Abstract
Costello syndrome (CS) is caused by heterozygous HRAS germline mutations. Most patients share the HRAS variant p.Gly12Ser that is associated with a typical, homogeneous phenotype. Rarer pathogenic HRAS variants (e.g., p.Thr56Ile) were identified in individuals with attenuated CS phenotypes. The obvious phenotypical variability reflects different dysfunctional consequences of distinct HRAS variants. We report on two boys with the novel de novo HRAS variant c.466 C > T p.(Phe156Leu). Both had severe feeding difficulties, airway obstruction and developmental delay, which are typical findings in CS. They showed subtle facial and dermatologic features consistent with attenuated CS. They significantly differed in their musculoskeletal, cardiovascular and endocrinologic manifestations underscoring the clinical variability of individuals with identical, in particular rarer pathogenic HRAS variants. Functional studies revealed enhanced effector-binding, increased downstream signaling activation and impaired growth factor-induced signaling dynamics in cells expressing HRASPhe156Leu. Our data further illustrate the molecular and phenotypic variability of CS.
Similar content being viewed by others
Introduction
RASopathies are syndromic conditions that result from germline alterations in genes coding for RAS pathway signaling proteins. Missense variants in the proto-oncogene HRAS underlie the RASopathy Costello syndrome (CS). Individuals with CS are predisposed to cancer and may have distinctive craniofacial features, cardiac anomalies, growth and developmental delays, as well as dermatological, orthopedic, ocular, and neurological issues [1]. Approximately 80% of CS-causing HRAS gene variants result in a p.Gly12Ser missense change and this variant is associated with the classic CS phenotype [1]. A more variable, milder or “attenuated” phenotype occurs with rarer pathogenic HRAS variants, such as p.Thr58Ile, p.Gly60Asp/Val or p.Ala146Thr/Val/Pro [1, 2].
Disease-associated changes of HRAS amino acid 12 or 13 impair intrinsic GTPase activity, confer resistance to GAPs and, thereby, trap HRAS in its active state independently from incoming signals. This results in increased activation of HRAS downstream signaling pathways, a functional consequence that is widely used to explain the pathobiology of CS. However, several studies demonstrated a more diverse spectrum of molecular defects of CS-associated HRAS variants, which result in dysregulated HRAS-dependent signaling dynamics [3,4,5,6,7].
Here, we aimed to characterize the clinical manifestations and functional consequences associated with the pathogenic HRAS variant c.466 C > T p.(Phe156Leu) and to compare our data to those for previously described CS-related HRAS variants.
Materials and methods
The patients’ parents provided written informed consent for the participation in the study, clinical data and specimen collection and genetic analysis according to the Declaration of Helsinki and national legal regulations. They signed informed consent regarding publishing their data and photographs.
A description of the laboratory methods is given in the Supporting Information.
Results
Clinical summaries
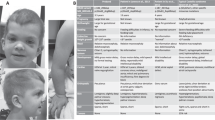
Patient 1. The pregnancy was complicated by maternal gestational diabetes, polyhydramnios, fetal overgrowth and caesarean section (37 weeks). Birth weight was above the 97th centile, length was on the 75th centile, and head circumference was greater than the 98th centile (Table 1). Limb shortening, varus posture of wrist joints, adducted thumbs, elbow contractures, rocker bottom feet, extended knees, hip dysplasia and macrocephaly were noted (Fig. 1A). At 5 weeks old the boy showed recurrent severe obstructive apneic episodes with cyanosis, loss of responsiveness, seizures and choking episodes during feeding. Pharyngeal and laryngeal obstruction required supraglottoplasty and recurrent apneic episodes and aspiration required permanent tracheostomy. Dysfunctional swallowing and feeding difficulties were noted. Weight gains improved with small frequent percutaneous endoscopic gastrostomy feeds. Details on tonic clonic seizures and treatment are given in the Supporting Results. Abdominal ultrasound detected no malignancy. The patient was following visually, smiling and breathing normally. Tracheostomy was removed at 9 months old. He had generalized developmental delay, was unable to roll or sit with marked head lag. Deep palmar and plantar creases were noted. At 2 2/3 years, weight and length were below the 3rd centile and head circumference was greater than the 95th centile (Table 1). Cardiac arrhythmia was documented, seizures persisted. Trio exome sequencing identified two likely pathogenic de novo variants, c.466 T > C p.(Phe156Leu) in HRAS (NM_005343.4) and c.4907 G > C p.(Arg1636Pro) in SCN1A (NM_001165963.4). The latter most probably underlies the seizures in this patient (OMIM *182389).
