bono molecular genetic testing services in India and the West Indies.
A definitive diagnosis beneficially resulted in the patients, their families, the community, and the inherited disease community as follows:
-
a.
Cost-effective disease management invalidates unnecessary empirically expensive therapies that could have serious side effects; prevents comorbidities; reduces morbidity, mortality, and secondary manifestations; and improves the quality of life and mental well-being.
-
b.
Neurologists are guided in understanding some of their most challenging patients who would have been left without answers and entangled in a diagnostic odyssey. Two patients in whom the physician initially suspected limb girdle muscular dystrophy (LGMD) and Pompe disease were later confirmed to have DMD via genetic testing.
-
c.
Potential prevention through genetic counseling and multidisciplinary treatment increases quality of life and life expectancy. An optimal treatment plan can promote prevention and early detection, allowing family planning. In our cohort, 41 cases (DMD, 9; SMA, 2; SCA, 17; and HD, 13), had affected siblings or family members. This indicates the need to establish carrier detection [23] and preimplantation genetic testing [24] within a proper legal framework, along with genetic and reproductive counseling, which is far from the current reality in Sri Lanka.
-
d.
Enrollment in clinical trial registries: 147 out of 343 (DMD,83; SMA,15; and HD, 49) patients were eligible for gene therapy [8, 25]. Fruitful discussions are underway with the Working Group consist of multiple stakeholders with expertise in the advocacy, diagnosis, treatment of rare neuromuscular disorders. The members include clinical experts from Sydney Children’s Hospital, Australia working in partnership with international patient advocate group, Advocacy Beyond Borders and diagnostic and therapeutic pharmaceutical stakeholders. The Committee members are self-funded with no financial or commercial conflicts of interest.
-
e.
Creation of National Wealth: established a neuro-biobank [26]: seventy six (76) aging human autopsy brains [27] and a DNA/serum repository of 2700 neurological disease patients: stroke (n-1200), Parkinson’s (n-350), neurodegenerative, neuromuscular, rare disease, and 500 controls. The rare disease cohort (Table 1) will be an asset for linking East and West, as 287 (46%) of the rare-disease cohort were negative for common mutations and a sporadic case of monozygotic twins genetically confirmed with DMD was identified. International collaborations to identify novel mutations and perform epigenetic analyses. Development of genetic networks in innovative multicenter translation therapeutic projects.
-
f.
Further cost reduction is possible by narrowing the number of SCA tests performed per patient based on geographical localization, which was based on an identified cluster (n = 29/61) of patients with SCA type 1 in three villages in the southern region of Sri Lanka.
-
g.
In our study multiplex PCR(mPCR) could provide molecular diagnosis for 55% (131/236) of the myopathy patients, Intriguingly 96% (131/136) of the patients with deletion mutations could be diagnosed by mPCR. Thus utilization of mPCR as an initial molecular diagnostic method is deemed cost-effective for nations with restricted resources, given its 96% rate of detection for deletions.
-
h.
Using serum samples of DMD patients from the biobank, Wijekoon et al. (2023) conducted a study correlating serum protein signatures with cognitive performance in DMD patients. Intriguingly, the study’s novel findings suggested a common pathogenic mechanism underlying for both Alzheimer’s disease and DMD cognitive impairment [27,28,29]. Further more promising fluid biomarkers for SCAs have not yet been validated, the authors were able to identify and validate novel serum biomarkers in SCA (Unpublished data).
Sri Lanka is served by 44 neurologists (1 neurologist per 500,000 people), reflecting the gross mismatch between the burden of neurological disorders and the availability of resources [30]. These numbers are much worse when the Indian subcontinent is considered; in India and Pakistan, there is one neurologist per one million people. This reflects a shortage of neurologists in regional countries [31, 32]. Patients affected by neurogenetic diseases in Sri Lanka must travel between 40 and 50 km to the closest regional hospital to seek specialty care. This results in the high cost of travel to hospitals for disabled or wheelchair-bound patients, which is greater than the monthly income of most families [33].
Most of Sri Lanka’s population (81.5%) live in rural areas [34]. A recent study identified that 42% (9.6 million people) lives below the poverty line [35]. Notably, the majority (80.9%) of the poor live in rural areas that are underdeveloped in healthcare, public transportation, and education. This has resulted in disparities in access to healthcare between rural and urban populations, mainly due to the lack of retention of healthcare workers in rural areas. This disparity has significantly affected the estate sector, as reflected by poorer health outcomes than in the urban and rural sectors in Sri Lanka. In line with this, it has been reported that maternal and child Health and nutritional and psychosocial indicators lag behind in the estate sector [36]. Moreover, the Multidimensional Poverty Index (MPI) of children reveals that 42.2% of children 0–4 y of age are poor, and one-third of young children are undernourished [37].
