Abstract
While prior studies have largely focused on family communication of diagnostic single-gene test results or specific types of cancer testing results, far less work has investigated family communication of cancer-related genetic results that include multi-gene panels, a broad array of cancer types/stages, and participants without family history of cancer. The study we report here examined individuals’ anticipated barriers and benefits to sharing genetic information with family members. An 80+ gene panel was performed on participants recruited from Mayo Clinic, diagnosed with different cancer types, who did not have a family history suggestive of an inherited risk. Participants completed a 49-item survey before receiving genetic test results. Family variant testing was provided to family members at no cost, allowing factors influencing intent to share to be examined in the absence of financial burdens. In all, 1721 of 2984 individuals who received genetic testing completed the survey (57.7% completion rate). Participants’ intent to share with parents, siblings, and children was inversely related to the number of anticipated barriers to sharing and directly related to the number of anticipated benefits to sharing. Of those participants who did not intend to share with parents, siblings, and adult children, 64.8%, 30.3%, and 67.6% reported that there were no barriers, while 17.1%, 24.5%, and 40.2.% reported there were no benefits. Findings indicate that barriers to sharing genetic information with family members vary across family member types, and an inability to identify at least one benefit of sharing with family members is a predictor of intent not to share.
Similar content being viewed by others
Introduction
Genetic testing results are relevant not only to individuals, but also their family members. In the cancer setting, where preventative screening and interventions are often available, sharing the results of a genetic test indicating a hereditary cancer mutation has the potential to change outcomes for at-risk family members. For example, a family member informed of a genetic risk for colon cancer may seek genetic testing, and if found to share a genetic risk factor, might undergo more frequent colonoscopies at a younger age than recommended for the general population [1].
Many studies have evaluated reasons individuals choose to share or not share genetic information with family members [2, 3]. Reasons individuals share with family members include the desire to alert family members of unknown risks, give family members the opportunity to make informed health decisions, and receive social and emotional support from family members. Reasons for not sharing include a lack of contact and/or emotional closeness to family members, the perception that genetic risk information is not of high relevance or significance to family members, and a desire to protect family members from potentially distressing information [4].
While many individuals share genetic risk information with at least one at-risk first-degree relative (FDR), the percentage of individuals who share genetic information with all of their at-risk living FDR is much smaller [5]. Little work has been done on family communication of cancer-related genetic test results that include multi-gene panels, a broad array of cancer types/stages and participants without a significant family history of cancer. Previous studies have focused largely on sharing (1) family communication of diagnostic single-gene test results or (2) family communication of very specific types of cancer testing results. For example, multiple studies have examined familial communication related to breast and ovarian-related genetic test results [6,7,8]. Additionally, while other studies have examined patient-reported barriers to sharing genetic information [8,9,10,11], very few studies have examined perceived or anticipated barriers and benefits of sharing [12].
This study aimed to evaluate individuals’ anticipated barriers and benefits to sharing genetic information with family members and to assess the interplay of these anticipated benefits and barriers on the intent to share with family members. In order to offer the best support to patients, it is critical for providers to understand factors that influence patients to share (or not share) genetic results with family members. The types of data we report here are relevant to oncologists who are ordering genetic testing on patients, as well as primary physicians and other providers who have patients who have received genetic test results and have family members who could benefit from being informed of such genetic risk information. This study examined individuals’ intent to share genetic risk information with different types of at-risk family members across different types of cancer, and the data reported here were collected prior to participants knowing their genetic test result.
Methods
This study was approved by the Mayo Clinic IRB (#18-000326). All individuals in this study agreed to participate and provided informed consent.
Individuals watched a video explaining hereditary cancer risk and the pros and cons of genetic testing prior to providing consent. An explanation of familial cancer risk and the potential medical implications of identifying a pathogenic variant were explained in the video. The importance of sharing genetic test results with at-risk family members were not discussed in the video, nor did the video discuss potential disruptions to family relationships as a potential harm that might be associated with genetic testing.
