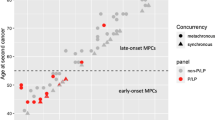
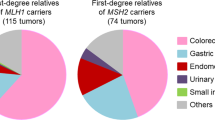
Abstract
Some patients with Lynch syndrome (LS) have extreme phenotypes, i.e. cancer before the recommended screening age, or cancer for which there are no screening guidelines. We made the hypothesis that additional germline variants in cancer susceptibility genes (CSG) could explain some of these phenotypes. We compared the prevalence of additional CSG variants in LS patients with a cancer diagnosis before age 30 (early-onset, EO group) and after 40 (usual-onset, UO group). While there was no overall difference, we did find an excess of pathogenic variants and variants of unknown significance in EO cases when only gastrointestinal CSG were considered (OR 2.25; 95% CI: 1.01–5.06, p value = 0.04). Four EO cases stood out: two with POLE/POLD1 variants in the key exonuclease domain, one with a BMPR1A duplication and one with an EPCAM deletion. Additional germline variants should be considered in future screening recommendations, as they might influence cancer risk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request, and pending a data transfer agreement between Assistance Publique-Hôpitaux de Paris and the applicant’s institution.
References
Seppälä TT, Latchford A, Negoi I, Sampaio Soares A, Jimenez-Rodriguez R, Sánchez-Guillén L, et al. European guidelines from the EHTG and ESCP for lynch syndrome: an updated third edition of the Mallorca guidelines based on gene and gender. Br J Surg. 2021;108:484–98.
Monahan KJ, Bradshaw N, Dolwani S, Desouza B, Dunlop MG, East JE, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut. 2020;69:411–44.
Kastrinos F, Ingram MA, Silver ER, Oh A, Laszkowska M, Rustgi AK, et al. Gene-specific variation in colorectal cancer surveillance strategies for lynch syndrome. Gastroenterol août. 2021;161:453–462. e15
Dominguez-Valentin M, Sampson JR, Seppälä TT, Ten Broeke SW, Plazzer JP, Nakken S, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the prospective lynch syndrome database. Genet Med. 2020;22:15–25.
Schamschula E, Kinzel M, Wernstedt A, Oberhuber K, Gottschling H, Schnaiter S, et al. Teenage-onset colorectal cancers in a digenic cancer predisposition syndrome provide clues for the interaction between mismatch repair and polymerase δ proofreading deficiency in tumorigenesis. Biomolecules 2022;12:1350.
Fernandez-Rozadilla C, Alvarez-Barona M, Schamschula E, Bodo S, Lopez-Novo A, Dacal A, et al. Early colorectal cancers provide new evidence for a lynch syndrome-to-CMMRD phenotypic continuum. Cancers. 2019;11:1081.
McGuigan A, Whitworth J, Andreou A, Hearn T, Genomics England Research Consortium, Tischkowitz M, et al. Multilocus inherited neoplasia allele syndrome (MINAS): an update. Eur J Hum Genet. 2022;30:265–70.
Bodo S, Colas C, Buhard O, Collura A, Tinat J, Lavoine N, et al. Diagnosis of constitutional mismatch repair-deficiency syndrome based on microsatellite instability and lymphocyte tolerance to methylating agents. Gastroenterology. 2015;149:1017–1029. e3
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28:1747–56.
Ricciuti B, Kravets S, Dahlberg SE, Umeton R, Albayrak A, Subegdjo SJ, et al. Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer. J Immunother Cancer. 2019;7:87.
Dhooge M, Baert-Desurmont S, Corsini C, Caron O, Andrieu N, Berthet P, et al. National recommendations of the French Genetics and Cancer Group—Unicancer on the modalities of multi-genes panel analyses in hereditary predispositions to tumors of the digestive tract. Eur J Med Genet. 2020;63:104080.
Ligtenberg MJL, Kuiper RP, Chan TL, Goossens M, Hebeda KM, Voorendt M, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3’ exons of TACSTD1. Nat Genet. 2009;41:112–7.
Seppälä TT, Latchford A, Negoi I, Soares AS, Jimenez‐Rodriguez R, Sánchez‐Guillén L, et al. European guidelines from the EHTG and ESCP for Lynch syndrome: an updated third edition of the Mallorca guidelines based on gene and gender. BJS (Br J Surg) [Internet]. [cité 9 déc 2020]; n/a(n/a). Disponible sur: http://bjssjournals.onlinelibrary.wiley.com/doi/abs/10.1002/bjs.11902
Downie JM, Riaz M, Xie J, Lee M, Chan AT, Gibbs P, et al. Incident cancer risk and signatures among older MUTYH carriers: analysis of population-based and genomic cohorts. Cancer Prev Res. 2022;15:509–19.
Jenkins MA, Buchanan DD, Lai J, Makalic E, Dite GS, Win AK, et al. Assessment of a polygenic risk score for colorectal cancer to predict risk of lynch syndrome colorectal cancer. JNCI Cancer Spectr. 2021;5:pkab022.
Llach J, Pellisé M, Monahan K. Lynch syndrome; towards more personalized management? Best Pr Res Clin Gastroenterol. 2022;58-59:101790.
Acknowledgements
We thank Pr Marie Pierre Buisine for her contribution to the revisions (concerning methylation of MSH2). No funding was received for this study.
Author information
Authors and Affiliations
Contributions
RV, PRB: designed the study, acquired and analyzed data, and wrote the manuscript. JH: data management, research governance. AP, FB, MT: complementary data collection and analysis. AL: statistical analyses. AD, FC: contribution to study design. CC, MD, SF, PLP, JM, DM,JN, GP: genetic counseling, patient care. NB, AC, CD, NH, MM, RN, AP, FC: germline sequencing and interpretation. All authors approved the final version, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
PRB has received honoraria from AstraZeneca, MSD and BMS. The other authors declare no competing interests.
Ethical approval
The study was approved by the ethics evaluation committee of Inserm, the Institutional Review Board (IRB00003888, IORG0003254, FWA00005831) of the French Institute of medical research and Health (Opinion number 22-878).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vibert, R., Hasnaoui, J., Perrier, A. et al. Lynch syndrome: influence of additional susceptibility variants on cancer risk. Eur J Hum Genet 31, 1078–1082 (2023). https://doi.org/10.1038/s41431-023-01367-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-023-01367-z
This article is cited by
-
Why don’t we all use genomic testing?
European Journal of Human Genetics (2023)