Abstract
Von Hippel-Lindau (VHL) disease is one of the most common cancer predisposition syndromes. Penetrance is high with around 20% of children presenting detectable and curable manifestations of the disease at 15 years old. VHL predictive genetic testing (PGT) is recommended during childhood from age 5 years in France. Insufficient compliance to surveillance of VHL pathogenic variant (PV) carriers is associated with severe outcome. PGT experienced by children and their parents is probably critical in influencing future acceptance of the result and adherence to surveillance. We conducted a retrospective study on minors tested (aged 5 to 16 years old) from 2010 to 2020, in a multidisciplinary oncogenetics consultation which follows a 3-step protocol based on psychological familial support. The objectives were to assess the adherence to follow-up within the National Expert Center for inherited predispositions to renal tumors (PREDIR) network of VHL PV carriers and its benefit through tumor detection and medical interventions. A VHL PGT was carried out in 34 children. Among the 16 children diagnosed as VHL PV carriers addressed to the PREDIR network, none had discontinued surveillance after a median of 41 months. Follow-up examinations detected 11 tumors in 6 children, 4 have been surgically treated. All had a favorable outcome. Our data suggest that a specific and adapted procedure for PGT in at-risk VHL children as well as a follow-up, organized within a specialized expert network, fosters a complete adherence to the surveillance protocol and thus lead to a favorable clinical outcome.
Similar content being viewed by others
Introduction
von Hippel-Lindau (VHL) disease is a multisystemic familial cancer syndrome, with dominant inheritance, caused by heterozygous germline pathogenic variant (PV) in the VHL tumor suppressor gene. It represents one of the most common familial cancer syndromes with approximatively one carrier in 36,000 live births. VHL is implicated in multiple cellular processes, particularly in the cellular response to hypoxia, explaining the development of highly vascular tumors. VHL disease tumor spectrum comprises both benign and malignant tumors including hemangioblastomas of the central nervous system (CNS, 60–80%) mainly in the cerebellum, retinal hemangioblastomas (60%), clear-cell renal cell carcinomas (24–70%), renal cysts, phaeochromocytomas and paragangliomas (10–20%), pancreatic neuroendocrine tumors (8–17%), pancreatic cysts and serous cystadenomas (17–56%), endolymphatic sac tumors (ELST, 3–15%), epididymal papillary cystadenomas (25–60%) and broad ligament cystadenomas (very rare) [1,2,3]. VHL disease has a high penetrance assessed up to 87% at age 65, with 16% of asymptomatic and 11% of symptomatic manifestations at 15 years old estimated on a cohort of 85 Danish VHL patients undergoing surveillance [4]. VHL-related tumors have been exceptionally reported as early as 2 years of age [5].
Before systematic medical surveillance the median survival of patients affected by VHL disease did not exceed 50 years of age [1]. Predictive genetic testing (PGT) identifies asymptomatic PV carriers who are eligible to a medical follow-up, with the aim of improving prognosis and overall survival, and non-PV carriers for whom surveillance is unnecessary. Due to the risk of tumors arising during childhood, VHL PGT is recommended from the age of 5 in France [6]. PGT in minors, defined as all subjects who have not reached the age of legal majority in health decisions, is allowed by the French bioethics’ legislation because VHL disease manifestations occurring in childhood can be effectively treated or prevented. VHL PGT is associated with an immediate medical benefit. Potential benefit of early detection of tumors outweighs the harms associated with the test. Parents or legal guardians should participate in the decision-making process regarding the health care of their children and are expected to decide to encounter the genetic test according to “the best interest of the child”. Medical genetic units should take a supportive role in this process [7]. PGT in minors is challenging and requires an adaptation of genetic counselling and of parents’ and children’s support throughout the testing procedure. That procedure is critical for preparing the announcement of the result of the test as it would influence the proper understanding of the genetic data and, for PV carriers, the future adherence to medical preventive actions.