The key findings, limitations and future directions
The key findings of this study are as follows: (i) The hindrance of patient participation in follow-up studies at the clinic can be effectively addressed by implementing mobile home visits. (ii) It is possible to achieve a diagnostic yield of 54% for patients with DMD, HD, SCA, and SMA, even in settings with minimal resources. (iii) Cost reduction can be achieved by utilizing mPCR as an initial molecular diagnostic method for DMD, as it has a 96% detection rate for deletions and by narrowing the number of SCA tests performed per patient based on their geographical localization.
The clinical evaluation conducted during mobile clinics at patients’ homes may be influenced by the infrastructure available at their residences. In such cases, it may be necessary to arrange for the patient’s transportation to the nearest hospital when deemed necessary. Furthermore, in Sri Lanka, it is observed that individuals affected by progressive neuro-degenerative genetic diseases often conceal their condition due to cultural and social factors. This is primarily driven by the stigma associated with these diseases. Consequently, once their condition becomes known, these patients are often isolated and excluded from society. Therefore, as a result of these factors, patients often experience underdiagnosis. Thirdly, the lack of a comprehensive patient recording system in many government hospitals for rare neurological diseases, coupled with the absence of a permanent solution, has resulted in a significant number of patients not being able to attend regular clinics. Consequently, it becomes challenging to provide adequate coverage for all patients.
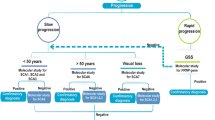
Although the current detection rate of our approach stands at 55%, there is potential for improvement through the implementation of a phenotype-based prioritization strategy for candidate genes. In this particular scenario, it is recommended to refine the initial clinical diagnosis of the patient by convening a “Genomic Odyssey Board”. (Fig. 2) This board will consist of esteemed professionals including Medical Geneticists, Neuroscientists, Clinicians, Neurologists/Pediatric Neurologists, Genetic Counselors, and International experts in the relevant field. The primary objective of this board is to reach a conclusive determination of the patient’s phenotype through a systematic process. Subsequently, the molecular diagnostics pertaining to the chosen phenotype will be conducted during the initial phase.
It is proposed to have multidisciplinary panel of experts including medical geneticists, Neuroscientists, Clinicians, Neurologists/ pediatric Neurologists, Genetic counselors and international experts in the relevant field. This approach will lead to molecular diagnostics pertaining to the chosen phenotype.
In conclusion, we show that establishing pro bono cost-effective molecular diagnostics is feasible in developing countries with limited resources and state funding with dedicated postgraduate students. Moreover, we show that accurate molecular diagnosis of DMD, HD, SCA, and SMA patients, a diagnostic yield of 54% (343/637), of which 43% (147/343) of the patients identified as amenable for available gene therapies using basic equipment, which can serve as a model for other developing countries, benefiting affected individuals with a definitive molecular diagnosis, their families, the health care systems and the community at large by genetic counseling and prevention. The work has resulted in establishing neurobiobank and a national registry in Sri Lanka, a rich and unique repository, a wealth creation for translational collaborative research and sharing of information in neurological diseases, as well as a lodestar for aspiring initiatives from other developing countries with limited resources.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Pradhan S, Sengupta M, Dutta A, Bhattacharyya K, Bag SK, Dutta C, et al. Indian genetic disease database. Nucleic acids Res. 2010;39:D933–D8.
Aggarwal S, Phadke SR. Medical genetics and genomic medicine in India: current status and opportunities ahead. Mol Genet Genom Med. 2015;3:160.
Kasthuri A. Challenges to healthcare in India-The five A’s. Indian J Commun Med: Off Publ Indian Assoc Prev Soc Med 2018;43:141.
Knight AW, Senior TP. The common problem of rare disease in general practice. Med J Aust. 2006;185:82–3.
Zurynski Y, Frith K, Leonard H, Elliott E. Rare childhood diseases: how should we respond? Arch Dis Child. 2008;93:1071–4.
Thong M-K, See-Toh Y, Hassan J, Ali J. Medical genetics in developing countries in the Asia-Pacific region: challenges and opportunities. Genet Med. 2018;20:1114–21.
Sobering AK, Li D, Beighley JS, Carey JC, Donald T, Elsea SH, et al. editors. Experiences with offering pro bono medical genetics services in the West Indies: Benefits to patients, physicians, and the community. Am J Med Genet C: Semin Med Genet; Wiley Online Library (2020).
Wijekoon N, Gonawala L, Ratnayake P, Sirisena D, Gunasekara H, Dissanayake A, et al. Gene therapy for selected neuromuscular and trinucleotide repeat disorders–An insight to subsume South Asia for multicenter clinical trials. IBRO Neurosci Rep. 2023;14:146–53.
Chamberlain JS, Gibbs RA, Rainer JE, Nguyen PN, Thomas C. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic acids Res. 1988;16:11141–56.