Patient 2. was recently mentioned [1]. The boy’s weigh and head circumference were on the 50th centile after a pregnancy complicated by polyhydramnios (Table 1). Bilious vomiting, hypoglycemia, macroglossia and poor feeding were noted on day 1 of life. Daily episodes of hypoglycemia associated with hyperinsulinism were treated with diazoxide and chlorothiazide. Severe hypoglycemia persisted with bolus feeds and continuous feeds were given via gastrostomy, with elemental formula due to cow’s milk protein intolerance. Laryngomalacia was diagnosed by endoscopy and echocardiography identified hypertrophy of the ventricular myocardium and mild thickening of the pulmonary valve. Metabolic/endocrinological investigations did not yield a diagnosis for the atypical hyperinsulinism. Glucose levels were unresponsive to octreotide, were more stable on diazoxide and improved with prednisone suggesting increased insulin sensitivity in addition to hyperinsulinism. Subtotal pancreatectomy was required at 8 months old. During a 5 months hospitalization hypertrophic cardiomyopathy and severe gastroesophageal reflux were diagnosed. The boy has subtle CS facial features with a prominent forehead, long philtrum and full cheeks (Fig. 1B). He has tight Achilles tendons and shows gait abnormalities. Dermatologic findings included thickened skin on elbows, excessive sweating, and sensitive skin. The patient has a mild ventriculomegaly and moderate global developmental delay. Unilateral strabismus (esotropia) of the right eye was noted. At 6 2/3 yrs old hypoglycaemia episodes were still significant with several low glucose episodes repeatedly per day caused by any form of excitement or stimulation. Trio exome sequencing identified the de novo HRAS c.466 C > T p.(Phe156Leu) variant.
Functional characterization
The strictly conserved HRAS amino acid Phe156 is not located within a distinct functional protein motif (Fig. 1C) [8]. However, it’s part of the α5-helix that is critical for overall HRAS structure [8]. Our functional studies support pathogenicity of the HRAS p.Phe156Leu variant: We used GST-fusion proteins of interacting motifs from RAS effectors RAF1, RALGDS, PIK3CA and PLCE1 and precipitated activated HA-tagged HRAS protein variants from cell extracts (Fig. 2A). Oncogenic HA-HRASGly12Val and CS-typical HA-HRASGly12Ser, but not the dominant-negative variant HA-HRASSer17Asn strongly co-precipitated with any effector under any culture condition tested (Fig. 2A). The amount of HA-HRASPhe156Leu was elevated compared to HA-HRASWT in all precipitates. These data suggest that HA-HRASPhe156Leu accumulates in the active form and forms stable complexes with effectors.
A HRASPhe156Leu co-precipitates with RAF1, RALGDS and PLCE1, PIK3CA. HRAS variants were expressed in HEK293T cells under serum-starved condition, normal growth condition, or serum-starved condition followed by 20 min EGF stimulation. HA-HRAS was precipitated from extracts by using GST-fused effector peptides and subjected to immunoblotting. B Expression of HRASPhe156Leu enhances downstream signaling. HRAS variants were expressed in HEK293T and MCF-7 cells. Cells were cultured under serum-starved, basal and stimulated conditions. Lysates were subjected to immunoblotting. C Expression of HRASPhe156Leu impairs epidermal growth factor sensitivity. HEK293T cells transiently expressing HRASWT, HRASGly12Val or HRASPhe156Leu were stimulated with EGF for various times (5, 15, 30 min) or left untreated (0 min) and phosphorylation levels were determined by immunoblotting (Fig. S3). Values are relative to maximum levels and represent the mean of three experiments; for untreated cells significance levels are specified between data points [∗, P < 0.05; (two-tailed t test)].
