Abstract
Whilst the finding of heritable susceptibility to disease was once relatively rare, mainstreaming of genetic testing has resulted in a steady increase. Patients are often encouraged to share their genetic test results with relevant relatives, but relatives may not receive this information, leaving them without knowledge of their own risk. Therefore, strategies to help communicate such information are important. This review aimed to explore the efficacy of existing interventions to improve the sharing of genetic test results. A synthesis without meta-analysis design was used. A systematic search of Medline, CINAHL, PsychINFO, and AMED was conducted, and five studies were identified worldwide. Data were extracted for each study regarding study aim, participant characteristics, condition, intervention details, comparison, study duration, outcome measures, theory and behaviour change techniques used. Limited efficacy and application of theory was found. Knowledge, motivation and self-efficacy were not increased in any intervention. No gender differences in communication behaviour were encountered in interventions that recruited men and women. Two studies reported an evaluation of acceptability, which showed that the interventions were well received by patients and health professionals. No study reported the involvement of the target population in any phase of intervention development. Given the lack of health psychology-informed interventions in this area of clinical genetics, we recommend genetic health professionals, health psychologists and patients collaborate on all stages of future interventions that involve the cascading of genetic health information within families. We also provide guidance regarding use of theory and intervention elements for future intervention development.
Similar content being viewed by others
Introduction
Genetic testing is being increasingly used to identify genetic predisposition to disease due to the mainstreaming of genetic testing through, for example, the National Health Service (England) genomic medicine service. Given that these predispositions are often inherited, the results of tests in one person (the proband) can reveal risk to that person’s relatives [1, 2]. For example, finding a BRCA2 variant (associated with an increased risk of breast, ovarian, and prostate cancer) in an individual could indicate that their close relatives might also benefit from such testing. However, these relatives will only be able to decide if they would like to take up testing, and any subsequent screening or preventative measures, if they are made aware of this possibility [3].
Research into familial communication suggests that patients often believe it is their responsibility to inform relatives of any relevant genetic information [4,5,6,7] but that some would like to do so with support from their health professional (HP) [8]. Guidelines about such communication generally outline the role for HPs as one of encouraging the proband to communicate relevant information to at-risk relatives and to offer support in doing so [9]. Probands are however, left to make their own judgements on when would be the best time to pass on this information, and in what way [10]. HP assistance often includes the use of a ‘family letter’, which outlines in general terms information about the genetic finding and how a relative might seek more information [11, 12]. How – or whether – family letters are used is unclear, with clinicians often left unsure as to whether the relevant information has been shared [12].
Although patients generally understand the importance of sharing information with family members, especially those with risks of diseases that can be prevented or treated [13], they also report difficulties in doing so [14,15,16]. It is estimated that many relatives do not receive such information in a timely fashion [14, 17,18,19,20,21,22,23] meaning that some remain unaware of their potential risks for longer than necessary and some never receive this information. Studies also show that when communication does occur, it may in fact be to the wrong relatives (those not at risk) or that the information passed on is insufficient or wrong [24,25,26,27,28].
There are many reasons why patients do or do not inform their at-risk relatives, or delay doing so [4, 20, 28,29,30]. Reasons include feelings of responsibility or guilt, family dynamics, perceived recipient reactions, perceived relevance of the information and the psychological burden of coping with their own result. This psychological distress or burden caused by giving or receiving such information has been shown to be often outweighed by the health advantages of having access to screening and appropriate healthcare [6, 31, 32]. The aim of informing relatives is so that they have an opportunity to make a more informed decision they could not make without such communication. Given that a range of studies has shown that not all at-risk relatives receive such information appropriately, interventions to improve this communication are needed [4, 17]. Yet resources to facilitate this are often limited [33,34,35] and there is no procedure in the UK that guarantees a relative – with whom the HP usually does not have direct contact – will receive information about their genetic risks [20].
Previous reviews in this area
Mendes et al., (2016) [34] summarised studies exploring the role of HPs in the communication of genetic health information within families and examined how such communication is addressed in clinic. The authors concluded that encouraging ‘reflective consideration’ and exploring ‘family dynamics and patterns of communication’ were helpful for HPs. Baroutsou et al., (2021) [35] reviewed interventions to facilitate familial communication of genetic health information, including interventions that focused on the gathering of a family history (i.e., [36]) and where no genetic test was conducted (i.e., [37, 38]). Zhao et al., (2022) [38] also reviewed family communication frameworks and included interventions that support the gathering of family histories. This review progresses from these broad reviews to a more detailed understanding of what interventions to facilitate communication exist, and whether, how and why they are effective. Our aims differ from previous reviews, in that they are focused on the sharing of a genetic test result; we look at the likelihood of performing the behaviour (sharing) and the quality of that behaviour (accuracy and effectiveness).
What is needed for an intervention to be successful in changing a behaviour?
Using a theory to guide the development of an intervention will make it more likely that the intervention will be successful [39] and the need for more theory-based interventions in the area of familial communication of genetic information has been highlighted [40]. Theory in this context should explain how, when and why a behaviour change intervention does, or does not, work [41]. An essential component to an efficacious intervention is ensuring the end user is involved in every step of the development of the intervention [42]. One approach to user involvement is the ‘person-based approach’ [43]. This approach focuses on involving the people the intervention is directed at, with the aim of understanding and integrating their needs and perspectives, which increases the likelihood of uptake and intervention engagement.