Beggs AH, Koenig M, Boyce FM, Kunkel LM. Detection of 98% of DMD/BMD gene deletions by polymerase chain reaction. Hum Genet. 1990;86:45–8.
Fratter C, Dalgleish R, Allen SK, Santos R, Abbs S, Tuffery-Giraud S, et al. EMQN best practice guidelines for genetic testing in dystrophinopathies. Eur J Hum Genet. 2020;28:1141–59.
Abbs S, Tuffery-Giraud S, Bakker E, Ferlini A, Sejersen T, Mueller CR. Best practice guidelines on molecular diagnostics in Duchenne/Becker muscular dystrophies. Neuromuscul Disord. 2010;20:422–7.
Sequeiros J, Martindale J, Seneca S. EMQN Best Practice Guidelines for molecular genetic testing of SCAs. Eur J Hum Genet. 2010;18:1173–6.
Losekoot M, Van Belzen MJ, Seneca S, Bauer P, Stenhouse SA, Barton DE. EMQN/CMGS best practice guidelines for the molecular genetic testing of Huntington disease. Eur J Hum Genet. 2013;21:480–6.
Darras BT, Menache-Starobinski CC, Hinton V, Kunkel LM. Dystrophinopathies. In: Darras BT, Jones HR Jr, Ryan MM, De Vivo DC, editors. Neuromuscular Disorders of Infancy, Childhood and Adolescence: A Clinician’s Approach. 2 ed. San Diego, CA: Academic Press; 2015, p. 551–92.
Angelini C. Calpainopathy. 2005 May 10 [Updated 2022 Dec 1]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1313/.
Prior TW, Leach ME, Finanger E. Spinal Muscular Atrophy. 2000 Feb 24 [Updated 2020 Dec 3]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1352/.
Caron NS, Wright GEB, Hayden MR. Huntington Disease. 1998 Oct 23 [Updated 2020 Jun 11]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1305/.
Aoki M, Takahashi T. Dysferlinopathy. 2004 Feb 5 [Updated 2021 May 27]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1303/.
Perlman S. Hereditary Ataxia Overview. 1998 Oct 28 [Updated 2023 Nov 16]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1138/.
Naseem N, Jawad U. Enabling the disabled: Call for intercepting disability surge in Pakistan. J Fam Med Prim Care. 2017;6:721.
Srivastava P, Malhotra KP, Husain N, Malhotra HS, Kulshreshtha D, Anand A. Diagnosing muscular dystrophies: comparison of techniques and their cost effectiveness: a multi-institutional study. J Neurosci Rural Pract. 2020;11:420–9.
Laing NG, Ong RW, Ravenscroft G. Genetic neuromuscular disorders: what is the best that we can do? Neuromuscul Disord. 2021;31:1081–9.
Blancato JK, Wolfe E, Sacks PC. Preimplantation genetics and other reproductive options in Huntington disease. Handb Clin Neurol. 2017;144:107–11.
Farrar MA, Calotes-Castillo L, De Silva R, Barclay P, Attwood L, Cini J, et al. Gene therapy-based strategies for spinal muscular atrophy—an Asia-Pacific perspective. Mol Cell Pediatr. 2023;10:1–11.
Wijekoon N, Gonawala L, Wijesinghe P, Steinbusch HW, Mohan C, de Silva KRD. A biobank in Sri Lanka that links East and West. Lancet Neurol. 2020;19:972.
Wijesinghe P, Shankar S, Yasha T, Gorrie C, Amaratunga D, Hulathduwa S, et al. Vascular contributions in Alzheimer’s disease-related neuropathological changes: First autopsy evidence from a South Asian aging population. J Alzheimer’s Dis. 2016;54:1607–18.
Wijekoon N, Gonawala L, Ratnayake P, Dissanayaka P, Gunarathne I, Amaratunga D, et al. Integrated genomic, proteomic and cognitive assessment in Duchenne Muscular Dystrophy suggest astrocyte centric pathology. Heliyon. 2023;9:e18530.
Wijekoon N, Gonawala L, Ratnayake P, Amaratunga D, Hathout Y, Mohan C, et al. Duchenne muscular dystrophy from brain to muscle: the role of brain dystrophin isoforms in motor functions. J Clin Med. 2023;12:5637.
LANKA, M.O.H.S. Annual Health Bulletin 2020; Ministry of Health, Sri Lanka: Colombo, Sri Lanka, 2020. https://www.health.gov.lk/wp-content/uploads/2023/09/Annual-Health-Bulletin-2020Updated-on-2023.02.02.pdf.
Farooq A, Venketasubramanian N, Wasay M. Stroke Care in Pakistan. Cerebrovasc Dis Extra. 2021;11:118–21.
