Abstract
Whole genome sequencing (WGS) allows the identification of human knockouts (HKOs), individuals in whom loss of function (LoF) variants disrupt both alleles of a given gene. HKOs are a valuable model for understanding the consequences of genes function loss. Naturally occurring biallelic LoF variants tend to be significantly enriched in “genetic isolates,” making these populations specifically suited for HKO studies. In this work, a meticulous WGS data analysis combined with an in-depth phenotypic assessment of 947 individuals from three Italian genetic isolates led to the identification of ten biallelic LoF variants in ten OMIM genes associated with known autosomal recessive diseases. Notably, only a minority of the identified HKOs (C7, F12, and GPR68 genes) displayed the expected phenotype. For most of the genes, instead, (ACADSB, FANCL, GRK1, LGI4, MPO, PGAM2, and RP1L1), the carriers showed none or few of the signs and symptoms typically associated with the related diseases. Of particular interest is a case presenting with a FANCL biallelic LoF variant and a positive diepoxybutane test but lacking a full Fanconi anemia phenotypic spectrum. Identifying KO subjects displaying expected phenotypes suggests that the lack of correct genetic diagnoses may lead to inappropriate and delayed treatment. In contrast, the presence of HKOs with phenotypes deviating from the expected patterns underlines how LoF variants may be responsible for broader phenotypic spectra. Overall, these results highlight the importance of in-depth phenotypical characterization to understand the role of LoF variants and the advantage of studying these variants in genetic isolates.
Similar content being viewed by others
Introduction
One of the best ways to investigate the function of a gene consists in studying the phenotypic consequences of a gene knockout event [1], in particular in animal models such as mice and rats, which share with humans approximately 80% of their genome. With the availability of high-throughput sequencing technologies, it has become feasible to sequence the entire genome of thousands of individuals opening new perspectives in the study of the effect of genes knockout events directly in humans [2]. Large-scale genome sequencing allowed the identification of loss of function (LoF) variants that include splice acceptor, splice donor, stop gained, stop lost, start lost, frameshift, transcript ablation, and transcript amplification variants. Individuals who carry biallelic LoF variants may be defined as human knockouts (HKOs) [3]. These LoF events may occur in genes already known to be implicated in severe genetic diseases or involve novel genes; such variants may be related to an extensive range of phenotypes, from disease-causing variants to variants responsible for the common inter-individual variability and even to variants that are beneficial to the carrier [4]. Recent studies have highlighted how each healthy subject may carry up to 100 LoF variants in his/her genome, most of them heterozygous, and thus presenting with 20 completely inactivated genes, some associated with Mendelian disorders and some other in “non-essential” genes. The absence of clinical signs could be explained by the role of modifier genes or by the possible presence in these individuals of other protective genetic pathways [5, 6].
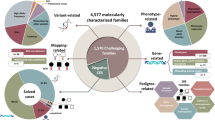
An efficient and cutting-edge approach to study HKOs consists in detecting these subjects in genetic isolates, i.e., populations characterized by few founders, small population size, high rate of inbreeding, and low rate of gene flow [7, 8]. These population characteristics lead to decreased genetic variability and can determine an enrichment in homozygous LoF variants, specifically in genes associated with recessive Mendelian genetic disorders [9]. The Italian Network of Genetic Isolates (INGI) includes several Italian isolated populations, characterized by a high endogamy rate and a particular genetic background, as previously demonstrated [10, 11]. Here, we investigated three INGI cohorts: Carlantino (CAR), a small village located in the Puglia region, Val Borbera (VBI), a valley in the Northwest of Italy, and the Friuli-Venezia Giulia (FVG) Genetic Park, which includes six villages in Northeastern Italy. A recent whole genome sequencing (WGS) study [12] provided an extensive characterization of these three isolated populations, describing their genetic features focusing on homozygous LoF variants. Here we describe the results obtained combining WGS data [12] and deep clinical phenotypes in Italian isolates with the main aim of increasing the knowledge on the role of the identified LoF variants in HKOs (Fig. 1).
The scheme represents the overall workflow of the study. WGS and clinical data from Italian isolated populations have been combined to select putative HKOs. In case of positive genotype–phenotype correlation, preventive strategies could be applied, whereas whenever the correlation results negative, functional studies are needed to better clarify the role of the identified variant.