Objectives
This review aimed to explore the efficacy of existing interventions that encourage patients to share genetic health information with their relatives and what intervention elements authors identified as making the performance of the behaviour more likely. We achieved this aim through the following objectives: 1) comparing study outcomes, 2) comparing behaviour change techniques (BCTs) used, 3) describing the extent to which interventions draw on theories of behaviour change, 4) describing the extent to which the views of people from the target population were incorporated into the development of the interventions, and 5) demonstrating how barriers and facilitators can be mapped onto theory.
Methods
A systematic review: synthesis without meta-analysis (SWiM) [44] design was used. This is an alternative method of synthesis from a meta-analysis, comprising of a narrative synthesis of effects. The studies included in this review were synthesised using this method due to methodological heterogeneity (i.e., RCTs and non-randomised and clinical diversity in relation to PICO (Population, Intervention, Comparison, Outcome)). This review follows the PRISMA Statement process [45] and the SWiM guidelines [44].
The protocol for this review can be accessed from www.crd.york.ac.uk/prospero with the registration number CRD42019121588.
Eligibility criteria
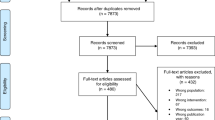
PICO was used to inform the eligibility criteria (see Fig. 1). We engineered the eligibility criteria to reflect a very specific behaviour - an adult with a genetic test result sharing that result with at-risk relatives under the advice of their HP. We therefore excluded interventions aimed at collecting family histories as - although they report on familial communication - the aims and challenges of collecting a family health history are different from sharing a genetic test result. We excluded interventions aimed at supporting parents to inform children under the age of 18 as the mode and method of communicating to children of varying ages will have to be very different to those used with adults. Finally, we excluded interventions aimed at HPs because the target behaviour is different, with a unique list of barriers and facilitators.
Information sources and search
Electronic databases Medline, CINAHL, PsychINFO, and AMED were searched in September 2019 for peer reviewed papers, the search was updated in March 2022. Grey literature searches and hand searching were performed for editorial letters, blog posts, conference proceedings and bulletins. No unpublished relevant literature was identified so no researchers/authors were contacted throughout this meta-synthesis. Supplementary papers were used, such as published protocols, to allow the coding of interventions.
Citation chaining (forward and backwards) was performed on the final articles selected for inclusion in this synthesis. After a preliminary search to refine the search terms the following terms were used: duty to inform OR family communication OR at-risk relative OR disclosure AND genetics OR genetic coun* OR genetic testing AND intervention OR randomi*ed controlled trial. Studies were selected based on the PICO characteristics in Fig. 1.
Study selection
Abstracts were screened independently by LMB and JF using the inclusion and exclusion criteria outlined in Fig. 1. Disagreements were discussed and resolved in all cases without the need for external involvement. The updated search was conducted by LMB based on the protocol and discussions from the original search.
Data collection process
Overall quality assessment and risk of bias was conducted using the EPHPP model (see Supplementary information) by two independent reviewers [46]. This tool leads to a rating of weak, moderate or strong based on an assessment of six categories. Data was extracted using the ‘Cochrane Data collection form for intervention reviews: RCTs and non-RCTs’. Data extracted included type of study, participants, type of intervention, theoretical basis and sections were added to the data extraction sheet for behaviour change techniques (BCTs) and evidence of researchers working with the target population in developing the intervention. BCTs were coded according to the behaviour change technique taxonomy (v1) of 93 hierarchically clustered techniques [47, 48].
Data analysis
We synthesised the data from each study by describing study characteristics of interest, theory and target group involvement. BCTs were coded by identifying all elements of each intervention and coding them according to the BCT taxonomy (V1). Once BCTs were identified for each study, we used the Kok et al., (2016) [48] intervention mapping tables, in which they have matched relevant theory with each BCT, to highlight suitable theories. Our synthesis aimed to provide rich descriptions about mediating factors, identification of similarities and differences across studies to inform the development of theory, and identification of what has worked and has not for whom and in what circumstances. We present the findings of this synthesis themed by patient outcomes.
Results
Study selection
1173 papers of potential relevance were found after duplicates were removed (see Fig. 2 for PRISMA flow diagram). After scanning titles and abstracts 1149 papers were removed. From the remaining 24 full text articles 19 were excluded.
Study characteristics
Table 1 summarises the study design and main findings for each study. The included papers were quantitative in design and were published between 2008 and 2018. The studies took place in different countries; two in Australia, two in the United States of America (USA) and one in the Netherlands. Studies looked at high risk single gene/mendelian disorders, where there were clear familial risks. They did not include, for example, moderate risk genes or polygenic scores where the risk to communicate to family members would be much less clear/certain. Three focused on heritable cancer syndromes and two looked more broadly at genetic conditions that had an implication for family members. Four studies used a randomised controlled trial design, and one used a controlled before and after study. Two studies (Eijzenga et al. 2018 [49] and Montgomery et al. 2013 [21]) included the communication of both positive and negative results (when a proband did have a variation and when they did not), whilst the remaining studies focused on the communication of results that indicated increased risk only. Overall study quality (see supplementary information) was assessed as being weak.