Participants
Participants in this study enrolled in the Interrogating Cancer Etiology using Proactive (Genetic) Testing (INTERCEPT) study. Participants were at least 18 years of age with an active diagnosis of cancer in one or more of the following sites: bladder, breast, cholangiocarcinoma, CNS/brain, colorectal, endometrial, esophageal, gastric, head/neck, hepatocellular, lunch, melanoma, ovarian, pancreas, prostate, renal, sarcoma, or small bowel. Patients were not selected based on cancer stage or family history of cancer. Patients who had prior germline cancer genetic testing within the last 24 months were excluded.
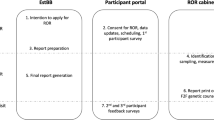
Participants were recruited from clinics in Medical Oncology, Radiation Oncology, Gynecology, Surgery, and Genetics across the Mayo Clinic Health system, including clinics in Phoenix, AZ; Jacksonville, FL; Rochester, MN; and Eau Claire, WI. Eligible patients were identified from clinical scheduling calendars and physician referrals. Study enrollment occurred from April 2018 through March 2020. More details regarding the study design can be found in Samadder et al. [13].
Study procedures
Cancer genetic testing was performed using a Clinical Laboratory Improvement Amendments certified, commercially available, 80+ gene panel available through Invitae. All patients found to have a pathogenic variant and were offered an in-person genetic counseling appointment. In cases where participants were not available to receive their genetic test results in person, results were reported by phone or postal mail. Participants’ oncologists were notified about genetic test results in parallel, regardless of whether the result indicated a pathogenic variant. Patients with no pathogenic variants or variants of unknown significance received their test results via a portal message or phone call.
The at-risk relatives of participants found to have a pathogenic variant were offered site specific genetic testing at no cost for 90 days following the date of result. Participants were notified of this option for their at-risk relatives during their consultation with their oncologist or with a genetic counselor when they received results indicating a pathogenic variant. It should be noted that some participants could not be reached and their results were returned via the patient portal. If participants did not review their results in the portal, these participants may not have received the information on how to obtain testing at no cost for family members.
Survey
Participants were asked to complete a brief survey following their decision to have genetic testing (and in all cases, before receiving their genetic test results). This survey included several items and measures from previous studies, including a cancer communication measure [14] and a familial support scale [15]. The 49-item survey examined participants’ intent to share genetic test results with different family members, anticipated barriers to sharing their results, family communication patterns, anticipated reactions of family members to receiving health information, and perceived usefulness of genetic information to family members. Demographic variables were collected during enrollment and from the electronic health record.
Participant intent to share with family members was assessed by a survey question that read “I am planning to share my results with…” followed by a list of family member types that included the following options: my spouse or partner, my father, my mother, at least one of my brothers, at least one of my sisters, at least one of my adult sons, at least one of my adult daughters. Each option listed the following response choices: “Yes”, “No”, “Unsure”, and “Not Applicable”.
Data collection
For the first seven months of data collection, a study coordinator called participants ~1 week after they consented to receive genetic testing and collected survey responses by phone. Data were entered into a REDCap database. In order to make completing the survey more convenient for participants, as well as decrease the study staff time required to complete the survey, in the eighth month of data collection, we began distributing the survey to participants via a web-based REDCap survey that was sent to participants by e-mail ~1 week following consent to the study. No repeat e-mail invitations were sent to survey nonresponders, but a study coordinator made phone calls to those individuals who did not respond to the survey link and attempted to collect their survey responses at that time.
Data analysis
Data were analyzed using JMP Pro 14 (2018 SAS Institute Inc.). We examined the proportions of patients who indicated an intent to share genetic test results with an at-risk family member. Participants who indicated that they did not have a living family member in a particular category (e.g., no living parent) are not included in the percentages we report.