Materials and methods
Ethical statement
All the experiments have been performed following relevant guidelines and regulations. The study was reviewed and approved by the Ethics Committee of the Institute for Maternal and Child Health – I.R.C.C.S. “Burlo Garofolo” of Trieste (Italy) (2007 242/07). The protocol conformed to the tenets of the Declaration of Helsinki.
Whole genome sequencing data generation
WGS data have been generated and analyzed, as previously reported by Cocca et al. [12]. Briefly, 947 DNA samples were randomly selected from the three cohorts, and WGS at 6–10× coverage was performed. Specifically, 381 individuals were selected from the Friuli-Venezia Giulia cohort, 433 from the Val Borbera cohort, and 133 from the Carlantino one. After extensive quality control, 926 samples were retained, and the generated data were aligned to the GRCh37/hg19 reference sequence. The aligned data were processed using GATK best practice pipelines [13] in order to generate germinal variant calls for both SNPs and INDELs. Functional annotation was performed using the Variant Effect Predictor tool [14]. Variants annotated as protein-truncating were selected as LoF. Specifically, the following categories were considered: frameshift, splice acceptor, splice donor, stop gained, stop lost, start lost, transcript ablation, and transcript amplification variants [3].
The genetic data described in this manuscript have been submitted to the European Variation Archive and are accessible in Variant Call Format at the following link: https://www.ebi.ac.uk/ena/data/view/PRJEB33648.
Human knockouts: functional selection and bioinformatic filters
The starting point of our work was a list of 506 LoF variants with a Combined Annotation Dependent Depletion score greater or equal to 20 [15] and for which at least one homozygous carrier was detected in our dataset, as described by Cocca et al. [12]. We first selected only variants in genes already known to be associated with Mendelian disorders, as reported in the Online Mendelian Inheritance in Man® (OMIM; https://www.omim.org/) free-access catalog of human genes and genetic disorders (Supplementary Table 1). In order to identify all the subjects with low-frequency biallelic LoF variants, a total allele frequency upper limit of 1% according to gnomAD (https://gnomad.broadinstitute.org/; date of the last update: May 24, 2020) was applied [16]. Furthermore, only variants affecting genes causative of autosomal recessive disorders were retained, resulting in 13 LoF variants in 13 distinct genes. Finally, for each selected variant, we confirmed whether it was a “total” or “partial” LoF, based on the number of the gene transcripts involved (https://www.ensembl.org/index.html). Specifically, each LoF variant has been classified as “total” if it falls on all coding transcript of a gene or as “partial” if it falls only on some coding transcripts. The ratio between the number of coding transcripts for which the variant is a LoF and the total number of coding transcripts of every gene is reported in Table 1.
Variants confirmation
All selected variants underwent Sanger sequencing confirmation. In order to amplify the DNA fragments, a touchdown polymerase chain reaction (PCR) was performed; the success of the PCR reaction was confirmed with electrophoresis and subsequent band visualization through a LED illuminator (FastGene® FAS V; Gel Documentation System). The amplified PCR products were then purified and labeled with BigDye® Terminators (ddNTPs) according to the manufacturer’s protocol. After a second purification step, the DNA fragments were sequenced (Applied Biosystem™ 3500 DX Genetic Analyzer; Thermo Fisher). The filtering and variants confirmation process is summarized in Fig. 2.
The scheme highlights the variants selection and filtering workflow. WGS data were obtained for 947 samples and processed applying GATK best practice pipelines to generate variant calls for both SNPs and INDELs. After functional annotation with the VEP tool, 506 LoF variants with at least one homozygous carrier and CADD score ≥20 were selected. Further filtering overlapping those variants with OMIM disease-associated genes reduced our target list to 62 variants. A minor allele frequency upper limit of 1% according to data from the gnomAD database (gnomad_AF field) was applied, to retain only low-frequency variants. A final filtering step to select variants in genes causative of autosomal recessive disorders (AR) was performed. The resulting variants underwent confirmation through Sanger Sequencing.
Clinical evaluation and follow-up
The initial clinical evaluation of all the subjects involved in the study comprised the assessment of hundreds of functional parameters, including (1) clinical biochemistry data (over 60 parameters inclusive of a complete blood count with differential, electrolytes, liver enzymes, serum protein, bilirubin, creatinine, insulin and lipase, cholesterol and triglycerides), (2) metabolomics data (obtained through 500 mHz nuclear magnetic resonance spectroscopy serum analysis), (3) bone densitometry, (4) an in-depth sensory evaluation that focused on the analysis of senses (hearing, taste, smell and vision), (5) a cardiovascular, a neurological and an orthodontic evaluation, and (6) a detailed personal and familial history with more than 200 questions asked to each subject. All parameters were systematically collected by professional and trained staff according to a standardized format. Since the parameters collected during the initial sampling were standard for all subjects, in some cases, a clinical follow-up was required in order to gather more details specific for the expected clinical phenotype.