Study results
Of the five interventions, three used prompting (i.e., calling to see how the proband is getting on with sharing their result with relevant relatives) as the main intervention along with problem solving support if they were struggling to share or had encountered any issues (Forrest et al., 2008 [18], Hodgson et al., 2016 [50], and Eijzenga et al., 2018). Kardashian et al. (2012) [19] used education about genetic findings and their relevance for relatives as the intervention and Montgomery et al. (2013) focused on building skills in communication of genetic test results, such as who, what and how to tell as well as identifying barriers to sharing. Three main outcomes were identified as being used by the studies in this review: 1) participant self-report of informing relatives (used in three studies (Kardashian et al., 2012; Montgomery et al., 2013; Eijzenga et al., 2018)); 2) relatives contacting genetics services (used in two studies (Forrest et al., 2008, and Hodgson et al., 2016)); and 3) relatives accessing testing (used in two studies (Forrest et al., 2008 and Kardashian et al., 2012)). One study found the intervention to be effective. There were also six additional outcome measures identified. Table 2 shows how the groupings were developed; each grouping is described in more detail below.
Self-report
Of the three studies that used self-reported sharing of information with relatives (Kardashian et al., 2012; Montgomery et al., 2013; Eijzenga et al., 2018) none found a significant difference between the intervention and control groups. Kardashian et al., (2012) and Montgomery et al., (2013) did show an effect in the direction of the intervention, however Eijzenga et al., (2018) found the direction of effect to be towards the control condition (see Table 3 for direction of effect).
Whether or not relatives contacted the genetic service
Two interventions used relatives of probands contacting the genetics services as an outcome measure (Forrest et al., 2008 and Hodgson et al., 2016). Forrest et al., (2008) was the only study where significantly more relatives of participants in the intervention group were informed of their risk and sought support from the genetic centre compared to the control group (χ2 = 6.52, P 0.01). However, there was no mention of a power calculation to determine sample size; the only acknowledgement of sample size was stating that the number of families in the study was ‘relatively small’. Hodgson et al.’s (2016) did not find a significant difference between the intervention and the control condition for proportion of relatives contacting the genetics service, but the direction of effect was towards the intervention group.
Relatives tested
Two studies measured whether relatives had taken up testing. Significantly more relatives underwent testing in the intervention group in the Forrest et al. (2008) study. Kardashian et al. (2012) did not find a significant difference and the direction of effect was negative (see Table 3).
Knowledge
Two studies used knowledge as an outcome measure; neither found that the intervention increase knowledge regarding whom to inform and what information to share (Kardashian et al. 2012 & Eijzenga et al., 2018).
Motivation, self-efficacy and distress
No intervention significantly increased motivation and self-efficacy (Eijzenga et al., 2018) and Montgomery et al., (2013) found no increase in distress.
Gender differences
Both Kardashian et al., (2012) and Montgomery et al., (2013) limited their studies to the recruitment of women. Other studies found that women are more likely to communicate genetic information to at-risk relatives than men [51], which may explain why the interventions were not found to be effective. Forrest et al., (2012) and Eijzenga et al., (2018) recruited both men and women probands, but found no gender differences. Moreover, there were differences reported for gender of relatives. For example, Forrest et al., (2008), Kardashian et al., (2012) and Montgomery et al., (2013) found that female at-risk relatives were more likely to be informed than male at-risk relatives. However, none of the studies were sufficiently powered for subgroup analysis.
Evaluation
Two studies reported feedback from HPs delivering the intervention and/or participants receiving it. Kardasian et al., (2012) found that even though the introduction of an educational tool in the form of a binder given to patients added 30 minutes to genetic counsellors’ workload, they were still positive about its use as it helped them structure their consultations and it was well received by patients. The intervention in the Eijzenga et al., (2018) study was found to be acceptable to patients; 96% of participants found the telephone counselling to be useful and 96% did not find the intervention to be confrontational.
Additional data synthesis based on review objectives
Involvement of the target group in intervention development
None of the studies involved the target population in the development of the intervention (see Table 2). Two studies, Kardashian et al., (2012) and Montgomery et al., (2013), consulted the HPs who would be delivering the intervention.
Behaviour change techniques (BCT) coded from each intervention
We coded each intervention to determine which BCTs were implemented using a published and widely used taxonomy (v1) (Michie et al., 2013) (tables with BCTs for each study are in supplementary information). De Vasconcelos et al., (2018) showed that effective interventions have a median number of nine BCTs (range 3-25). Our review found that the maximum number of techniques used was seven. The Forrest et al. (2008) intervention was clearly explained, making coding of techniques easier, and used the most BCTs (seven). Eijzenga et al.’s (2018) intervention was well described making it easier to identify the five BCTs used. Hodgson et al. (2016), Montgomery et al. (2013) and Kardashian et al. (2012) did not describe the intervention in sufficient detail, making the identification of BCTs difficult. From the details provided, we identified that the Hodgson et al. (2016) intervention included six BCTs and Montgomery et al. (2013) and Kardashian et al. (2012) four. Kardashian et al. (2012) also did not justify any of the intervention components, or a link between the target behaviour and the intervention.