Families affected by VHL disease encounter psychological, social, and practical challenges, which can lead to an insufficient compliance to surveillance resulting in screening delays [8, 9]. It has been shown that as many as 60% of identified VHL PV carriers were lost to follow-up 5 years after testing [10], urging the need for solutions to improve adherence to surveillance protocols. The French guidelines recommended that a regular screening for asymptomatic PV carriers begins at 5 years old for retinal lesions and catecholamine-producing pheochromocytoma (PHEO) or paraganglioma by annual physical examination and blood pressure measurement, metanephrines measurements, dilated eye examination and abdominal ultrasonography (until 18 years old and then in alternance with abdominal MRI). This surveillance also comprises, biennially, an audiology assessment for searching ELST. A biennially cranial MRI for detecting CNS hemangioblastomas is performed from the age of 15, Ultrasound of the epididymis is now only performed in case of symptoms [11].
Since 2010, the oncogenetics multidisciplinary consultation unit (Genetics department of European Georges Pompidou Hospital in Paris, France) follows a 3-step protocol for VHL PGT in minors, firstly developed and validated for children at risk of hereditary paraganglioma/ PHEO [12]. That consultation is the referral genetic unit for the PGT in minors at-risk of VHL disease of the National Expert Center for inherited predispositions to renal tumors (the PREDIR network, see https://predir.org/), which is in charge of the medical follow-up of VHL patients and of asymptomatic PV carriers. Herein, we evaluate the adherence to the surveillance proposed by the PREDIR network to minors after a positive PGT carried out with the 3-step protocol from 2010 to 2020 and the benefits of screening through tumor detection and medical intervention.
Subjects and methods
Patients
All minor subjects (age under 18 years old) addressed for VHL PGT at the Genetics department of the European Georges Pompidou Hospital (Paris, France) from 2010 to 2020 were included in our retrospective study.
Three-step protocol of PGT
The complete procedure is drawn on Fig. 1. The first step is a preparatory step, only addressed to the parents. During this step, parents receive information on genetic testing, on the PGT procedure and on the medical preventive actions that will be immediately recommended in case of a positive test. They first meet the geneticist (A-P.G-R) and subsequently the psychiatrist (K.L-L) who both provide support during the decision-making process and help for informing their child. For instance, parents can express their anxiety about the consequences of the genetic result on their child or, sometimes, their feeling of guilt about disease transmission. Parents are advised on how to best inform their child, in tailoring the information to their age-related capacities. The affected parent is encouraged to talk about his/her own disease and its hereditary transmission. Personalized advices are given to parents to find the best words for explaining the risk of transmission and the interest of prevention. An informative booklet written for children is given to parents as information support (https://www.vhlfrance.org/2018/10/26/vivre-avec-le-vhl/). More than one preparatory consultation can be organized if necessary.
Design of the 3-step protocol for predictive genetic testing in minors at risk of Von Hippel–Lindau disease. During the first step, the parents without the child meet successively the geneticist and the psychiatrist who provide information and advice on the test and how to inform the child. During the second and third steps, the parents and the child meet with the geneticist and the psychiatrist for the test and for the announcement of the result.
The second step, leading to the test, is organized when parents are ready to undertake the testing of their child, who has been properly informed and prepared. The child accompanied by his parents meet the geneticist and the psychiatrist during a combined consultation. The correct understanding of the genetic test by the minor is checked and eventually the information is completed. Consent forms are delivered and explained by the geneticist, who then leaves the consultation room for allowing a dedicated consultation of both parents and the child with the psychiatrist. During this time, subjective feelings regarding the result of the genetic test are raised and potential emotional consequences following the result are anticipated. Lastly, the child is seen alone by the psychiatrist, to give the child the possibility to express personal worries about the consequences of the genetic result. When the decision to undergo the test is taken, both parents and the minor if considered mature enough, sign the consent form for genetic testing in presence of the psychiatrist and blood is withdrawn subsequently.
