Abstract
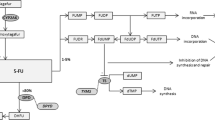
The Dutch Pharmacogenetics Working Group (DPWG) aims to facilitate PGx implementation by developing evidence-based pharmacogenetics guidelines to optimize pharmacotherapy. This guideline describes the starting dose optimization of the anti-cancer drug irinotecan to decrease the risk of severe toxicity, such as (febrile) neutropenia or diarrhoea. Uridine diphosphate glucuronosyl transferase 1A1 (UGT1A1 encoded by the UGT1A1 gene) enzyme deficiency increases risk of irinotecan-induced toxicity. Gene variants leading to UGT1A1 enzyme deficiency (e.g. UGT1A1*6, *28 and *37) can be used to optimize an individual’s starting dose thereby preventing carriers from toxicity. Homozygous or compound heterozygous carriers of these allele variants are defined as UGT1A1 poor metabolisers (PM). DPWG recommends a 70% starting dose in PM patients and no dose reduction in IM patients who start treatment with irinotecan. Based on the DPWG clinical implication score, UGT1A1 genotyping is considered “essential”, indicating that UGT1A1 testing must be performed prior to initiating irinotecan treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
All data and material are either included in the supplementary information or publicly available (i.e. the published articles, PubMed). The guidelines and background information are available on the website of the Royal Dutch Pharmacists Association (KNMP) (Pharmacogenetic Recommendation Text. Available from: https://www.knmp.nl/).
Change history
09 February 2023
Supplementary table 3 has been corrected.
16 February 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41431-023-01315-x
References
Swen J, Wilting I, Goede A, de, Grandia L, Mulder H, Touw D, et al. Pharmacogenetics: From Bench to Byte. Clin Pharm Ther. 2008;83:781–7. https://doi.org/10.1038/sj.clpt.6100507.
Guchelaar H-J. Pharmacogenomics, a novel section in the European Journal of Human Genetics. Eur J Hum Genet. 2018;26:1399–400. https://doi.org/10.1038/s41431-018-0205-4.
Lunenburg CATC, van der Wouden CH, Nijenhuis M, Crommentuijn-van Rhenen MH, de Boer-Veger NJ, Buunk AM, et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene–drug interaction of DPYD and fluoropyrimidines. Eur J Hum Genet. 2020;28:508–17. https://doi.org/10.1038/s41431-019-0540-0.
Matic M, Nijenhuis M, Soree B, de Boer-Veger NJ, Buunk A-M, Houwink EJF, et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2D6 and opioids (codeine, tramadol and oxycodone). Eur J Hum Genet. 2021. https://doi.org/10.1038/s41431-021-00920-y.
Brouwer JMJL, Nijenhuis M, Soree B, Guchelaar H-J, Swen JJ, van Schaik RHN, et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2C19 and CYP2D6 and SSRIs. Eur J Hum Genet. 2021. https://doi.org/10.1038/s41431-021-01004-7.
Rougier P, Bugat R. CPT-11 in the treatment of colorectal cancer: clinical efficacy and safety profile. Semin Oncol. 1996;23:34–41.
Rothenberg ML. Efficacy and toxicity of irinotecan in patients with colorectal cancer. Semin Oncol. 1998;25:39–46.
Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s Syndrome. N. Engl J Med. 1995;333:1171–5. https://doi.org/10.1056/nejm199511023331802.
UGT1A1 allele nomenclature n.d. https://www.pharmacogenomics.pha.ulaval.ca/wp-content/uploads/2015/04/UGT1A1-allele-nomenclature.html (accessed September 6, 2021).
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. BioRxiv. 2019:531210. https://doi.org/10.1101/531210.
Akiyama Y, Fujita K, Nagashima F, Yamamoto W, Endo H, Sunakawa Y, et al. Genetic testing for UGT1A1*28 and *6 in Japanese patients who receive irinotecan chemotherapy. Ann Oncol. 2008;19:2089–90. https://doi.org/10.1093/annonc/mdn645.
Teh LK, Hashim H, Zakaria ZA, Salleh MZ. Polymorphisms of UGT1A1*6, UGT1A1*27 & UGT1A1*28 in three major ethnic groups from Malaysia. Indian J Med Res. 2012;136:249–59.
Sung C, Lee PL, Tan LL, Toh DSL. Pharmacogenetic risk for adverse reactions to irinotecan in the major ethnic populations of Singapore. Drug Saf. 2011;34:1167–75. https://doi.org/10.2165/11594440-000000000-00000.
Akaba K, Kimura T, Sasaki A, Tanabe S, Ikegami T, Hashimoto M, et al. Neonatal hyperbilirubinemia and mutation of the bilirubin uridine diphosphate-glucuronosyltransferase gene: a common missense mutation among Japanese, Koreans and Chinese. Biochem Mol Biol Int. 1998;46:21–6. https://doi.org/10.1080/15216549800203512.
de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S. Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet. 2018;57:1229–54. https://doi.org/10.1007/s40262-018-0644-7.
Goetz MP, McKean HA, Reid JM, Mandrekar SJ, Tan AD, Kuffel MA, et al. UGT1A1 genotype-guided phase i study of irinotecan, oxaliplatin, and capecitabine. Invest N. Drugs. 2013;31:1559–67. https://doi.org/10.1007/s10637-013-0034-9.
Denlinger CS, Blanchard R, Xu L, Bernaards C, Litwin S, Spittle C, et al. Pharmacokinetic analysis of irinotecan plus bevacizumab in patients with advanced solid tumors. Cancer Chemother Pharm. 2009;65:97–105. https://doi.org/10.1007/s00280-009-1008-7.