Use of behaviour change theory for intervention development
Once the BCTs were coded, we used the Kok et al., (2016) intervention mapping tables, in which relevant theory is matched with each BCT, to highlight suitable theories (see supplementary information). From their ‘methods to change awareness and risk perception’ table, Kok et al. (2016) summarise that the Health Belief Model, Precaution-Adoption Process Model, Trans-Theoretical Model (Stages of Change) as potential theories to facilitate providing information regarding the causes and consequences of a behaviour being performed (or not). The Precaution-Adoption Model also facilitates functions such as cost/benefit analysis of action and inaction and constructing images of future gains and losses. Framing – from the Protection Motivation Theory - may also be a useful concept for the sharing of genetic health information, whereby messages from HPs are gain-framed (the advantages of performing the behaviour) and loss-framed (the disadvantages of not performing the behaviour). Because the target behaviour here is one that affects another person (and often more than one person) theories that facilitate the shifting of perspectives, such as Theories of Stigma and Discrimination, could help the proband to take on the perspective of their relative. Similarly, so could Theory of Planned Behaviour; Reasoned Action Approach; Social Comparison Theory, all of which have elements of considering others’ approval or disapproval.
Montgomery et al., (2013) was the only study reporting the use of theory. They used the Theory of Planned Behaviour (TPB) and measured components such as, attitudes, subjective norms, perceived behavioural control. They showed that three TPB variables predicted intention to inform, though in a further analysis they found that attitude did not predict actual sharing, whereas social norms and perceived control did. The social norms and perceived control variables were strong predictors of behaviour. However, there was no clear explanation as to how the theoretical components related to the intervention. Hodgson et al., (2016) did describe the development of the intervention being informed by the Reciprocal Engagement Model of genetic counselling, however, we did not classify this as a behaviour change theory because it described standard practice. In addition, Eijzenga et al., (2018) stated that their intervention was based on Motivational Interviewing. We did not classify this as a behaviour change theory as it is more of a ‘counselling style’ and it does not explain how the intervention leads to change.
Moving this field of enquiry forward
Given the lack of intervention development and use of theory in this area we have completed an additional step to this review by demonstrating how to develop a theory-informed intervention, using the COM-B model (see Fig. 3), which theorises that capability (C), opportunity (O) and motivation (M) need to be present for a behaviour (B) to happen. There are many behaviour change theories as listed above, but many are complex making them inaccessible to many researchers and HPs [52]. Michie and colleagues developed the Behaviour Change Wheel (COM-B and the Theoretical Domains Framework) to address some of these challenges (Ibid). The COM-B model can be broken down further into 14 domains, which are organised under the Theoretical Domains Framework (see Fig. 4) [53, 54]. We used the four steps outlined in French et al., (2012) to demonstrate how to apply a behaviour change model to the patient behaviour of sharing genetic health information with relatives indicated by their HP, which can been seen in Table 4. Here we conducted a behavioural analysis based on previous literature (separate from the coding of BCTs in the interventions included in this review) and then mapped the BCTs onto the COM-B model. The left column of Table 4 details barriers and facilitators identified from the wider literature, presented in sections corresponding with the COM-B model. The right column matches behaviour change techniques to the particular barrier or facilitator. For example, if a researcher identified that only being advised once to share results with at-risk relatives reduces the likelihood that behaviour will be performed, they may wish to build prompts and cues into the intervention.
The three key concepts are divided into two further categories to capture distinct elements of each concept [61].
It is broken down into 14 domains to enhance specificity [62].
Discussion
Our review explored interventions designed to increase the likelihood that probands shared relevant genetic health information with their appropriate relatives. We identified five such studies. Each had identified some weaknesses to their approach, and we highlighted further ones. We recognise that the behaviour of sharing genetic test results with relatives is a difficult behaviour to firstly influence and then to measure the effectiveness of that influence. It will always be difficult to measure communication among people who are not in direct contact with the health system, furthermore, the urgency to do so will vary considerably depending on the condition and the ages and relatedness of family members. Some relatives may have been well informed but chosen not to pursue genetic testing. Arguably, at least in some cases the intervention will have been helpful, but relatives made informed decisions not to seek a referral.
The Forrest et al., (2008) intervention, was the only one to find a significant difference in favour of the intervention. However, the sample was small and no power calculation was reported, meaning the significance of this is uncertain [55]. In addition, the authors highlighted that the geographical features of the clinic meant there is typically little migration and that this may influence the generalisability of the intervention. The intervention was relatively simple, indicating that prompting could work as an intervention element. No significant difference was found in the studies using relatives undergoing testing as the primary outcome measure, however one study found more relatives in the intervention condition underwent testing. Synthesising the secondary outcomes found that knowledge, motivation and self-efficacy were not increased by any intervention, but neither were negative consequences such as distress. No gender differences were observed in interventions that recruited both men and women. Regarding evaluation of interventions, none reported the involvement of the target population in any phase of intervention development. Two studies reported a basic evaluation, indicating the interventions were well received by patients and HPs.
We found limited application of theory and very few BCTs in intervention descriptions. When designing interventions to make it more or less likely a behaviour is performed, many interventions are designed using the ‘ISLAGIATT’ principle [56]. This principle, coined by Martin Eccles, stands for “It seemed like a good idea at the time”. ISLAGIATT reflects an implicit common-sense approach consisting of personal experience and a brief analysis of the behaviour. For an evidence-based approach, a thorough behavioural analysis and a theory or model should be used to inform the intervention content or “active ingredients” [53]. Theory in this context should explain how, when and why a behaviour change intervention does, or does not, work [41]. Using a theory to guide the development of an intervention will make it more likely that the intervention will be successful [39]. The systematic application of theory means that theory should inform the design; the theoretical constructs should guide the choice of intervention components, and the evaluation of the intervention (Ibid).