The third step corresponds to the announcement of the genetic test result, by the geneticist to the minor and his/her parents, in presence of the psychiatrist. Afterwards the child is seen alone by the psychiatrist, then with his/her parents to provide all family members an opportunity to express together their immediate reaction to the result. Following a positive result, a subsequent consultation at the PREDIR reference center (Bicêtre Hospital, Le Kremlin-Bicêtre, France) is promptly organized. A specific attention is turned to close relations between the Genetics department and the PREDIR center to facilitate the booking of the first appointment with the PREDIR physician coordinator (S.R) who sees VHL at-risk minors in priority.
A multidisciplinary meeting between the geneticist and the psychiatrist is organized after each step of the protocol, in order to precisely prepare the next step. Throughout the whole procedure, parents and children have the possibility to benefit from additional consultations with the psychiatrist and/or the geneticist or to postpone appointments. One of the main objectives of the 3-step protocol is to prepare and to accompany parents and children as best as possible throughout the PGT procedure and appropriately to possible life events, such as hospitalization of the affected parent, etc.
Genetic testing
Two blood samples are collected during the second step. Each blood sample is processed independently in order to deliver a single definitive result at the third step. Leucocyte DNA is extracted from the blood samples in the molecular genetic laboratory of the Genetics department of European Georges Pompidou Hospital (Paris, France). Targeted Sanger sequencing or MLPA (SALSA MLPA Probemix P016 VHL- MRC Holland) is performed for the detection of the VHL PV or of the large VHL deletion previously identified in the index case of every family.
Follow-up of VHL PV carriers within the PREDIR network
Our department is part of the PREDIR network, which comprises multiple specialists with specific skills in charge of establishing a diagnosis and optimizing the monitoring and treatment of VHL patients and at-risk family members. It is composed of a reference center coordinated by the Bicêtre hospital at Assistance-Publique Hôpitaux de Paris and 11 competence centers spread throughout France. All positive-tested children are addressed to a PREDIR center for explanation on the recommendations for an initial screening for VHL disease-related tumors and then an optimal VHL follow-up. Prescriptions for follow-up exams are sent prior to surveillance consultations. All children have a follow-up file updated regularly by the reference center and reminder letters are sent in case of delayed follow-up.
Endpoints
The adherence to medical follow-up has been evaluated with three different criteria: 1. consultation in the PREDIR center after the genetics result; 2. effective surveillance; 3. regularity of follow-up.
The surveillance benefits have been assessed on the number of lesions detected by follow-up screening exams and on medical outcome of at-risk VHL minors.
Results
Population
From 2010 to 2020, a total of 23 parents, from 21 different VHL families, came to oncogenetics consultation for asking a VHL PGT for their children (Fig. 2). The protocol was stopped after the first step for 4 out of 23 parents. One mother was addressed to another genetic consultation in a French oversea department, where she lived, for organizing the PGT of their children. Three additional parents with difficult contexts did not pursue the procedure. One affected mother had discontinued her medical follow-up for 7 years when she came for the preparatory consultation. She was encouraged to restart her own surveillance before organizing the PGT for her child. One patient diagnosed for VHL disease only one year ago, came alone to receive information on PGT for their child and finally disagreed with the proposed 3-step protocol. One unaffected mother was still in mourning of her husband who died of the VHL disease in a context of bilateral renal cancer. Geneticist and psychiatrist recommended to take more time in order to be psychologically able to accompany her child during the PGT procedure. The procedure was thus pursued for 19 parents, from 18 unrelated families, for which the surveillance had been carried out within the PREDIR network (median of follow-up: 3 years, ranging from 0 to 19 years). Difficult familial contexts were also encountered among these latter, including two fathers that had lost their spouse of the VHL disease. Among the 36 children in age of being tested within the families who went along with the procedure, two had not yet been tested. One was planned to benefit from the PGT during the year 2021. The other, aged of 9 years in 2020, was in second position in the sibling but up to now his parents did not yet come back to genetic consultation.
