Abstract
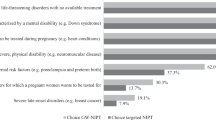
In the Netherlands, genome-wide non-invasive prenatal testing (NIPT) is offered to all pregnant women as part of the nationwide TRIDENT-2 study. Findings other than trisomy 21, 18, or 13, which are called “additional findings”, are reported only on request of the pregnant woman. This study examined: (1) women’s pre-test perceptions and reasons to opt for additional findings and (2) women’s experiences with- and the psychological impact of being informed about an additional finding. A questionnaire, consisting of the anxiety measure State-Trait Anxiety Inventory (STAI), distress measure Impact of Event Scale (IES) and questions developed specifically for this study, was retrospectively administered to 402 women who received an additional finding. A total of 227 (56.5%) women completed the questionnaire. Most (60.2%) chose to know additional findings because they wanted as much information as possible about the health of their fetus. Almost all (92%) stated that receiving the additional finding was unexpected, a shock, and/or they were in disbelief, for 85% it caused a lot of worry. Post-test, high anxiety (STAI) levels were reported in 15.5% of women, and 7.5% reported severe distress (IES). Women who gave birth to an affected child (n = 10) experienced most psychological impact (STAI and IES). Eighty-six percent of women with a fetal aberration would opt for additional findings again, compared to 49.2% of women whose result was confined to the placenta. Pre-test counseling should focus on explaining the different results NIPT can generate. Post-test counseling should focus on guiding pregnant women through this uncertain and anxious time.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Lo YMD, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CWG, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–7.
Warsof SL, Larion S, Abuhamad AZ. Overview of the impact of noninvasive prenatal testing on diagnostic procedures. Prenat Diagn. 2015;35:972–9.
Ravitsky V, Roy M-C, Haidar H, Henneman L, Marshall J, Newson AJ, et al. The Emergence and Global Spread of Noninvasive Prenatal Testing. Annu Rev Genom. Hum Genet. 2021;22:309–38.
van Opstal D, Van Maarle MC, Lichtenbelt K, Weiss MM, Schuring-Blom H, Bhola SL, et al. Origin and clinical relevance of chromosomal aberrations other than the common trisomies detected by genome-wide NIPS: results of the TRIDENT study. Genet Med. 2018;20:480–5.
Fiorentino F, Bono S, Pizzuti F, Duca S, Polverari A, Faieta M, et al. The clinical utility of genome‐wide non invasive prenatal screening. Prenat Diagn. 2017;37:593–601.
Pescia G, Guex N, Iseli C, Brennan L, Osteras M, Xenarios I, et al. Cell-free DNA testing of an extended range of chromosomal anomalies: clinical experience with 6,388 consecutive cases. Genet Med. 2017;19:169–75.
Pertile MD, Halks-Miller M, Flowers N, Barbacioru C, Kinnings SL, Vavrek D, et al. Rare autosomal trisomies, revealed by maternal plasma DNA sequencing, suggest increased risk of feto-placental disease. Sci Transl Med. 2017;9:1–11.
van der Meij KRM, Sistermans EA, Macville MVE, Stevens SJC, Bax CJ, Bekker MN, et al. TRIDENT-2: National Implementation of Genome-wide Non-invasive Prenatal Testing as a First-Tier Screening Test in the Netherlands. Am J Hum Genet. 2019;105:1091–101.
Lannoo L, van Straaten K, Breckpot J, Brison N, De Catte L, Dimitriadou E, et al. Rare autosomal trisomies detected by non-invasive prenatal testing: an overview of current knowledge. Eur J Hum Genetics. 2022;30:1323–30.
van Prooyen Schuurman L, Sistermans EA, Van Opstal D, Henneman L, Bekker MN, Bax CJ, et al. Clinical impact of additional findings detected by genome-wide non-invasive prenatal testing: follow-up results of the TRIDENT-2 study. Am J Hum Genet. 2022;109:1140–52.
Gammon BL, Jaramillo C, Riggan KA, Allyse M. Decisional regret in women receiving high risk or inconclusive prenatal cell-free DNA screening results. J Matern Fetal Neonatal Med. 2018;33:1412–8.
Jani JC, Gil MM, Benachi A, Prefumo F, Kagan KO, Tabor A, et al. Genome‐wide cfDNA testing of maternal blood. Ultrasound Obstet Gynecol. 2020;55:13–4.
RIVM. Draaiboek prenatale screening downsyndroom en Structureel Echoscopisch Onderzoek. 2018. Available from: https://www.rivm.nl/Documenten_en_publicaties/Professioneel_Praktisch/Draaiboeken/Preventie_Ziekte_Zorg/Draaiboek_prenatale_screening_downsyndroom_en_Structureel_Echoscopisch_Onderzoek
(RIVM) RvVeM. Screeningen tijdens de zwangerschap: RIVM; 2021. Available from: pns.nl/prenatale-screeningen.
Marteau TM, Bekker H. The development of a six‐item short‐form of the state scale of the Spielberger State—Trait Anxiety Inventory (STAI). Br J Clin Psychol. 1992;31:301–6.
van der Bij AK, de Weerd S, Cikot RJLM, Steegers EAP, Braspenning JCC. Validation of the Dutch short form of the state scale of the Spielberger State-Trait Anxiety Inventory: considerations for usage in screening outcomes. Public Health Genom. 2003;6:84–7.