Only one study (Montgomery et al., 2013) used a theory of behaviour change to inform the intervention functions. The authors found that social norms, such as perceiving relatives to be supportive of testing, are a strong predictor of behaviour. Perceived control was another predictor, potentially indicating proband confidence in communicating with relatives may lead to high rates of sharing. In this study, BCTs were minimal and there was no clear justification for the intervention components. Some intervention descriptions were too brief to be able to confidently identify the BCTs used. Given the minimal use of theory, the limited reference to health psychology, behaviour change and implementation science literature and the large body of literature detailing the barriers and facilitators to sharing genetic health information with relatives we have applied a theoretical framework to this behaviour, described in detail above and summarised in Table 4.
Recommendations
-
Interventions in clinic are acceptable and feasible - accounts were positive from those delivering and those receiving the interventions, indicating promise that it is feasible and acceptable to implement interventions into the clinical setting.
-
Simple solutions to avoid psychological overburdening - Eijzenga et al., (2018) found that 60% of participants reported informing relatives who were not at increased risk, as patients were often unaware of the exact message they were meant to be communicating, leading to a ‘better safe than sorry’ approach. Informing more relatives than necessary is overburdening the patient and their relatives. Interventions that make it very clear which relatives to inform and what information to share are needed [28]. One such example is myKinMatters [57], a web application which supports the proband to create a family tree, have their clinician indicate on the tree who to contact, upload their test results and electronically send them to the indicated relatives.
-
Digital interventions should be explored – none of the interventions reviewed had a digital element, although they were situated within genetics clinics and in addition, not particularly current. Digital behaviour change interventions are increasingly being used in healthcare as they have evidence-based potential to improve health, can be easily tailored, are generally cheaper than HP-delivered interventions and can be rolled out at scale without much human resource [58].
-
Target interventions - two interventions recruited women participants exclusively, and also reported high amounts of communication in both control and intervention conditions, indicating that future interventions could be targeted towards groups of patients who are less likely to share genetic health information with relatives i.e., men.
-
Involve the end user in all elements of intervention development – studies in the review contained very little involvement of the target group in intervention development. It is essential that the target user is involved in the whole intervention development process, this will increase the likelihood of adoption, engagement and health outcomes [42]. Guidance regarding the previously mentioned Person-Based Approach can be found here: https://www.personbasedapproach.org/ which contains information, tutorials and resources.
Future research
Future development of interventions in this area should consider the use of behaviour change theories and models. Genetics HPs could work alongside behavioural scientists to gain support regarding the use of behaviour change theory in intervention development [59]. Behaviour change research has been successfully applied to other areas of clinical genetic practice, such as, the study of personalised genetic risk information and health behaviour change [60]. The interventions in this review did not significantly increase Motivation or Capability (self-efficacy), however, we have demonstrated how the COM-B model can be used to develop interventions and what BCTs would be most helpful. Exploring the notion of responsibility to inform is also required to understand HP and patient roles, perspectives and assumptions. Moreover, a professional and social duty to inform at-risk relatives exists, with the legal and ethical aspects of both being qualitatively different. Comparing the practice of cascading health information in other areas of healthcare, such as infectious disease and sexual health, may also evolve thinking in this area. In addition, we must acknowledge that theories beyond behaviour change, and implementation science may be relevant and require exploration given the multilevel barriers within cascade screening in the clinical setting.
Limitations
When examining the primary outcome measures, we found issues with each. Self-report could have an interventional function (i.e., participants are more likely to inform relatives if prompted) and also suffer from response bias (the tendency for a participant to falsely report the outcome they think the researcher wants to hear). Relatives contacting the genetic service and relatives being tested as outcome measures are problematic as this approach misses those relatives who were told but who made an informed decision not to be tested, as well as those relatives that were referred to a geographically different genetic service. However, it could be argued that the ‘gold standard’ is that every relative would have a counselling session with the genetic service. This highlights that interventions may need to target other outcomes in addition to a numerical count of relatives informed i.e., capability, opportunity and motivation [41]. And importantly – different outcomes (i.e., information sharing verses relatives contacting the service) will likely have different target behaviours.
There are several limitations at a review-level. Firstly, we included only studies published in English, due to resource constraints, meaning that articles could have been missed. Secondly, due to the heterogenous nature of the outcome measures used we were unable to conduct a meta-analysis, therefore we conducted a narrative synthesis of interventions.
Conclusion
It is surprising that in the rapidly expanding field of genetics, which receives so much political attention, that very few studies – and not one in the UK - have paid attention to supporting probands in the communication of relevant findings to relatives. However, it is less surprising that those developing these interventions have little experience of behaviour change or implementation science theory and practice due to disciplinary silos. Health psychology has a lot to offer this relatively untapped area of medicine and we recommend health psychologists; genetics HPs and their patients work together to make the cascading of genetic health information more effective in reducing morbidity and mortality. This review has highlighted factors of importance, which we hope will focus attention on this much neglected area.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
NHS. Genetic and genomic testing: NHS. 2019.