Iyer L, Das S, Janisch L, Wen M, Ramírez J, Karrison T, et al. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J. 2002;2:43–7. https://doi.org/10.1038/sj.tpj.6500072.
de Jong FA, Kehrer DFS, Mathijssen RHJ, Creemers G, de Bruijn P, van Schaik RHN, et al. Prophylaxis of irinotecan‐induced diarrhea with neomycin and potential role for UGT1A1*28 genotype screening: a double‐blind, randomized, placebo‐controlled study. Oncologist. 2006;11:944–54. https://doi.org/10.1634/theoncologist.11-8-944.
Paoluzzi L, Singh AS, Price DK, Danesi R, Mathijssen RHJ, Verweij J, et al. Influence of genetic variants in UGT1A1 and UGT1A9 on the in vivo glucuronidation of SN-38. J Clin Pharm. 2004;44:854–60. https://doi.org/10.1177/0091270004267159.
Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol. 2004;22:1382–8. https://doi.org/10.1200/JCO.2004.07.173.
Hulshof EC, de With M, de Man FM, Creemers GJ, Deiman BALM, Swen JJ, et al. UGT1A1 genotype-guided dosing of irinotecan: a prospective safety and cost analysis in poor metaboliser patients. Eur J Cancer. 2022;162:148–57. https://doi.org/10.1016/J.EJCA.2021.12.009.
Hu ZY, Yu Q, Pei Q, Guo C. Dose-dependent association between UGT1A1*28 genotype and irinotecan-induced neutropenia: Low doses also increase risk. Clin Cancer Res. 2010;16:3832–42. https://doi.org/10.1158/1078-0432.CCR-10-1122.
Liu X, Cheng D, Kuang Q, Liu G, Xu W. Association of UGT1A1*28 polymorphisms with irinotecan-induced toxicities in colorectal cancer: a meta-analysis in Caucasians. Pharmacogenomics J. 2014;14:120–9. https://doi.org/10.1038/tpj.2013.10.
Lu CY, Huang CW, Wu IC, Tsai HL, Ma CJ, Yeh YS, et al. Clinical implication of UGT1A1 promoter polymorphism for irinotecan dose escalation in metastatic colorectal cancer patients treated with bevacizumab combined with FOLFIRI in the first-line setting. Transl Oncol. 2015;8:474–9. https://doi.org/10.1016/j.tranon.2015.11.002.
Minami H, Sai K, Saeki M, Saito Y, Ozawa S, Suzuki K, et al. Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet Genomics. 2007;17:497–504. https://doi.org/10.1097/FPC.0b013e328014341f.
Pirmohamed M, Hughes DA. Pharmacogenetic tests: the need for a level playing field. Nat Rev Drug Discov. 2013;12:3–4. https://doi.org/10.1038/nrd3921.
Swen JJ, Huizinga TW, Gelderblom H, de Vries EGE, Assendelft WJJ, Kirchheiner J, et al. Translating pharmacogenomics: challenges on the road to the clinic. PLoS Med. 2007;4:e209. https://doi.org/10.1371/journal.pmed.0040209.
Swen JJ, Nijenhuis M, van Rhenen M, de Boer-Veger NJ, Buunk AM, Houwink EJF, et al. Pharmacogenetic information in clinical guidelines: The European perspective. Clin Pharm Ther. 2018;103:795–801. https://doi.org/10.1002/cpt.1049.
Etienne-Grimaldi MC, Boyer JC, Thomas F, Quaranta S, Picard N, Loriot MA, et al. UGT1A1 genotype and irinotecan therapy: general review and implementation in routine practice. Fundam Clin Pharm. 2015;29:219–37. https://doi.org/10.1111/fcp.12117.
AIOM - SIF. Raccomandazioni par analisi farmacogenetiche n.d. https://www.aiom.it/raccomandazioni-2019-per-analisi-farmacogenetiche/ (accessed November 15, 2021).
Stanford University & St. Jude Children’s Research Hospital. Clinical Pharmacogenetics Implementation Consortium. PharmGKB PGRN 2020. https://cpicpgx.org/ (accessed May 8, 2020).
Funding
The U-PGx consortium received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 668353. The DPWG received funding from the Royal Dutch Pharmacists Association.
Author information
Authors and Affiliations
Contributions
ECH and MJD drafted the manuscript. JJS and HJG supervised drafting of the manuscript and contributed to conceiving the work and interpretation of the results. MN performed most of the literature searches and article summaries, and suggested clinical decision support texts. BS had the clinical decision support texts translated in English and published them. NBV, AB, EJHF, AR, GAR, RHNS, DT, JW, and RW contributed to conceiving the work and interpretation of the results. VHMD led the meetings in which the DPWG decided about the article summaries and clinical decision supports texts and contributed to conceiving the work and interpretation of the results. In addition, all authors revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Supplementary table 3 has been corrected.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hulshof, E.C., Deenen, M.J., Nijenhuis, M. et al. Dutch pharmacogenetics working group (DPWG) guideline for the gene–drug interaction between UGT1A1 and irinotecan. Eur J Hum Genet 31, 982–987 (2023). https://doi.org/10.1038/s41431-022-01243-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-022-01243-2
This article is cited by
-
Personalizing adjuvant therapy for patients with colorectal cancer
Nature Reviews Clinical Oncology (2024)
-
Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction of CYP2C9, HLA-A and HLA-B with anti-epileptic drugs
European Journal of Human Genetics (2024)
-
Why don’t we all use genomic testing?
European Journal of Human Genetics (2023)
-
Towards UGT1A1 guided irinotecan dosing
European Journal of Human Genetics (2023)