Results
Of the 3004 participants who met eligibility criteria and were invited to participate in the INTERCEPT study, 2984 responded to the study invitation, consented to participate, and elected to pursue genetic testing. Among consenting participants, 1721 completed the survey (57.7% completion rate). Table 1 summarizes demographic characteristics of individuals who participated in INTERCEPT study and who completed the survey before receiving a genomic test result. Our survey sample was comprised largely of white (93.5%) and older (mean age = 61.8) individuals, with more men (52.5%) represented than women (47.5%). A survey non-completer analysis was performed and revealed that individuals who did not complete the survey were significantly more likely to be Hispanic or Latino (p < 0.0001).
The data we report here were collected before participants received their genetic test results.
Table 2 summarizes participant responses to survey items measuring anticipated barriers and benefits to sharing. The most commonly cited anticipated barrier to sharing among participants was that family members would struggle to understand their genetic results (indicated by 11.6% of participants), while the least commonly cited anticipated barrier to sharing among participants was that family members would be upset when they heard about their results (indicated by 4.4% of participants). The most commonly cited anticipated benefit among participants was that family members would be glad they shared their results with them (indicated by 83.4% of participants), while the least commonly cited anticipated benefit among participants was that sharing genetic results would bring their family closer together (indicated by 43.6% of participants).
Table 3 summarizes the number of participants who reported intent to share with parents, siblings, and children and the number of barriers and benefits reported by those participants. Participants’ intent to share with parents, siblings, and children was inversely related to the number of anticipated barriers to sharing reported by participants, and directly related to the number of anticipated benefits to sharing reported by participants. Thus, participants who anticipated fewer barriers were more likely to share with parents, siblings, and children, and participants who anticipated more benefits were more likely to share with parents, siblings, and children.
The frequency of anticipated barriers and benefits of sharing with family members and intent to share with family members is shown in Table 4. Of the 216 participants who did not intend to share with a parent, 37 (17.1%) reported no anticipated benefits of sharing. Overall, 24.5 and 40.2% of participants who did not intend to share with a sibling or child also reported no anticipated benefits of sharing, respectively. The proportion of participants who intended to share but reported zero anticipated barriers was much lower. In all, 3.5% of participants who reported zero anticipated benefits intended to share with their parents, 5.1% with siblings, and 4.7% with children. Of those participants who did not intend to share with parents, siblings, and adult children, 64.8%, 30.3%, and 67.6%, respectively, reported that there were no barriers, while 17.1%, 24.5%, and 40.2%, respectively reported there were no benefits to sharing.
Discussion
This study examined anticipated barriers and benefits of sharing of cancer-related genetic test results with family members, and how the interplay of those anticipated barriers and benefits impact intent to share with family members. Our data were collected prior to individuals receiving their genetic test results, and suggest three notable findings. First, barriers to sharing genetic information with family members vary across types of family members. Second, individual’s inability to identify at least one benefit of sharing genetic information with family members was a strong predictor of the lack of sharing with family members. Third, intent to share with family members was largely the same across cancer types. These findings have several implications moving forward, which apply to both research and clinical practice.
Our data indicate that anticipated barriers to sharing genetic information vary across family members. Notably, much of the existing literature has assumed that barriers to sharing genetic information are the same across family member types (i.e., many studies have focused on the number of relatives that individuals share genetic test results with, rather than the specific type of biological relationship between the individual and family member(s)) [8, 16]. Although studies examining types of biological relationships do exist, these studies primarily focus on sharing behaviors in closer versus distant relatives and general sharing behaviors across family members [17, 18], rather than the anticipated benefits and barriers that influence the sharing of genetic information with specific family member types. Going forward, when designing research or clinical interventions that specifically focus on decreasing barriers to family sharing of genetic information, it may be beneficial to the consider which barriers are especially relevant to sharing results with particular types of family members.
While a majority of individuals reported at least one barrier to sharing genetic information with family members, a comparatively smaller proportion of participants did not recognize “any” benefits to sharing. However, when an individual did not recognize at least one benefit to sharing with family members, this was a strong predictor of individuals intending not to share genetic information with family members. In other words, a lack of appreciation for the potential benefits of sharing genetic information with family members is a significant predictor of lower intent to share information.