Gorrie A, Archibald AD, Ioannou L, Curnow L, McClaren B. Exploring approaches to facilitate family communication of genetic risk information after cystic fibrosis population carrier screening. J Commun Genet. 2018;9:71–80. https://www.ncbi.nlm.nih.gov/pubmed/28971321.
Mendes A, Metcalfe A, Paneque M, Sousa L, Clarke AJ, Sequeiros J. Communication of information about genetic risks: putting families at the center. Fam Process. 2018;57:836–46. https://www.ncbi.nlm.nih.gov/pubmed/28714147.
Smit AK, Bartley N, Best MC, Napier CE, Butow P, Newson AJ, et al. Family communication about genomic sequencing: A qualitative study with cancer patients and relatives. Patient Educ Couns. 2021;104:944–52. https://doi.org/10.1016/j.pec.2020.10.022.
Hallowell N, Jenkins N, Douglas M, Walker S, Finnie R, Porteous M, et al. Patients’ experiences and views of cascade screening for familial hypercholesterolemia (FH): a qualitative study.
Leenen CH, Heijer M, van der Meer C, Kuipers EJ, van Leerdam ME, Wagner A. Genetic testing for Lynch syndrome: family communication and motivation. Fam Cancer]. 2016;15:63–73. https://www.ncbi.nlm.nih.gov/pubmed/26446592.
van El C, Baccolini V, Piko P, Cornel M. Stakeholder views on active cascade screening for familial hypercholesterolemia. Healthcare 2018;6:108.
Ballard LM, Horton RH, Dheensa S, Fenwick A, Lucassen AM Exploring broad consent in the context of the 100,000 Genomes Project: a mixed methods study. Eur J Hum Genet. 2020. https://www.ncbi.nlm.nih.gov/pubmed/31919452.
Forrest LE, Delatycki MB, Skene L, Aitken MA. Communicating genetic information in families - A review of guidelines and position papers. Eur J Hum Genet. 2007;15:612–8.
Foster C, Herring J, Boyd M. Testing the limits of the ‘joint account’ model of genetic information: a legal thought experiment. J Med Ethics. 2015;41:379–82.
de Geus E, Eijzenga W, Menko FH, Sijmons RH, de Haes HCJM, Aalfs CM, et al. Design and feasibility of an intervention to support cancer genetic counselees in informing their at-risk relatives. J Genet Couns. 2016;25:1179–87.
Dheensa S, Lucassen A, Fenwick A. Limitations and pitfalls of using family letters to communicate genetic risk: a qualitative study with patients and healthcare professionals. J Genet Couns. 2018;27:689–701. https://www.ncbi.nlm.nih.gov/pubmed/29094272.
Dheensa S, Fenwick A, Lucassen A. “Is this knowledge mine and nobody else’s? I don’t feel that.” Patient views about consent, confidentiality and information-sharing in genetic medicine. J Med Ethics. 2016;42:174–9. http://www.ncbi.nlm.nih.gov/pubmed/26744307.
Burns C, Mcgaughran J, Davis A, Semsarian C, Ingles J. Factors influencing uptake of familial long QT syndrome genetic testing. Am J Med Genet Part A. 2016;170:418–25.
Dean M, Tezak AL, Johnson S, Pierce JK, Weidner A, Clouse K, et al. Sharing genetic test results with family members of BRCA, PALB2, CHEK2, and ATM carriers. Patient Educ Couns. 2021;104:720–5. https://doi.org/10.1016/j.pec.2020.12.019.
Gaff CL, Clarke AJ, Atkinson P, Sivell S, Elwyn G, Iredale R, et al. Process and outcome in communication of genetic information within families: A systematic review. Eur J Hum Genet. 2007;15:999–1011.
Bowen DJ, Makhnoon S, Shirts BH, Fullerton SM, Larson E, Ralston JD, et al. What improves the likelihood of people receiving genetic test results communicating to their families about genetic risk? Patient Educ Couns. 2021;104:726–31. https://doi.org/10.1016/j.pec.2021.01.001.
Forrest LE, Burke J, Bacic S, Amor DJ. Increased genetic counseling support improves communication of genetic information in families. Genet Med. 2008;10:167–72. http://www.ncbi.nlm.nih.gov/pubmed/18344705.
Kardashian A, Fehniger J, Creasman J, Cheung E, Beattie M. A pilot study of the sharing risk information tool (ShaRIT) for families with hereditary breast and ovarian cancer syndrome. Hered Cancer Clin Pr. 2012;10:1–10.
Martin AP, Downing J, Collins B, Godman B, Alfirevic A, Greenhalgh KL, et al. Examining the uptake of predictive BRCA testing in the UK; findings and implications. Eur J Hum Genet. 2021;29:699–708.
Montgomery SV, Barsevick AM, Egleston BL, Bingler R, Ruth K, Miller SM, et al. Preparing individuals to communicate genetic test results to their relatives: report of a randomized control trial. Fam Cancer. 2013;12:537–46. http://www.ncbi.nlm.nih.gov/pubmed/23420550.
Roshanai AH, Rosenquist R, Lampic C, Nordin K. Does enhanced information at cancer genetic counseling improve counselees’ knowledge, risk perception, satisfaction and negotiation of information to at-risk relatives?-a randomized study. Acta Oncol. 2009;48:999–1009. http://www.ncbi.nlm.nih.gov/pubmed/19636983.