Thirty-four asymptomatic children, with a median age of 8.5 years old (ranging from 5 to 16), were genetically tested for VHL disease following the 3-step protocol. A positive VHL status was announced to 20 children (58.8%). Apart from four children that were living abroad, all the other children (n = 16) were subsequently addressed to the PREDIR center that organized the first screening and then an annual follow-up. All children except one, who pursued her follow-up within a PREDIR regional competence center (Nantes, C.A), were followed by the PREDIR national expert center at Kremlin Bicêtre (S.R). All 16 children were seen for a first consultation within the PREDIR center or network and/or realized a first follow-up exam in a median of one month (ranging from 0 to 10 months) after the announcement of the positive VHL result.
Adherence to follow-up protocol
All at-risk children who received the result of their PGT before or in 2019 (n = 13), pursued a regular follow-up within the PREDIR center or network. No child was lost to follow-up with at least one surveillance exam performed during 2020, or at the end of 2019 for one child. Two individuals tested carriers (patients #1 and #13) had reached adult age at last follow-up, notably the first child tested in 2010 aged 23 years old at last follow-up, who had been regularly followed for ten years. The median duration of follow-up of all 16 children addressed to the PREDIR center, calculated from the date of the result of the genetic test and the last consultation/follow-up exam, was 41 months, ranging from 29 days to 113 months.
Screening results
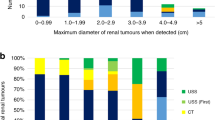
Follow-up exams have detected 11 tumors (4 PHEO, 4 CNS and 2 retinal hemangioblastomas, 1 ELST) in 6 children (median age at diagnosis of the first syndromic tumor of 14.5 years, range 11–17) for a total of 687 person-months, representing a theoretical detection of 192 tumors in a population of 1,000 VHL PV carriers followed during a year (Table 1). One child with a 3 cm PHEO had an abnormal level of metanephrines and the child with ELST had a loss of hearing. In addition, microcysts (size inferior to 1 cm) were detected in the epididymis (4 patients, at a median age of 12.5 years old), in the kidneys (2 patients, at age of 12 and 18, respectively) and in the pancreas (2 patients, at 17 and 18 of age, respectively). All detected tumors were in line with the expected tumor spectrum according to the affected parent’s presumed VHL subtype (defined by mutation type and clinical manifestations, Table 1).
Medical outcome
All PHEO and the ELST were surgically cured. Laser treatment was applied on the two detected retinal hemangioblastomas (V.K). Follow-up evaluation after surgery or laser treatment was normal. Tumor cell growths of the four CNS hemangioblastomas, as well as of microcysts, were followed regularly. All at-risk VHL children have experienced a favorable outcome and did not present any new lesions after the last follow-up.
Discussion
VHL is a potentially severe disease associated with premature deaths or severe disabilities in unscreened and unfollowed members of affected families. Parents, who are theoretically in favor of prevention by testing their child, face great difficulty in carrying it out in practice. They find it difficult to expose the hereditary disease during childhood, fearing to distress the child and harm his development. A supportive procedure to guide parents throughout the PGT of their children is thus required. Our procedure, based on a strong psychological familial support, was associated with a complete adherence to the surveillance protocol. Our genetics department had initially developed a 3-step multidisciplinary protocol for PGT in minors at risk of paraganglioma or PHEO in the early 2000 s [12]. The procedure has progressively been improved with experience and was further applied, from 2010, in the PGT in minors at risk of VHL disease. This protocol was approved by the great majority of parents who came with the aim of testing their child and said to be reassured by the inclusion and the accompaniment throughout a well-defined procedure. It should be emphasized that the 3-step protocol was adaptive to each situation, with the possibility of organizing additional consultations with personalized advice, depending on the situation of the parent affected by the disease. The main objective was to ensure a long-life follow-up to PV carriers diagnosed during childhood.