Results
Homozygous LoF variants selection and validation
Considering the starting list of 506 LoF variants, only those in genes already known to be associated with autosomal recessive Mendelian disorders were selected (Supplementary Table 1). These variants were filtered as detailed in Materials and methods, obtaining 13 variants in 13 genes. Finally, 10 out of 13 variants were confirmed by Sanger sequencing, and their role was further investigated, looking at the corresponding phenotypes (Table 1). Of note, according to gnomAD, all the selected genes show evidence of LoF tolerance (pLI = 0). However, among them, three (FANCL, PGAM2, and RP1L1) should be more prone to accumulate LoF variants, with an observed vs. expected LoF ratio >1.1. The other seven genes (C7, F12, ACADSB, GRK1, LGI4, MPO, and GPR68) are supposedly less prone to accumulate LoF variants, with an observed vs. expected LoF ratio <1.
Phenotypical characterization of the carriers of the selected loss of function variants
The phenotypes of the subjects carrying homozygous LoF variants have been deeply investigated and compared to the expected ones (Table 2). A brief description of the diseases associated with LoF variants in the selected genes and the relevant clinical findings is reported below.
HKOs subjects presenting with the expected phenotype
C7 gene
Biallelic LoF variants in this gene have been associated with C7 deficiency, a rare immunological defect characterized by increased susceptibility to systemic infections, mainly caused by encapsulated bacteria [17]. Individual_1, carrying the known NM_000587.2:c.2350+2T>C splicing variant [18], presented with the typical clinical features of C7 deficiency. Specifically, the patient suffered from a meningococcal meningitis episode and reported a long history of gastritis related to Helicobacter pylori infection, pericarditis, pneumonia, bronchopneumonia, and a peculiar soft tissue infection of the tip of the nose. Despite the presence of clear signs and symptoms, the disease was never diagnosed, and a genetic test was never requested by the physicians who took care of this patient.
F12 gene
Biallelic LoF variants in this gene may cause Factor XII deficiency, which is usually not associated with any clinical symptom, but causes prolonged whole-blood clotting time [19]. All the LoF variants in this gene described in literature are associated with Factor XII deficiency, except for a small insertion and a gross deletion, causative of hereditary angioedema [20]. Here, the NM_000505.3:c.1681-1G>A splicing variant was detected in Individual_2 at the homozygous state. Blood coagulation tests were not performed during the initial evaluation and no other peculiarities emerged from the patient’s clinical assessment, but during the follow-up visit, the subject reported a history of extended coagulation time with an activated partial thromboplastin time of 200–300 s (average values: 30–40 s). Despite the altered coagulation time, Factor XII deficiency was never suspected, and a genetic test was never performed.
GPR68 gene
Biallelic LoF variants in this gene have been associated with Amelogenesis imperfecta type IIA6, characterized by enamel hypomineralization, which causes early functional failure [21]. Individual_3 is a homozygous carrier of the NM_003485.3:c.1006G>T nonsense variant in the GPR68 gene, and at follow-up reported a history of multiple caries and recurrent tooth decay since childhood; the subject has been wearing dentures since the age of 20 years. Also, in this case, despite the presence of clinical features characteristic of Amelogenesis imperfecta, the subject was still lacking the precise clinical diagnosis and subsequently had never undergone genetic testing.
HKOs subjects not presenting with the expected phenotype
ACADSB gene
Biallelic LoF variants in the ACADSB gene cause 2-methylbutyrylglycinuria, a metabolic disorder characterized by impaired isoleucine degradation. This disorder may be detected via newborn screening; it is often clinically asymptomatic, but some individuals have been reported to be affected by developmental delay and neurological signs and symptoms including hypotonia and seizure [22]. A homozygous splicing variant, NM_001609.3:c.303+1G>A, has been detected in Individual_4 and Individual_5, two sisters from our cohorts. This variant has not previously been associated with 2-methylbutyrylglycinuria; another nucleotide change involving the same splicing site (NC_000010.10:g.124797366A>G) has been described as causative of this disease [23]. None of the two subjects presented with neurological alteration during our assessment nor reported developmental difficulties during childhood.