Neurology WFo. The Future of Neurology in India 2020 [Available from: https://worldneurologyonline.com/article/the-future-of-neurology-in-india/.
Samaranayake N, Dissanayaka P, Gunarathna I, Gonawala L, Wijekoon N, Rathnayake P, et al. What we fail to see in neuro-genetic diseases: a bird’s eye view from the developing world. Ann Neurosci. 2020;27:91–7.
Census Do, Statistics. Household income and expenditure survey 2012/13. Colombo, Sri Lanka: Child Youth and Cultural Affairs Sri Lanka; 2015. http://www.statistics.gov.lk/Resource/en/IncomeAndExpenditure/HouseholdIncomeandExpenditureSurvey2012-2013FinalReport.pdf.
FT D. Nearly 10 m Sri Lankans affected by poverty: Study: Daily FT; 2022 [Available from: https://www.ft.lk/front-page/Nearly-10-m-Sri-Lankans-affected-by-poverty-Study/44-740898.
Department of Census and Statistics SL. Household income and expenditure survey 2016. Colombo: Ministry of National Policies and Economic Affairs. (2018).
Department of Census and Statistics SL. Multidimensional Poverty in Sri Lanka. (2019).
Acknowledgements
We sincerely thank the patients and their family members for taking part in the study. Special thanks to the members of the Pro bono Research team of ICIBN of the University of Sri Jayewardenepura that included; Dr. Ruwani Wijayakoon, Dr. Vindika Suriyakumara, Dr. Chamara Jayasinghe, Dr. Beneeta Hettiarachchi, Dr. Roshani Karunarathne, Dr. Kasunka Gamage, Dr. Jayantha Udurawana, Dr. Navami Samaranayake, Dr. Gayatri Wijeweera, Dr. Kasinathar Lokajini, Dr. Pulasthi Dissanayaka Mr. Yoonus Imran, and Ms. Shamali Wasala, for providing support.
Funding
The Corresponding author in Sri Lanka received funding from the Muscular Dystrophy Association, Washington DC, USA (Grant Number FMS/7090/2010); the World Health Organization (Grant Number 2010/81594-0); the World Class University Grant Project (University of Sri Jayewardenepura, Sri Lanka; Grant Number WCUP/Ph. D./19/2013 and WCUP/Ph.D./19B 2013); the Ministry of Primary Industries, Sri Lanka (Grant Number SP/CIN/2016/02); the University of Sri Jayewardenepura (Grant Numbers ASP/06/RE/2010/07, ASP/06/RE/2012/18, ASP/06/RE/2013/28); General Sir John Kotelawala Defence University, Sri Lanka (Grant Numbers KDU/RG/2021/CARE/005 and KDU/RG/2021/CARE/006); and the Interdisciplinary Center for Innovation in Biotechnology and Neuroscience, University of Sri Jayewardenepura (ICIBN/ USJ). The equipment was donated by the National Institutes of Health (Bethesda, MD, USA) through IBRO-APRC and by the Chinese Neuroscience Society. Moreover, the corresponding author received funding from the IBRO-APRC and the International Society for Neurochemistry for international training scholarships for postgraduates and neuroscience workshops in Sri Lanka.
Author information
Authors and Affiliations
Contributions
LG and NW are PhD scholars who contributed equally to the establishment of pro bono molecular diagnostic services by performing molecular diagnostic tests, interpreting genomic data, collecting data through mobile clinics/home visits, and co-writing the manuscript. DA provided support for the administrative coordination of the project. PR, DS, HG, AD, AK contributed in performing the clinical diagnosis and clinical evaluation of patients. CM contributed reagents and consumables, conceptualization, analyzed and interpreted the data of proteomic study. HS, EH, and AD are co-supervisors of LG and NW, provided academic guidance. AD trained LG and NW in molecular diagnostics. KRD was the principal investigator of the project, conceptualization and pioneering the concept of pro bono molecular diagnostic services and developing the manuscript from its inception to the final version. LG NW carried out the research activities and analysis and wrote the manuscript under the supervision of KRD. AD and EH provided necessary assistance for neurogenetic testing.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interest to report.
Ethical approval
This study adheres to the ethical standards of Sri Lankan institutional review boards that follow the Helsinki Declaration (Ethical Approval Nos. 449/09, 38/19, and 34/14 from The Ethics Review Committee, Faculty of Medical Sciences, University of Sri Jayewardenepura, and Ethical Approval No. LRH/D/06/2007 Lady Ridgeway Hospital for Children, Sri Lanka).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gonawala, L., Wijekoon, N., Attanayake, D. et al. Diagnostic outcome of pro bono neurogenetic diagnostic service in Sri Lanka: A wealth creation. Eur J Hum Genet (2024). https://doi.org/10.1038/s41431-023-01525-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41431-023-01525-3