By analyzing binding between HA-HRASPhe156Leu and the RAS-specific NF1 GTPase activating protein (GAP), we detected moderately enhanced GAP binding efficiency compared to HA-HRASWT; however, this increase was less pronounced than observed for HA-HRASGly12Ser and HA-HRASGly12Val (Supporting Results, Figure S1). This suggests that p.Phe156Leu does not negatively interfere with NF1 GAP binding.
To gain insight into consequences of p.Phe156Leu on signal traffic, we measured levels of phosphorylated MEK1/2, ERK1/2 and AKT (Fig. 2B). Expression of HA-HRASGly12Val or HA-HRASGly12Ser but not HA-HRASSer17Asn promoted MEK1/2 and ERK1/2 phosphorylation in HEK293T cells under any cell culture condition. Likewise, HA-HRASPhe156Leu enhanced phosphorylation of MEK1/2 and ERK1/2 compared to HA-HRASWT (Fig. 2B). AKT phosphorylation was marginally increased in HEK293T cells expressing HA-HRASPhe156Leu, HA-HRASGly12Val or HA-HRASGly12Ser (Supporting Results, Fig. S2); however, HA-HRASPhe156Leu, HA-HRASGly12Val or HA-HRASGly12Ser induced stronger AKT phosphorylation than HA-HRASWT in MCF-7 cells (Fig. 2B). Our data suggest that the p.Phe156Leu change intensifies HRAS downstream signal flux.
Impaired signaling dynamics rather than a simple static hyperactivation of RAS-dependent signaling may underlie the development of CS [3, 4]. We compared the intensity of EGF-induced signaling over time. EGF addition induced a strong ERK1/2 phosphorylation response in HA-HRASWT cells after 5 min followed by a decrease after 15 to 30 min (Figs. S3 and 2C). In contrast, cells expressing HA-HRASPhe156Leu or HA-HRASGly12Val showed enhanced basal ERK1/2 phosphorylation (0 min EGF) and little further increase (Figs. S3 and 2C). Similarly, AKT phosphorylation was slightly stimulated upon EGF treatment in cells expressing HA-HRASWT but not or marginally in cells expressing HA-HRASGly12Val and HA-HRASPhe156Leu, respectively (Figs. S3 and 2C). These data suggest that the HRAS p.Phe156Leu variant impairs EGF-induced signal transduction efficiency within cells.
Discussion
Phenotype associated with HRAS c.466 T > C p.(Phe156Leu)
Our patients presented with prenatal polyhydramnios, severe feeding difficulties, musculoskeletal manifestations as well as gastroenterologic, endocrinologic and metabolic findings, which are common or frequent findings associated with both the typical CS-associated p.Gly12Ser variant and rarer HRAS variants (Table 1) [1,2,3, 7, 9,10,11,12,13,14,15,16]. Hyperinsulinism and hypoglycemia have been repeatedly reported in individuals with CS;[1, 5] however, in patient 2 these findings are uncommonly severe in terms of clinical presentation, management challenge and durability. Respiratory and otolaryngologic findings as well as cardiovascular abnormalities have also been described in patients with CS (Table 1) [1]. Notably, airway problems requiring tracheostomy has been reported as rare complication in patients with CS and different HRAS variants;[1, 14, 17] thus, this finding is not related to specific (rare or common) variants. On the other hand, both individuals showed milder facial features than patients with HRAS p.Gly12Ser; this is more in keeping with findings seen in individuals with rare HRAS variants affecting residues Thr58, Gly60, Lys117 or Ala146 (Table 1) [1,2,3, 5, 7, 9,10,11, 18]. The same is true for the dermatologic findings in both children (Table 1); however, since the skin phenotype varies with age, a final assessment should only be made in adulthood. Body length and height was in a normal range in patient 2, which has also been reported for individuals with HRAS variants p.Thr58Ile, p.Gly60Asp and p.Gly13Cys (Table 1) [5, 7, 10, 19]. Overall, the phenotypic presentation of the individuals described here may be best classified as less severe, with the exception of otolaryngology involvement, attenuated CS similar to that previously described for rare pathogenic HRAS variants [1,2,3, 5, 7, 9,10,11, 18].