It is concerning that even though family sharing was a large component of our study, 10% of our participants did not see benefits to sharing their results with family members. This indicates that, at least in the context of pursing genetic testing in the cancer setting, more emphasis on the value of sharing results with family members is needed.
Prior work has found that individuals do not always realize that their genetic or genomic test results are relevant to family members, or the importance of sharing results with family members [16, 19]. Findings by Tan et al. [20] revealed that cancer patients have poor understanding of the screening guidelines for relatives after their own cancer diagnoses, and efforts to promote sharing of genetic risk information to family members and preventative screening of family members are lacking among medical professionals.
Thus, more emphasis on the value of sharing genetic risk information is needed for both providers and individuals. Because providers who are the ones to recommend, explain, order, and return results of genetic tests in the oncology setting, they have the potential to significantly increase patient understanding of the value and relevance of results to family members, if they clearly understand this themselves. While genetic counselors are well-aware of the importance and value of genetic risk information to family members, such understanding is not always as clear among other providers [21]. Therefore, providing additional training to oncologists about the value of sharing genetic risk information with family members in the form of short videos, online training modules, or other means of education may be beneficial. Findings indicate there is currently a great need for substantive genomic education for cancer physicians specifically, and this can happen on two levels. First, medical schools will need to keep updating their curricula to ensure they are abreast to the rapid developments in medical genetics and genomics, and second, continuing education opportunities for working oncologists must be provided. Options for continuing education might include workshops at conferences as well as options for self-paced, independent learning at home [21].
Provider emphasis on the value and importance of sharing genetic information with family members can occur during both the consent and return of results processes. An initial explanation during the consent process followed by a reiteration of the importance during the return of the results process may be beneficial. Receiving information pretest gives individuals time to start thinking about who to share results with, how to share, and also provides a chance for providers to address follow-up questions and clarify understanding during posttest discussions [22]. Individuals may also benefit from videos, online modules, and other mechanisms of education that further emphasize and explain the values of sharing information with their family members.
A final key point is that our data report the intent of participants with many different types of cancer to share with family members. This is notable because previous studies have examined very specific types of cancer testing results, and have also largely focused on participants with known family hereditary cancer mutations. For example, there have been many studies that focus on family sharing after receiving genetic test results indicating a pathogenic BRCA 1/2 variant [23, 24], or a pathogenic DNA mismatch repair variant linked to Lynch Syndrome [25, 26]. These data and data from future research on family sharing across different types of cancer are important for research or clinical interventions designed to increase family sharing of genetic risk information in the cancer setting going forward. Future research is needed in a variety of settings, population types, variant types, and cancer types to further investigate and validate these findings.
Limitations
While all providers were initially given the same information about this study, and all individuals watched the same video before being consented, the dialogues between providers and potential participants about this study are uncertain. Specifically, it is uncertain whether, and the degree to which, providers emphasized the benefits of sharing genetic information with family members. It is possible that providers saw the benefits of this study as providing information that had the potential to influence clinical management of their patient, the opportunity for their patients to receive genetic testing at no cost, or to contribute to research that may advance the field. Thus, participants may have received widely variable information about this study and its potential benefits.
Another limitation of this study was that due to the survey nature of data collection, it is possible that a barrier or benefit to sharing genetic information was anticipated by an individual, but not listed as a response on our survey. Additionally, if individuals anticipated a barrier to sharing genetic information with one member of the family, they may ultimately choose not to share with any family members due to the fluid nature of families, and the possibility that one family member would tell others. This was not listed as a response option on our survey.
The barriers and benefits we report here were not linked to a specific family member, so it is possible that reported barriers and benefits were limited to one or more family members, but not others. It is also possible that individual clinicians may have discussed these and other familial implications of cancer-related genetic testing with participants or counseled participants on the potential value of genetic testing to family members.
Finally, while this study examined individuals anticipated barriers and benefits of sharing of genetic risk information with family members, and how the interplay of those anticipated barriers and benefits impact intent to share with family members, it was not possible for us to tease out the barriers and benefits of sharing genetic risk information separately from the barriers and benefits of sharing a cancer diagnosis, since individuals may not think about these topics independently. Additionally, our sample included several individuals who were recently diagnosed with cancer and who may or may not have shared their cancer diagnosis with family members. These experiences could influence their thoughts about the pros and cons of sharing genetic risk information.