The PREDIR network is in charge of French VHL families and patients (adults and children) as well as of the follow-up of asymptomatic VHL PV carriers detected by PGT. In that situation, the tumors detected by screening exams can benefit from an early minimal treatment, and /or scrupulous surveillance. These assumptions are in line with our data, in which all treated children by surgery or photocoagulation did not present new lesions at last follow-up without any therapeutic sequels. From our data, we have estimated that in a theoretical population of 1,000 VHL PV carriers diagnosed and followed during childhood, 192 curable tumors would be detected, during one year of follow-up, highlighting the interest and the benefit of VHL PGT and subsequent regular follow-up in children. Furthermore, all detected tumors fell within the expected spectrum of their parent’s presumed VHL subtype, suggesting a good match between the parent’s and the child’s phenotype, which could help guide management and genetic counselling. Nevertheless, current French surveillance guidelines do not distinguish between subtypes for the routine clinical management.
Rasmussen et al. [10] had tested individuals for VHL PV from 17 unrelated families including 43 children under the age of 18. Thirty-six individuals were tested positive in their study and after 5 years only 38.9% of PV carriers continued the tumor surveillance program. Among these individuals, 8 asymptomatic VHL-positive children were diagnosed but only one child had pursued follow-up after five years (14.3%). A hemangioblastoma was diagnosed in one child that had discontinued follow-up five years after the test. For their PGT procedure, they used the same procedure for adults and children, with pre-test counseling including interviews with the geneticist, a social worker, and a clinical psychologist experienced in PGT. If significant distress was noted, a further evaluation was performed by an experienced psychiatrist and, if necessary, the test was postponed. All PV-carriers underwent an initial screening for VHL disease-related tumors and appointments were programmed annually by the social worker upon completion of the precedent screening. Although the follow-up time in our study was shorter, of about three and half years, the absence of VHL positive child lost to follow-up emphasizes the importance of a dedicated protocol to children for the PGT, different from the one of adults, as previously discussed in the literature [13, 14].
In agreement with our procedure, it has been recently reported that bibliotherapy using stories, such as our booklet intended for children, helping children understand inherited cancer predisposition syndromes, was useful for children aged 5–10 years old at risk of either Li-Fraumeni syndrome or hereditary PHEO and paraganglioma syndrome, by improving the process of communication between parents and children [15].
A key value of our work is the validation of our 3-step protocol that can be applied by other multidisciplinary teams carrying PGT for children at risk of genetic predisposition. In our case, the protocol was conceived and developed jointly by the geneticist and the psychiatrist who had been working together for years, based on their experience. To date several French psychologists have been trained by our team and have integrated other multidisciplinary teams performing PGT for children according to a similar 3-step protocol, for different genetic predispositions such as inherited cardiomyopathies. A limit of our study was the impossibility to consider all parents that could have been referred to our consultation for the testing of their children but who did not come to our consultation. Difficult contexts were identified for all parents who did not pursue the PGT procedure for their child, underlining the importance of detecting this kind of context and possibly trying to improve the management in these cases. Nevertheless, similar difficult contexts were also encountered among parents who pursued the procedure. Moreover, all parents that pursued with our procedure went through the whole process without interruption.
In conclusion, our study demonstrates for the first time the benefits of VHL PGT during childhood, of a specific and adaptive procedure for organizing PGT in children at risk of VHL, of a follow-up organized within a well-defined specialized network for this rare predisposition to cancers. A longer follow-up period will be required to examine the continuation of follow-up during adulthood and the long-time clinical outcome of patients identified as VHL PV carriers during their infancy. Finally, our data show that the 3-step protocol dedicated to PGT in children might be applied for other genetic predispositions.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. von Hippel-Lindau disease. Lancet 2003;361:2059–67.
Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet. 2011;19:617–23.
Dornbos D, Kim HJ, Butman JA, Lonser RR. Review of the Neurological Implications of von Hippel–Lindau Disease. JAMA Neurol. 2018;7:620–7.