FANCL gene
Biallelic LoF variants in this gene have been associated with Fanconi anemia (FA), a severe condition usually lethal in childhood [24]. In Individual_6, we detected a rare biallelic LoF variant, NM_018062.3:c.2T>C, which has been described as causative of breast cancer in males [25]. At the initial clinical evaluation, the woman reported a history of head and neck carcinoma and short stature, both possible signs of FA. At follow-up, the diepoxybutane (DEB) chromosome fragility test, pathognomonic of FA [26], resulted positive, as shown in Fig. 3, even though no classical hematological FA pattern was found in the subject both at first sampling and at follow-up (white blood cell count: 5.16 × 103/μl (normal values: 3.7–11.7 × 103/μl); red blood cell count: 4.67 × 106/μl (normal values: 3.88–5.78 × 106/μl); platelets: 277 × 103/μl (normal values: 172–400 × 103/μl)). Moreover, three relatives of Individual_6 were also investigated (her two children, a 48-year-old man and his sister of 51 years of age, and her brother, a 71-year-old man). They are carriers of the variant at the heterozygous state, and, as expected, none of them presented any peculiar phenotype nor a positive DEB test.
GRK1 gene
The majority of the biallelic LoF variants in this gene are responsible for Oguchi disease type 2, a congenital stationary night blindness in which every other visual function—visual acuity, visual field, and color vision—are usually normal [27]. Moreover, one of the nonsense variants and one of the small insertion previously described have been linked to autosomal recessive retinal dystrophy [28] and retinitis pigmentosa [29], respectively. In Individual_7, we identified the NM_002929.2:c.699+1G>A splicing variant at the homozygous state. The analysis of the clinical and instrumental data carried out during the initial assessment on the subject excludes the presence of retinal disease or any other signs of Oguchi disease type 2 since he did not specifically report any visual alteration in dark adaptation. Unfortunately, no recent clinical data are available since the subject died and it has not been possible to perform a follow-up visit.
LGI4 gene
Biallelic LGI4 LoF variants may cause a rare form of neurogenic Arthrogryposis multiplex congenita due to a specific myelin defect, a severe disease characterized by prenatal onset (reduced fetal mobility, club feet, camptodactyly), which often results in stillbirth. Live-born children present multiple joint contractures and usually die within a few days of respiratory failure secondary to pulmonary hypoplasia [30]. The investigated HKO, Individual_8, did not present any clinical features associated with this specific disease, reporting only hypertension and dying at 81. Further investigations of the detected ENST00000591633.1:c.636del variant highlighted that it does not impact the canonical LGI4 transcript, and it involves only one protein-coding transcript out of the four reported in the Ensembl database. This transcript is the one with the shortest protein product, and its median mRNA expression, assessed using RNA-seq data from the Genotype-Tissue Expression (GTEx) project [31], is very low compared to the one of the canonical transcript (1.7 transcripts per million vs. 14.8 transcripts per million) (Fig. 4). All four LoF variants already described in the literature as causative of Arthrogryposis multiplex congenita fall outside our transcript of interest. Moreover, among the remaining five missense variants identified, only one (i.e., NM_139284.2:c.200A>C) affects our transcript and, to our knowledge, no disease-causing variants specifically affecting the coding region of this isoform have ever been described.
MPO gene
Biallelic LoF variants in this gene have been associated with Myeloperoxidase deficiency, a primary immunodeficiency due to a defect in innate immunity, which may lead to an increased incidence of fungal infection, particularly candidiasis [32]. Individual_9, who carries the known NM_000250.1:c.1552_1565del variant [33], is an MPO HKO who did not report episodes of recurrent candidiasis or other severe infections, neither at the first clinical assessment nor at follow-up.
PGAM2 gene
Biallelic LoF variants in the PGAM2 gene may be responsible for muscle phosphoglycerate mutase deficiency, known as well as glycogen storage disease X [34], or for rhabdomyolysis [35]. Affected individuals may complain of exercise intolerance, intense exertion pain, and muscle cramps; they may also present with elevated serum creatine phosphokinase (CPK) and occasional myoglobinuria [36]. In our cohorts, Individual_10 carries the NM_000290.3:c.532del variant, previously associated with muscle phosphoglycerate mutase deficiency [37]. The PGAM2 KO subject’s blood tests only showed increased lactate dehydrogenase values (429UI/l (normal values: 140–280 UI/l)) with CPK within the normal range (165 UI/l (normal values: 24–204 UI/l)); anamnestic and clinical data did not suggest exercise intolerance or exertion pain.