Conclusion
This study examined individuals’ anticipated barriers and benefits of sharing of genetic risk information with family members, and the interplay of those anticipated barriers and benefits on intent to share with family members. Our findings indicate that barriers to sharing genetic information with family members vary across family member types, and individual’s inability to identify at least one benefit of sharing genetic information with family members predicts the lack of intent to share with family members.
Going forward, future research should evaluate similarities and differences in anticipated barriers to sharing genetic information across different family member and cancer types, as well as consider these factors when designing interventions targeted to increase family sharing of genetic risk information. The potential for cancer genetic testing to facilitate genetic testing of other family members who may be at risk requires that patients who test positive share their test results with others. Understanding why family sharing does not always happen, and addressing potential barriers that may be preventing individuals from sharing their cancer genetic test results with family members, is critical to the goals of preventive cancer care.
Future research and interventions should also focus on providing both providers and individuals with more education on the potential benefits of sharing genetic information with family members, in hopes to promote more family sharing of genetic information.
References
Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Gastroenterology. 2014;147:502–26.
Li ST, Sun S, Lie D, Met‐Domestici M, Courtney E, Menon S, et al. Factors influencing the decision to share cancer genetic results among family members: an in‐depth interview study of women in an Asian setting. Psychooncology. 2018;27:998–1004.
Nycum G, Avard D, Knoppers BM. Factors influencing intrafamilial communication of hereditary breast and ovarian cancer genetic information. Eur J Hum Genet. 2009;17:872–80.
McCann S, MacAuley D, Barnett Y, Bunting B, Bradley A, Jeffers L, et al. Family communication, genetic testing and colonoscopy screening in hereditary non‐polyposis colon cancer: a qualitative study. Psychooncology. 2009;18:1208–15.
Stuttgen K, Bollinger J, McCague A, Dvoskin R, Mathews D. Family communication patterns and challenges of huntington’s disease risk, the decision to pursue presymptomatic testing, and test results. J Huntingtons Dis. 2020;9:265–74.
Cheung EL, Olson AD, Tina MY, Han PZ, Beattie MS. Communication of BRCA results and family testing in 1,103 high-risk women. Cancer Epidemiol Biomarkers Prev. 2010;19:2211–9.
Conley CC, Ketcher D, Reblin M, Kasting ML, Cragun D, Kim J, et al. The big reveal: family disclosure patterns of BRCA genetic test results among young Black women with invasive breast cancer. J Genet Couns. 2020;29:410–22.
Healey E, Taylor N, Greening S, Wakefield CE, Warwick L, Williams R, et al. Quantifying family dissemination and identifying barriers to communication of risk information in Australian BRCA families. Genet Med. 2017;19:1323–31.
MacDonald DJ, Sarna L, van Servellen G, Bastani R, Giger JN, Weitzel JN. Selection of family members for communication of cancer risk and barriers to this communication before and after genetic cancer risk assessment. Genet Med. 2007;9:275–82.
Forrest K, Simpson SA, Wilson BJ, Van Teijlingen ER, McKee L, Haites N, et al. To tell or not to tell: barriers and facilitators in family communication about genetic risk. Clin Genet. 2003;64:317–26.
Foster C, Evans DG, Eeles R, Eccles D, Ashley S, Brooks L, et al. Non-uptake of predictive genetic testing for BRCA1/2 among relatives of known carriers: attributes, cancer worry, and barriers to testing in a multicenter clinical cohort. Genet Test. 2004;8:23–9.
Kinney AY, Gammon A, Coxworth J, Simonsen SE, Arce-Laretta M. Exploring attitudes, beliefs, and communication preferences of Latino community members regarding BRCA1/2 mutation testing and preventive strategies. Genet Med. 2010;12:105–15.