Wynn J, Milo Rasouly H, Vasquez-Loarte T, Saami AM, Weiss R, Ziniel SI, et al. Do research participants share genomic screening results with family members? J Genet Couns. 2022;31:447–58.
Clarke S, Butler K, Esplen MJ. The phases of disclosing BRCA1/2 genetic information to offspring. Psychooncology. 2008;17:797–803. https://doi.org/10.1002/pon.1344.
Foster C, Eeles R, Ardern-Jones A, Moynihan C, Watson M. Juggling roles and expectations: dilemmas faced by women talking to relatives about cancer and genetic testing. Psychol Health. 2004;19:439–55.
Hamilton RJ, Bowers BJ, Williams JK. Disclosing genetic test results to family members. J Nurs Scholarsh. 2005;37:18–24. https://doi.org/10.1111/j.1547-5069.2005.00007.x.
Himes DO, Davis SH, Lassetter JH, Peterson NE, Clayton MF, Birmingham WC, et al. Does family communication matter? Exploring knowledge of breast cancer genetics in cancer families. J Commun Genet. 2019. https://www.ncbi.nlm.nih.gov/pubmed/30877488.
Srinivasan S, Won NY, Dotson WD, Wright ST, Roberts MC. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28:1631–44. https://doi.org/10.1038/s41431-020-00725-5.
Bleiker EM, Esplen MJ, Meiser B, Petersen HV, Patenaude AF. 100 years Lynch syndrome: what have we learned about psychosocial issues? Fam Cancer. 2013;12:325–39. https://www.ncbi.nlm.nih.gov/pubmed/23670341.
Chivers Seymour K, Addington-Hall J, Lucassen AM, Foster CL. What facilitates or impedes family communication following genetic testing for cancer risk? A systematic review and meta-synthesis of primary qualitative research. J Genet Couns. 2010;19:330–42. http://www.ncbi.nlm.nih.gov/pubmed/20379768.
Landsbergen KM, Prins JB, Brunner HG, van Duijvendijk P, Nagengast FM, van Krieken JH, et al. Psychological distress in newly diagnosed colorectal cancer patients following microsatellite instability testing for Lynch syndrome on the pathologist’s initiative. Fam Cancer. 2012;11:259–67. https://doi.org/10.1007/s10689-012-9510-1.
Manne SL, Chung DC, Weinberg DS, Vig HS, Catts Z, Cabral MK, et al. Knowledge and attitudes about microsatellite instability testing among high-risk individuals diagnosed with colorectal cancer. Cancer Epidemiol Biomark Prev. 2007;16:2110–7. https://www.ncbi.nlm.nih.gov/pubmed/17932359.
Burns C, Yeates L, Spinks C, Semsarian C, Ingles J. Attitudes, knowledge and consequences of uncertain genetic findings in hypertrophic cardiomyopathy. Eur J Hum Genet. 2017;25:809–15. https://doi.org/10.1038/ejhg.2017.66.
Mendes A, Paneque M, Sousa L, Clarke A, Sequeiros J. How communication of genetic information within the family is addressed in genetic counselling: a systematic review of research evidence. Eur J Hum Genet. 2016;24:315–25. https://www.ncbi.nlm.nih.gov/pubmed/26264439.
Baroutsou V, Underhill-Blazey ML, Appenzeller-Herzog C, Katapodi MC. Interventions facilitating family communication of genetic testing results and cascade screening in hereditary breast/ovarian cancer or lynch syndrome: A systematic review and meta-analysis. Cancers (Basel). 2021;13:1–25.
Bodurtha JN, McClish D, Gyure M, Corona R, Krist AH, Rodríguez VM, et al. The KinFact intervention - a randomized controlled trial to increase family communication about cancer history. J Women’s Health (Larchmt). 2014;23:806–16.
Dekker N, Hermens RP, de Wilt JH, van Zelst-Stams WA, Hoogerbrugge N, Nagengast F, et al. Improving recognition and referral of patients with an increased familial risk of colorectal cancer: Results from a randomized controlled trial. Color Dis. 2015;17:499–510.
Zhao J, Guan Y, McBride CM A systematic review of theory-informed strategies used in interventions fostering family genetic risk communication. Patient Educ Couns. 2022. https://doi.org/10.1016/j.pec.2022.03.009.
Christmas S, Michie S, West R. Thinking about behaviour change: an interdisciplinary dialogue. London: UK.: Silverback Publishing; 2015.
Dwyer AA, Hesse-Biber S, Flynn B, Remick S. Parent of origin effects on family communication of risk in brca+ women: A qualitative investigation of human factors in cascade screening. Cancers (Basel). 2020;12:1–16.
Michie S, West R, Campbell R, Brown J, Gainforth H. An ABC of behaviour change theories. London: UK.: Silverback Publishing; 2014.
Morrison L, Muller I, Yardley L, Bradbury K. The person-based approach to planning, optimising, evaluating and implementing behavioural health interventions. Eur Heal Psychol. 2018;20:464–9.
Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: Application to digital health-related behavior change interventions. J Med Int Res. 2015;17:e30.
Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020;368:1–6.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:332–6. https://doi.org/10.1136/bmj.b2535.
Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004;1:176–84.
Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46:81–95. http://www.ncbi.nlm.nih.gov/pubmed/23512568.