RP1L1 gene
Specific biallelic LoF variants in this gene may cause autosomal recessive Retinitis pigmentosa; moreover, other variants in this gene may cause occult macular dystrophy. Symptoms of patients carrying LoF variants usually include night blindness, tunnel vision, slowly progressive decreased central vision, decreased visual acuity, visual field alteration, dyschromatopsia, and alterations at the fundus oculi examination [38]. Here, we identified a HKO (Individual_11), carrying the NM_178857.5:c.326_327insT variant, associated with syndromic retinal dystrophy in a previously described patient carrying another in cis RP1L1 nonsense variant (NM_178857.5:c.326_327insA) together with a nonsense variant in C2orf71, thus suggesting a digenic effect [39]. Our subject did not report any history of ophthalmologic disorders.
Discussion
One of the major goals in biomedicine consists in understanding the function of every gene of the human genome. An interesting approach to achieve this is represented by the study of putative LoF variants that disrupt both copies of a specific gene. A key point in studying HKOs consists in the identification of populations that may be enriched in these rare and possibly disease-causing LoF variants, such as genetic isolates. Only a few research groups have so far focused on this specific kind of population. For example, Saleheen et al. [40]. have recently described a series of Pakistani adult HKOs detected during a study aimed at identifying variants influencing cardiovascular disease. In 2015, more than 1100 homozygous LoF variants were detected in a cohort of over 100,000 Icelanders [41], and the following year over 780 HKOs have been identified in a cohort of consanguineous British adults [42]. One overall advantage of this kind of study is the possibility to perform in-depth phenotyping with accurate follow-up to link the identified LoF variants to a specific clinical outcome [4].
In this study, we describe the results of the first Italian screening of HKOs by combining WGS data and deep phenotyping. This work represents a further detailed characterization of the initial analysis of knockout variants carried out by Cocca et al. [12]. In particular, we focused on LoF variants involving OMIM disease-associated genes, and specifically on those linked to autosomal recessive disorders, in order to be able to objectively assess whether the identified variants were associated with a well-known clinical condition. Our results may be summarized in two classes: (1) HKOs presenting the expected phenotype, in most cases not diagnosed, and (2) HKOs that, despite carrying biallelic homozygous LoF variants, do not display the supposed clinical outcome.
The carriers of biallelic LoF variants in three genes, C7, F12, and GPR68, belong to the first group. As regards C7 deficiency, the investigated subject reported the typically increased infection rate. Despite the clear clinical signs, a genetic condition was never suspected, thus not allowing the patient to benefit from preventive medical strategies such as meningococcal vaccination or plasma transfusion. The F12 gene KO individual presented a history of extended coagulation time without any other relevant clinical problem. In this case, as well, no genetic condition was suspected, and the patient’s surgical procedures were repeatedly delayed because of the impossibility to understand the reason of the coagulation defect correctly. Furthermore, a GPR68 HKO reported Amelogenesis imperfecta distinctive clinical manifestations. Again, this individual never received a genetic disease diagnosis, which could have led to an early therapy based on enamel protection and specific dental surgery.