Samadder NJ, Riegert-Johnson D, Boardman L, Rhodes D, Wick M, Okuno S, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7:230–7.
Mesters I, Van Den Borne H, McCormick L, Pruyn J, De Boer M, Imbos T. Openness to discuss cancer in the nuclear family: scale, development, and validation. Psychosom Med. 1997;59:269–79.
Procidano ME, Heller K. Measures of perceived social support from friends and from family: three validation studies. Am J Community Psychol. 1983;11:1–24.
Gaff CL, Clarke AJ, Atkinson P, Sivell S, Elwyn G, Iredale R, et al. Process and outcome in communication of genetic information within families: a systematic review. Eur J Hum Genet. 2007;15:999–1011.
Claes E, Evers‐Kiebooms G, Boogaerts A, Decruyenaere M, Denayer L, Legius E. Communication with close and distant relatives in the context of genetic testing for hereditary breast and ovarian cancer in cancer patients. Am J Med Genet A. 2003;116:11–9.
Stoffel EM, Ford B, Mercado RC, Punglia D, Kohlmann W, Conrad P, et al. Sharing genetic test results in Lynch syndrome: communication with close and distant relatives. Clin Gastroenterol Hepatol. 2008;6:333–8.
Rowland E, Metcalfe A. Communicating inherited genetic risk between parent and child: a meta-thematic synthesis. Int J Nurs Stud. 2013;50:870–80.
Tan KK, Lim TZ, Chan DK, Chew E, Chow WM, Luo N, et al. Getting the first degree relatives to screen for colorectal cancer is harder than it seems—patients’ and their first degree relatives’ perspectives. Int J Colorectal Dis. 2017;32:1065–8.
Chow-White P, Ha D, Laskin J. Knowledge, attitudes, and values among physicians working with clinical genomics: a survey of medical oncologists. Hum Resour Health. 2017;15:42.
Starkings R, Shilling V, Jenkins V, Fallowfield L. A systematic review of communication interventions to help healthcare professionals discuss genetic testing for breast cancer. Breast Cancer Res Treat. 2020;183:9–21.
Costalas JW, Itzen M, Malick J, Babb JS, Bove B, Godwin AK, et al. Communication of BRCA1 and BRCA2 results to at‐risk relatives: a cancer risk assessment program’s experience. Am J Med Genet C. 2003;119:11–8.
Hallowell N, Ardern‐Jones A, Eeles R, Foster C, Lucassen A, Moynihan C, et al. Communication about genetic testing in families of male BRCA1/2 carriers and non‐carriers: patterns, priorities and problems. Clin Genet. 2005;67:492–502.
Aktan-Collan KI, Kääriäinen HA, Kolttola EM, Pylvänäinen K, Järvinen HJ, Haukkala AH, et al. Sharing genetic risk with next generation: mutation-positive parents’ communication with their offspring in Lynch syndrome. Fam Cancer. 2011;10:43–50.
Leenen CH, Den Heijer M, van der Meer C, Kuipers EJ, van Leerdam ME, Wagner A. Genetic testing for Lynch syndrome: family communication and motivation. Fam Cancer. 2016;15:63–73.
Funding
The study was funded by a Mayo Clinic Transform the Practice Grant, the Mayo Clinic Center for Individualized Medicine, Desert Mountain CARE Foundation, and the David and Twila Woods Foundation. These funding sources did not play a role in the design, conduct, or reporting of the study or in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Finn, K.S., Pacyna, J., Azevedo Tsou, C. et al. Patient-reported anticipated barriers and benefits to sharing cancer genetic risk information with family members. Eur J Hum Genet 30, 53–61 (2022). https://doi.org/10.1038/s41431-021-00890-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-021-00890-1
This article is cited by
-
Patients’ perceptions and practices of informing relatives: a qualitative study within a randomised trial on healthcare-assisted risk disclosure
European Journal of Human Genetics (2024)
-
New year, new issue
European Journal of Human Genetics (2022)
-
Communication processes about predictive genetic testing within high-risk breast cancer families: a two-phase study design
Scientific Reports (2021)