Functional consequences of HRAS p.Phe156Leu
Our data demonstrate that p.Phe156Leu results in the accumulation of active HRAS and dysregulated HRAS-dependent signaling dynamics. Phe156 is located in the α5-helix within the hydrophobic core of HRAS and, hence, it has a crucial role in its structural stability [8]. Structural considerations suggested that substitution of HRAS Phe156 by leucine affects contacts with surrounding residues and changes intrinsic functions of HRAS [20]. Indeed, p.Phe156Leu induced major structural changes, weakened HRAS contacts with Mg2+ and guanine nucleotides and increased GDP/GTP dissociation rates and levels of GTP-bound HRAS [8]. This is in contrast to the typical CS-associated HRAS p.G12S variant that perturbs GTPase activity. The germline variant p.Phe156Leu has also been identified in KRAS of patients with RASopathy and functional consequences of KRASPhe156Leu were determined (Supporting Discussion). Summarizing available data, the main molecular defect of HRASPhe156Leu is a strong acceleration of nucleotide exchange, but interaction with effectors and regulators is still functional. We conclude that HRAS c.466 T > C p.(Phe156Leu) is pathogenic. The p.Phe156Leu variant reduces growth factor sensitivity of HRAS, which is detectable as decreased stimulus-dependent increment of downstream signaling pathway activation. This is a common finding for CS-associated HRAS variants and underscores that impaired signaling dynamics is the central pathomechanism for CS (and related RASopathies) [3, 4].
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files]. The variant and the associated phenotype have been documented in the ClinVar database (Accession: VCV001327492.1; Variation ID: 1327492; Submission ID: SUB10779819).
References
Gripp KW, Morse LA, Axelrad M, Chatfield KC, Chidekel A, Dobyns W, et al. Costello syndrome: Clinical phenotype, genotype, and management guidelines. Am J Med Genet A. 2019;179:1725–44.
Chiu AT, Leung GK, Chu YW, Gripp KW, Chung BH. A novel patient with an attenuated Costello syndrome phenotype due to an HRAS mutation affecting codon 146-Literature review and update. Am J Med Genet A. 2017;173:1109–14.
Gripp KW, Kolbe V, Brandenstein LI, Rosenberger G. Attenuated phenotype of Costello syndrome and early death in a patient with an HRAS mutation (c.179G>T; p.Gly60Val) affecting signalling dynamics. Clin Genet. 2017;92:332–7.
Lorenz S, Lissewski C, Simsek-Kiper PO, Alanay Y, Boduroglu K, Zenker M, et al. Functional analysis of a duplication (p.E63_D69dup) in the switch II region of HRAS: new aspects of the molecular pathogenesis underlying Costello syndrome. Hum Mol Genet. 2013;22:1643–53.
Gripp KW, Sol-Church K, Smpokou P, Graham GE, Stevenson DA, Hanson H, et al. An attenuated phenotype of Costello syndrome in three unrelated individuals with a HRAS c.179G>A (p.Gly60Asp) mutation correlates with uncommon functional consequences. Am J Med Genet A. 2015;167A:2085–97.
Gripp KW, Baker L, Robbins KM, Stabley DL, Bellus GA, Kolbe V, et al. The novel duplication HRAS c.186_206dup p.(Glu62_Arg68dup): clinical and functional aspects. Eur J Hum Genet. 2020;28:1548–54.
Gripp KW, Hopkins E, Serrano A, Leonard NJ, Stabley DL, Sol-Church K. Transmission of the rare HRAS mutation (c. 173C>T; p.T58I) further illustrates its attenuated phenotype. Am J Med Genet A. 2012;158A:1095–101.
Quilliam LA, Zhong S, Rabun KM, Carpenter JW, South TL, Der CJ, et al. Biological and structural characterization of a Ras transforming mutation at the phenylalanine-156 residue, which is conserved in all members of the Ras superfamily. Proc Natl Acad Sci USA. 1995;92:1272–6.