In the second category, for four HKOs (ACADSB, MPO, and PGAM2 genes, respectively) the expected clinical phenotype was not detected. According to literature data, only 10% of the subjects carrying biallelic LoF variants in the ACADSB gene develop early childhood symptoms, especially when exposed to increased catabolic stress, which may lead to metabolic decompensation [43]. Similarly, MPO HKOs are usually asymptomatic, not displaying an increased susceptibility to infections, unless specific comorbidities occur (i.e., diabetes mellitus) [44]. Regarding PGAM2, LoF variants carriers become symptomatic only during strenuous physical exercise and are otherwise asymptomatic [45]. Therefore, the absence of clinical signs and symptoms in these four HKOs may be due to the specific incomplete penetrance of the underlying diseases. In this category, other interesting results are represented by the discovery of two different subjects carrying biallelic LoF variants in the GRK1 and RP1L1 genes, both involved in retinal diseases. The detected KO carriers did not report a history of ophthalmologic disorder or visual alteration in dark adaptation. Again, in this case, literature data suggest that both conditions are mild and non-progressive. In this light, since these pathologies peculiar clinical signs might have been missed, it would be proper to perform a deep and updated ophthalmological evaluation on the RP1L1 HKO (i.e., fundus oculi assessment). We additionally identified an LGI4 HKO who did not present the expected clinical features. The finding was striking since biallelic LoF variants in this gene cause a severe disease that often results in stillbirth or neonatal death. However, meticulous analysis of the variant genomic context showed that it does not impact the canonical LGI4 transcript, which still seems able to generate the full-length protein, thus explaining the typical clinical phenotype absence in the detected HKO. Finally, the most intriguing case is represented by discovering an HKO for the FANCL gene, which, when mutated, causes FA, a severe genetic disease often lethal in childhood. The KO carrier we identified is a 74-year-old individual characterized by short stature who reported having suffered from a brain tumor and a head and neck carcinoma without showing the classical FA spectrum phenotype (i.e., not presenting any hematological abnormalities) but with a positive DEB test. The reasons for the mild clinical presentation of this HKO are still unclear. Several hypotheses may be proposed: (a) the possible presence of other variants in the FANCL gene that might allow the transcription of a shorter transcript leading to the production of a smaller but still partly functioning protein, and (b) the possibility that this individual carries other variants/genes able to compensate for the detrimental effects of the disease-related FANCL allele. Future in vitro and in vivo studies will clarify if this “genetic resilience” is related to a secondary variant that bypassed the mutant phenotype or a gene over-expression that rescued the mutant phenotype.
In conclusion, the present findings remark the importance of a deep phenotypical characterization when trying to understand the role of LoF variants, performing, when required, a specific clinical follow-up on all HKOs. The detection of KO subjects presenting the expected phenotype highlights how often the lack of a correct diagnosis, including a genetic one, may lead to inappropriate or delayed treatment. On the other hand, the identification of subjects that, despite carrying biallelic LoF, do not display a conventional clinical presentation, underlines how LoF variants may be responsible for a broader phenotypic spectrum than previously expected, raising awareness toward the discovery of putatively protective variants that may become the cornerstone of new therapeutic approaches. Overall, studying HKOs in genetic isolates represents an intriguing and not commonly employed opportunity to investigate genotype–phenotype correlations, with still undiscovered potential in helping the clinical decision-making process regarding preventive, diagnostic, and therapeutic approaches.
References
Perlman RL. Mouse models of human disease: an evolutionary perspective. Evol Med Public Heal. 2016;2016:170–6.
Callaway E. Geneticists tap human knockouts. Nature. 2014;514:548–548.
Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91.
Alkuraya FS. Natural human knockouts and the era of genotype to phenotype. Genome Med. 2015;7:48.
MacArthur DG, Balasubramanian S, Frankish A, Huang N, Morris J, Walter K, et al. A systematic survey of loss-of-function variants in human protein-coding genes. Science. 2012;335:823–8.
Narasimhan VM, Xue Y, Tyler-Smith C. Human knockout carriers: dead, diseased, healthy, or improved? Trends Mol Med. 2016;22:341–51.
de la Chapelle A, Wright FA. Linkage disequilibrium mapping in isolated populations: the example of Finland revisited. Proc Natl Acad Sci USA. 1998;95:12416–23.
Erzurumluoglu AM, Shihab HA, Rodriguez S, Gaunt TR, Day INM. Importance of genetic studies in consanguineous populations for the characterization of novel human gene functions. Ann Hum Genet. 2016;80:187–96.
Peltonen L, Jalanko A, Varilo T. Molecular genetics of the Finnish disease heritage. Hum Mol Genet. 1999;8:1913–23.
Esko T, Mezzavilla M, Nelis M, Borel C, Debniak T, Jakkula E, et al. Genetic characterization of northeastern Italian population isolates in the context of broader European genetic diversity. Eur J Hum Genet. 2013;21:659–65.
Traglia M, Sala C, Masciullo C, Cverhova V, Lori F, Pistis G, et al. Heritability and demographic analyses in the large isolated population of Val Borbera suggest advantages in mapping complex traits genes. PLoS One. 2009;4:e7554.
Cocca M, Barbieri C, Concas MP, Robino A, Brumat M, Gandin I, et al. A bird’s-eye view of Italian genomic variation through whole-genome sequencing. Eur J Hum Genet. 2020;28:435–44.
Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, del Angel G, Levy-Moonshine A, et al. From FastQ data to high-confidence variant calls: the genome analysis toolkit best practices pipeline. Curr Protoc Bioinforma. 2013;43:11.10.1–11.10.33.
McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GRS, Thormann A, et al. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17:122.
Xue Y, Mezzavilla M, Haber M, McCarthy S, Chen Y, Narasimhan V, et al. Enrichment of low-frequency functional variants revealed by whole-genome sequencing of multiple isolated European populations. Nat Commun. 2017;8:15927.
Gibson G. Rare and common variants: twenty arguments. Nat Rev Genet. 2012;13:135–45.
Barroso S, Sánchez B, Alvarez AJ, López-Trascasa M, Lanuza A, Luque R, et al. Complement component C7 deficiency in two Spanish families. Immunology. 2004;113:518–23.
Fernie BA, Hobart MJ. Complement C7 deficiency: seven further molecular defects and their associated marker haplotypes. Hum Genet. 1998;103:513–9.
Maas C, Govers-Riemslag JWP, Bouma B, Schiks B, Hazenberg BPC, Lokhorst HM, et al. Misfolded proteins activate Factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008;118:3208–18.
Bork K, Wulff K, Meinke P, Wagner N, Hardt J, Witzke G. A novel mutation in the coagulation factor 12 gene in subjects with hereditary angioedema and normal C1-inhibitor. Clin Immunol. 2011;141:31–5.
Parry DA, Smith CEL, El-Sayed W, Poulter JA, Shore RC, Logan CV, et al. Mutations in the pH-sensing G-protein-coupled receptor GPR68 cause amelogenesis imperfecta. Am J Hum Genet. 2016;99:984–90.
Sass JO, Ensenauer R, Röschinger W, Reich H, Steuerwald U, Schirrmacher O, et al. 2-Methylbutyryl-coenzyme A dehydrogenase deficiency: functional and molecular studies on a defect in isoleucine catabolism. Mol Genet Metab. 2008;93:30–5.
Matern D, He M, Berry SA, Rinaldo P, Whitley CB, Madsen PP, et al. Prospective diagnosis of 2-methylbutyryl-CoA dehydrogenase deficiency in the Hmong population by newborn screening using tandem mass spectrometry. Pediatrics. 2003;112:74–8.
Vetro A, Iascone M, Limongelli I, Ameziane N, Gana S, Della ME. et al. Loss-of-function FANCL mutations associate with severe Fanconi anemia overlapping the VACTERL association. Hum Mutat. 2015;36:562–8.
Fostira F, Saloustros E, Apostolou P, Vagena A, Kalfakakou D, Mauri D, et al. Germline deleterious mutations in genes other than BRCA2 are infrequent in male breast cancer. Breast Cancer Res Treat. 2018;169:105–13.
Esmer C, Sánchez S, Ramos S, Molina B, Frias S, Carnevale A. DEB test for Fanconi anemia detection in patients with atypical phenotypes. Am J Med Genet Part A. 2004;124A:35–9.
Yamamoto S, Sippel KC, Berson EL, Dryja TP. Defects in the rhodopsin kinase gene in the Oguchi form of stationary night blindness. Nat Genet. 1997;15:175–8.
Li L, Chen Y, Jiao X, Jin C, Jiang D, Tanwar M, et al. Homozygosity mapping and genetic analysis of autosomal recessive retinal dystrophies in 144 consanguineous Pakistani families. Invest Ophthalmol Vis Sci. 2017;58:2218–38.
Yamamoto S, Khani SC, Berson EL, Dryja TP. Evaluation of the Rhodopsin kinase gene in patients with retinitis pigmentosa. Exp Eye Res. 1997;65:249–53.
Xue S, Maluenda J, Marguet F, Shboul M, Quevarec L, Bonnard C, et al. Loss-of-function mutations in LGI4, a secreted ligand involved in Schwann cell myelination, are responsible for arthrogryposis multiplex congenita. Am J Hum Genet. 2017;100:659–65.
Carithers LJ, Ardlie K, Barcus M, Branton PA, Britton A, Buia SA, et al. A novel approach to high-quality postmortem tissue procurement: the GTEx project. Biopreserv Biobank. 2015;13:311–9.
Goedken M, McCormick S, Leidal KG, Suzuki K, Kameoka Y, Astern JM, et al. Impact of two novel mutations on the structure and function of human myeloperoxidase. J Biol Chem. 2007;282:27994–8003.