Kok G, Gottlieb NH, Peters GJY, Mullen PD, Parcel GS, Ruiter RAC, et al. A taxonomy of behaviour change methods: an Intervention Mapping approach. Health Psychol Rev. 2016;10:297–312. https://doi.org/10.1080/17437199.2015.1077155.
Eijzenga W, de Geus E, Aalfs CM, Menko FH, Sijmons RH, de Haes HCJM, et al. How to support cancer genetics counselees in informing at-risk relatives? Lessons from a randomized controlled trial. Patient Educ Couns. 2018;101:1611–9.
Hodgson J, Metcalfe S, Gaff C, Donath S, Delatycki MB, Winship I, et al. Outcomes of a randomised controlled trial of a complex genetic counselling intervention to improve family communication. Eur J Hum Genet. 2016;24:356–60. http://www.ncbi.nlm.nih.gov/pubmed/26130486.
De Vasconcelos S, Toskin I, Cooper B, Chollier M, Stephenson R, Blondeel K, et al. Behaviour change techniques in brief interventions to prevent HIV, STI and unintended pregnancies: A systematic review. PLoS One. 2018;13:e0204088.
Cowdell F, Dyson J. How is the theoretical domains framework applied to developing health behaviour interventions? A systematic search and narrative synthesis. BMC Public Health. 2019;19:1–10.
Michie S, Atkins L, West R The Behaviour Change Wheel: A Guide to Designing Interventions. Great Britain: Silverback Publishing; 2014.
French SD, Green SE, O’Connor DA, McKenzie JE, Francis JJ, Michie S, et al. Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the Theoretical Domains Framework. Implement Sci. 2012;7:38. https://doi.org/10.1186/1748-5908-7-38.
Banerjee A, Chitnis UB, Jadhav SL, Bhawalkar JS, Chaudhury S. Hypothesis testing, type I and type II errors. Ind Psychiatry J 2009;18:127–31.
Whittal A, Atkins L, Herber OR What the guide does not tell you: reflections on and lessons learned from applying the COM-B behavior model for designing real life interventions. Transl Behav Med. 2020. https://www.ncbi.nlm.nih.gov/pubmed/33200792.
Ballard LM, Fenwick A, Lucassen AM myKinMatters intervention: developing an online intervention to support patients in communicating relevant health information to at-risk relatives (oral). In: European Society for Human Genetics. Gothenburg, Sweden; 2019.
Michie S, Yardley L, West R, Patrick K, Greaves F. Developing and evaluating digital interventions to promote behavior change in health and health care: recommendations resulting from an international workshop. J Med Internet Res. 2017;19:e232. http://www.jmir.org/2017/6/e232/.
Taylor N, Healey E, Morrow A, Greening S, Wakefield CE, Warwick L, et al. Aligning intuition and theory: enhancing the replicability of behaviour change interventions in cancer genetics. Implement Sci Commun. 2020;1:1–10.
French DP, Cameron E, Benton JS, Deaton C, Harvie M. Can communicating personalised disease risk promote healthy behaviour change? A systematic review of systematic reviews. Ann Behav Med. 2017;51:718–29.
West R, Michie S. A brief introduction to the COM-B Model of behaviour and the PRIME Theory of motivation. Qeios. 2020;
Atkins L, Francis J, Islam R, O’Connor D, Patey A, Ivers N, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci. 2017;12:77. https://www.ncbi.nlm.nih.gov/pubmed/28637486.
Acknowledgements
LMB was funded by a Research Fellowship from Health Education England Genomics Education Programme and the National Institute for Health Research Biomedical Research Centre Southampton. This work was supported by funding from a Wellcome Trust collaborative award [grant number 208053/B/17/Z (to AL)]. The views expressed in this publication are those of the author(s) and not necessarily those of the HEE GEP. We wish to thank Jade Ford for her work on the initial review process completed as part of a MSc in Genomic Medicine and Dr Kate Morton for her helpful comments on an earlier draught.
Funding
LMB was funded by a Research Fellowship from Health Education England Genomics Education Programme. LMB and AML are supported by the National Institute for Health Research Biomedical Research Centre Southampton. This work was also supported by funding from a Wellcome Trust collaborative award [grant number 208053/Z/17/Z (to AML)].
Author information
Authors and Affiliations
Contributions
All authors conceived and/or designed the work that led to the submission, acquired data, and/or played an important role in interpreting the results, draughted or revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ballard, L.M., Band, R. & Lucassen, A.M. Interventions to support patients with sharing genetic test results with at-risk relatives: a synthesis without meta-analysis (SWiM). Eur J Hum Genet 31, 988–1002 (2023). https://doi.org/10.1038/s41431-023-01400-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-023-01400-1
This article is cited by
-
Patients’ perceptions and practices of informing relatives: a qualitative study within a randomised trial on healthcare-assisted risk disclosure
European Journal of Human Genetics (2024)
-
The experience of receiving a letter from a cancer genetics clinic about risk for hereditary cancer
European Journal of Human Genetics (2024)
-
Public attitudes challenge clinical practice on genetic risk disclosure in favour of healthcare-provided direct dissemination to relatives
European Journal of Human Genetics (2024)
-
Cascade genetic counseling and testing in hereditary syndromes: inherited cardiovascular disease as a model: a narrative review
Familial Cancer (2024)
-
Why don’t we all use genomic testing?
European Journal of Human Genetics (2023)