Tluczek A, Henriques JB, Brown RL. Support for the reliability and validity of a six-item state anxiety scale derived from the State-Trait Anxiety Inventory. J Nurs Meas. 2009;17:19.
Horowitz M, Wilner N, Alvarez W. Impact of event scale: a measure of subjective stress. Psychosom Med. 1979;41:209–18.
van der Ploeg E, Mooren T, Kleber RJ, van der Velden PG, Brom D. Construct validation of the Dutch version of the impact of event scale. Psychol Assess. 2004;16:16.
Hutchings ED, G.J. Impact of Event Scale (IES): Clintools. Available from: http://www.clintools.com/victims/resources/assessment/ptsd/ies.html#:~:text=The%20IES%20scale%20consists%20of,a%20total%20subjective%20stress%20score.
Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Health. 2004;11:12.
Fransen MP, Van Schaik TM, Twickler TB, Essink-Bot ML. Applicability of internationally available health literacy measures in the Netherlands. J Health Commun. 2011;16:134–49.
Carleton RN, Norton MAPJ, Asmundson GJG. Fearing the unknown: a short version of the Intolerance of Uncertainty Scale. J Anxiety Disord. 2007;21:105–17.
Helsen K, Van den Bussche E, Vlaeyen JWS, Goubert L. Confirmatory factor analysis of the Dutch Intolerance of Uncertainty Scale: comparison of the full and short version. J Behav Ther Exp Psychiatry. 2013;44:21–9.
van Schendel RV, Dondorp WJ, Timmermans DRM, van Hugte EJH, de Boer A, Pajkrt E, et al. NIPT‐based screening for Down syndrome and beyond: what do pregnant women think? Prenat Diagn. 2015;35:598–604.
van der Meij KRM, Njio A, Martin L, Gitsels-van der Wal JT, Bekker MN, van Vliet-Lachotzki EH, et al. Routinization of prenatal screening with the non-invasive prenatal test: pregnant women’s perspectives. Eur J Hum Genet. 2022;30:661–8.
van der Steen SL, Diderich KEM, Riedijk SR, Verhagen‐Visser J, Govaerts LCP, Joosten M, et al. Pregnant couples at increased risk for common aneuploidies choose maximal information from invasive genetic testing. Clin Genet. 2015;88:25–31.
Labonté V, Alsaid D, Lang B, Meerpohl JJ. Psychological and social consequences of non-invasive prenatal testing (NIPT): a scoping review. BMC Pregnancy Childbirth. 2019;19:1–14.
Van Opstal D, Srebniak MI. Cytogenetic confirmation of a positive NIPT result: evidence-based choice between chorionic villus sampling and amniocentesis depending on chromosome aberration. Expert Rev Mol Diagn. 2016;16:513–20.
Rosen NO, Knäuper B, Sammut J. Do individual differences in intolerance of uncertainty affect health monitoring? Psychol Health. 2007;22:413–30.
Rosen NO, Knäuper B, Di Dio P, Morrison E, Tabing R, Feldstain A, et al. The impact of intolerance of uncertainty on anxiety after receiving an informational intervention about HPV: a randomised controlled study. Psychol Health. 2010;25:651–68.
Nambot S, Sawka C, Bertolone G, Cosset E, Goussot V, Derangère V, et al. Incidental findings in a series of 2500 gene panel tests for a genetic predisposition to cancer: Results and impact on patients. Eur J Med Genet. 2021;64:104196.
Cheung F, Birch P, Friedman JM, Study C, Gen CS, Elliott AM, et al. The long‐term impact of receiving incidental findings on parents undergoing genome‐wide sequencing. J Genet Couns. 2022;31:887–900.
Bekker M, Henneman L, Macville M, Sistermans E, Galjaard R-J. Benefit vs potential harm of genome-wide prenatal cfDNA testing requires further investigation and should not be dismissed based on current data. Ultrasound Obstet Gynecol. 2020;55:695–6.
Acknowledgements
The authors would like to thank all participating women for completing the questionnaire. This study was made possible using data from Peridos, the Dutch national digital registration system for prenatal screening. The authors would like to thank all members of the NIPT consortium.
Funding
This work was partly supported by a grant from the Netherlands Organization for Health Research and Development (ZonMw, No. 543002001).
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: Study conception and design: IMB, LH, EHv, MNB & RJHG. Data collection: IMB. Analysis and interpretation of results: IMB, MGP, LH, MNB & RJHG. Draft manuscript preparation: IMB & RJHG. Revised manuscript: LH, EHv, LM, JTG, MGP, MNB & RJHG. All authors reviewed the results and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The research ethics review committee of Erasmus MC, University Medical Center Rotterdam exempted this questionnaire study (MEC-2018-1685). All participants signed informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bakkeren, I.M., Henneman, L., van Vliet-Lachotzki, E.H. et al. Psychological impact of additional findings detected by genome-wide Non-Invasive Prenatal Testing (NIPT): TRIDENT-2 study. Eur J Hum Genet 32, 302–308 (2024). https://doi.org/10.1038/s41431-023-01504-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-023-01504-8
This article is cited by
-
Solving medical mysteries with genomics
European Journal of Human Genetics (2024)