Romano M, Dri P, Dadalt L, Patriarca P, Baralle FE. Biochemical and molecular characterization of hereditary myeloperoxidase deficiency. Blood. 1997;90:4126–34.
Tsujino S, Shanske S, Sakoda S, Fenichel G, DiMauro S. The molecular genetic basis of muscle phosphoglycerate mutase (PGAM) deficiency. Am J Hum Genet. 1993;52:472–7.
Wu L, Brady L, Shoffner J, Tarnopolsky MA. Next-generation sequencing to diagnose muscular dystrophy, rhabdomyolysis, and hyperCKemia. Can J Neurol Sci. 2018;45:262–8.
DiMauro S, Miranda AF, Khan S, Gitlin K, Friedman R. Human muscle phosphoglycerate mutase deficiency: newly discovered metabolic myopathy. Science. 1981;212:1277–79.
Sidhu M, Brady L, Vladutiu GD, Tarnopolsky MA. Novel heterozygous mutations in the PGAM2 gene with negative exercise testing. Mol Genet Metab Rep. 2018;17:53–5.
Davidson AE, Sergouniotis PI, Mackay DS, Wright GA, Waseem NH, Michaelides M, et al. RP1L1 variants are associated with a spectrum of inherited retinal diseases including retinitis pigmentosa and occult macular dystrophy. Hum Mutat. 2013;34:506–14.
Liu YP, Bosch DGM, Siemiatkowska AM, Rendtorff ND, Boonstra FN, Möller C, et al. Putative digenic inheritance of heterozygous RP1L1 and C2orf71 null mutations in syndromic retinal dystrophy. Ophthalmic Genet. 2017;38:127–32.
Saleheen D, Natarajan P, Armean IM, Zhao W, Rasheed A, Khetarpal SA, et al. Human knockouts and phenotypic analysis in a cohort with a high rate of consanguinity. Nature. 2017;544:235–9.
Sulem P, Helgason H, Oddson A, Stefansson H, Gudjonsson SA, Zink F, et al. Identification of a large set of rare complete human knockouts. Nat Genet. 2015;47:448–52.
Narasimhan VM, Hunt KA, Mason D, Baker CL, Karczewski KJ, Barnes MR, et al. Health and population effects of rare gene knockouts in adult humans with related parents. Science. 2016;352:474–7.
Porta F, Chiesa N, Martinelli D, Spada M. Clinical, biochemical, and molecular spectrum of short/branched-chain acyl-CoA dehydrogenase deficiency: two new cases and review of literature. J Pediatr Endocrinol Metab. 2019;32:101–8.
Pahwa R, Jialal I. Myeloperoxidase deficiency. Treasure Island (FL); 2020.
Salameh J, Goyal N, Choudry R, Camelo-Piragua S, Chong PST. Phosphoglycerate mutase deficiency with tubular aggregates in a patient from panama. Muscle Nerve. 2013;47:138–40.
Acknowledgements
We gratefully acknowledge Prof. Daniela Toniolo for raising awareness about the study and involving the Val Borbera population in this project.
Funding
This research was supported by BENEFICENTIA Stiftung to GG, D70-RESRICGIROTTO to GG, and SENSAGING—Sensory decays and ageing (D70-PRINSENSAGING-19: CUP J94I19000930006) to PG.
Author information
Authors and Affiliations
Contributions
BS analyzed the clinical data and wrote the manuscript with support from FF, MC, MF, RP, AM, and GG. MC performed and coordinated WGS data analysis with support from CB, MF, and MM. AM and GP performed Sanger sequencing variants confirmation. GG and PG conceived the study and supervised the project. All authors discussed the results and critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spedicati, B., Cocca, M., Palmisano, R. et al. Natural human knockouts and Mendelian disorders: deep phenotyping in Italian isolates. Eur J Hum Genet 29, 1272–1281 (2021). https://doi.org/10.1038/s41431-021-00850-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-021-00850-9
This article is cited by
-
Recent advances in acid sensing by G protein coupled receptors
Pflügers Archiv - European Journal of Physiology (2024)
-
From target discovery to clinical drug development with human genetics
Nature (2023)
-
Ontologizing health systems data at scale: making translational discovery a reality
npj Digital Medicine (2023)
-
A new impact factor for European Journal of Human Genetics
European Journal of Human Genetics (2021)