Zampino G, Pantaleoni F, Carta C, Cobellis G, Vasta I, Neri C, et al. Diversity, parental germline origin, and phenotypic spectrum of de novo HRAS missense changes in Costello syndrome. Hum Mutat. 2007;28:265–72.
Gripp KW, Innes AM, Axelrad ME, Gillan TL, Parboosingh JS, Davies C, et al. Costello syndrome associated with novel germline HRAS mutations: an attenuated phenotype? Am J Med Genet A. 2008;146A:683–90.
Hiippala A, Vasilescu C, Tallila J, Alastalo TP, Paetau A, Tyni T, et al. The rare Costello variant HRAS c.173C>T (p.T58I) with severe neonatal hypertrophic cardiomyopathy. Am J Med Genet A. 2016;170:1433–8.
Leoni C, Giorgio V, Onesimo R, Kuczynska E, Zampino G. Impact of Costello syndrome on growth patterns. Am J Med Genet A. 2020;182:2797–9.
Leoni C, Romeo DM, Pelliccioni M, Di Gia M, Onesimo R, Giorgio V, et al. Musculo-skeletal phenotype of Costello syndrome and cardio-facio-cutaneous syndrome: insights on the functional assessment status. Orphanet J Rare Dis. 2021;16:43.
Choi N, Ko JM, Shin SH, Kim EK, Kim HS, Song MK, et al. Phenotypic and genetic characteristics of five korean patients with Costello syndrome. Cytogenet Genome Res. 2019;158:184–91.
Vuralli D, Kosukcu C, Taskiran E, Simsek-Kiper PO, Utine GE, Boduroglu K, et al. Hyperinsulinemic hypoglycemia in a patient with costello syndrome: an etiology to consider in hypoglycemia. Mol Syndromol. 2020;11:207–16.
Carpentieri G, Leoni C, Pietraforte D, Cecchetti S, Iorio E, Belardo A, et al. Hyperactive HRAS dysregulates energetic metabolism in fibroblasts from patients with Costello syndrome via enhanced production of reactive oxidizing species. Hum Mol Genet. 2022;31:561–75.
Gomez-Ospina N, Kuo C, Ananth AL, Myers A, Brennan ML, Stevenson DA, et al. Respiratory system involvement in Costello syndrome. Am J Med Genet A. 2016;170:1849–57.
Denayer E, Parret A, Chmara M, Schubbert S, Vogels A, Devriendt K, et al. Mutation analysis in Costello syndrome: functional and structural characterization of the HRAS p.Lys117Arg mutation. Hum Mutat. 2008;29:232–9.
Gripp KW, Hopkins E, Sol-Church K, Stabley DL, Axelrad ME, Doyle D, et al. Phenotypic analysis of individuals with Costello syndrome due to HRAS p.G13C. Am J Med Genet A. 2011;155A:706–16.
Gremer L, Merbitz-Zahradnik T, Dvorsky R, Cirstea IC, Kratz CP, Zenker M, et al. Germline KRAS mutations cause aberrant biochemical and physical properties leading to developmental disorders. Hum Mutat. 2010;32:33–43.
Funding
This study was supported by the German Federal Ministry of Education and Research (BMBF) (funding code 01GM1902E to GR). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
GR designed and supervised the study. SLT and ME collected and curated the clinical data. VR and TN performed and evaluated functional assays. GR prepared all tables and figures. SLT, ME and GR wrote the original draft of the manuscript. GR revised and edited the original draft of the manuscript. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Medical Chamber Hamburg (vote no. PV7038).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lindsey-Temple, S., Edwards, M., Rickassel, V. et al. A novel HRAS c.466C>T p.(Phe156Leu) variant in two patients with attenuated features of Costello syndrome. Eur J Hum Genet 30, 1088–1093 (2022). https://doi.org/10.1038/s41431-022-01139-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-022-01139-1
This article is cited by
-
Guidelines, guidelines everywhere—and still I’m not sure what to do
European Journal of Human Genetics (2022)