Launbjerg K, Bache I, Galanakis M, Bisgaard ML, Binderup MLM. von Hippel-Lindau development in children and adolescents. Am J Med Genet A. 2017;173:2381–94.
Ridley M, Green J, Johnson G. Retinal angiomatosis: the ocular manifestations of von Hippel-Lindau disease. Can J Ophthalmol. 1986;21:276–83.
Levy M, Richard S. Attitudes of von Hippel-Lindau disease patients towards presymptomatic genetic diagnosis in children and prenatal diagnosis. J Med Genet. 2000;37:476–8.
European Society of Human Genetics. Genetic testing in asymptomatic minors: Recommendations of the European society of human genetics. Eur J Hum Genet. 2009;17:720–1.
Kasparian NA, Rutstein A, Sansom-Daly UM, Mireskandari S, Tyler J, Duffy J, et al. Through the looking glass: An exploratory study of the lived experiences and unmet needs of families affected by Von Hippel-Lindau disease. Eur J Hum Genet. 2015;23:34–40.
Lammens CRM, Aaronson NK, Hes FJ, Links TP, Zonnenberg BA, Lenders JWM, et al. Compliance with periodic surveillance for Von-Hippel-Lindau disease. Genet Med. 2011;13:519–27.
Rasmussen A, Alonso E, Ochoa A, De Biase I, Familiar I, Yescas P, et al. Uptake of genetic testing and long-term tumor surveillance in von Hippel-Lindau disease. BMC Med Genet. 2010;11:4.
Nielsen SM, Rhodes L, Blanco I, Chung WK, Eng C, Maher ER, et al. Von Hippel-Lindau Disease: Genetics and role of genetic counseling in a multiple neoplasia syndrome. J Clin Oncol. 2016;34:2172–81.
Lahlou-Laforêt K, Consoli SM, Jeunemaitre X, Gimenez-Roqueplo AP. Presymptomatic genetic testing in minors at risk of paraganglioma and pheochromocytoma: our experience of oncogenetic multidisciplinary consultation. Horm Metab Res. 2012;44:354–8.
Godino L, Turchetti D, Jackson L, Hennessy C, Skirton H. Impact of presymptomatic genetic testing on young adults: a systematic review. Eur J Hum Genet. 2016;24:496–503.
Godino L, Turchetti D, Jackson L, Hennessy C, Skirton H. Presymptomatic genetic testing for hereditary cancer in young adults: a survey of young adults and parents. Eur J Hum Genet. 2019;27:291–9.
Schlub GM, Crook A, Barlow-Stewart K, Fleming J, Kirk J, Tucker K, et al. Helping young children understand inherited cancer predisposition syndromes using bibliotherapy. J Genet Couns. 2021;30:1119–32.
Acknowledgements
This work was supported in part by INCa and the Association VHL-France. We thank Anne-Marie Birot and Matthieu Bruzzi for their contribution to the childcare circuit.
Funding
No funding to declare.
Author information
Authors and Affiliations
Contributions
RV, KLL, MS, VK, TB, LA, NB, CA, SR, APGR: conceived and designed the work that led to the submission, acquired data and played an important role in interpreting the results. Drafted or revised the manuscript. Approved the final version. Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The research was approved by the AP-HP Centre Ethics Committee (IRB registration: #00011928; Reference:2021-10-03).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vibert, R., Lahlou-Laforêt, K., Samadi, M. et al. Minors at risk of von Hippel-Lindau disease: 10 years’ experience of predictive genetic testing and follow-up adherence. Eur J Hum Genet 30, 1171–1177 (2022). https://doi.org/10.1038/s41431-022-01157-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-022-01157-z
This article is cited by
-
2022: the year that was in the European Journal of Human Genetics
European Journal of Human Genetics (2023)
-
Happy 30th birthday to the European Journal of Human Genetics!
European Journal of Human Genetics (2022)