Abstract
Chronic non-communicable diseases are the leading cause of morbidity and mortality worldwide. Developing and implementing effective preventive strategies is the best way to ensure the overall metabolic health status of the population and to counter the global burden of non-communicable diseases. Predisposition to obesity and other non-communicable diseases is due to a combination of genetic and environmental factors throughout life, but the early environment, particularly the environment during the fetal period and the early years of life, is crucial in determining metabolic health, hence the concept of ‘fetal programming’. The origins of this causal link between environmental factors and disease lie in epigenetic mechanisms. Among the environmental factors, diet plays a crucial role in this process. Substantial evidence documented the key role of macronutrients in the programming of metabolic diseases early in life. Recently, the effect of maternal micronutrient intake on offspring metabolic health in later life emerged. The purpose of this narrative review is to bring to light available evidence in the literature on the effect of maternal micronutrient status on offspring metabolic health and underlying epigenetic mechanisms that drive this link to highlight its potential role in the prevention of non-communicable diseases.
Similar content being viewed by others
Introduction
Obesity is the most common nutritional disease of children and adolescents and a major health concern increasing worldwide [1]. Obesity onset in childhood has a high risk of persisting into adulthood and leading to cardiometabolic diseases and complications, such as type 2 diabetes (T2D), metabolic syndrome, and cardiovascular disease [1]. Insulin resistance, hypertension, dyslipidaemia, and central distribution of body adiposity associated to hepatic steatosis are detrimental risk factors for the development of cardiometabolic disease. Among these, insulin resistance, which consists of a reduced target tissues response to the action of insulin causing impaired glucose disposal and compensatory hyperinsulinemia, plays a key role in the development and progression of cardiometabolic risk factors and diseases, particularly dyslipidaemia and T2D [2].
Chronic non-communicable diseases (NCDs) are the leading cause of morbidity and mortality worldwide and cardiometabolic diseases and diabetes account for ~20 million deaths annually [3]. Developing and implementing effective preventive strategies since early life is the best way to ensure the overall metabolic health status of the population and to counter the global burden of NCDs [4].
The fetal programming hypothesis suggests that unfavourable environmental factors during the early stages of life (periconceptional, intrauterine, and postnatal) lead to a high incidence of chronic NCDs [5]. This would represent an attempt by the foetus to adapt to adverse conditions experienced in utero, resulting in adaptations that will be harmful when such conditions do not prevail later in life [6]. This concept has gradually evolved into the Developmental Origins of Health and Disease hypothesis, and the mechanisms underlying this theory, that link environmental factors to diseases, are explained by epigenetics [5,6,7].
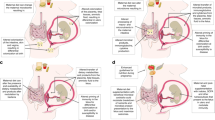
Epigenetics refers to the changes in gene expression without the modification of the nucleotide sequence [8]. The main contributors so far known to epigenetic modifications of gene expression are DNA methylation, histone post-translational modifications and non-coding RNAs regulation, represented in Fig. 1. While microRNAs regulate the expression of genes at the post-transcriptional level, DNA methylation and histone modifications modify gene expression before transcription initiation, inducing a remodelling of the chromatin structure or modifying the genes accessibility to transcription factors, through the action of epigenetic enzymes, such as methyltransferases, methyl-binding proteins, acetyltransferases, and deacetylases [9]. Together, these modifications constitute the epigenome, which continues to change in dynamic and reversible remodelling during developmental, physiological, and pathogenic processes throughout life [7]. There is increasing evidence supporting that epigenetic modifications can be stable and inherited, making them potentially important pathogenic and hereditary mechanisms in complex metabolic diseases, such as T2D and cardiovascular disease [9].
Maternal macronutrient deprivation and excess during early life have been demonstrated to play a crucial role in programming metabolic diseases in later-life [10]. Historical experiments following significant periods of famine have demonstrated that offspring born from women who experienced energy and protein deprivation in specific periods of pregnancy have an increased risk of developing obesity, T2D and coronary heart diseases [11, 12].
Micronutrients are classified into minerals, trace elements, and vitamins, which play an important role in biochemical reactions and functions, and although they are needed in small amounts, they are essential to ensure the body’s homeostasis [13]. Micronutrient deficiency usually leads to health problems during pregnancy and throughout life and constitutes a threatening problem worldwide, especially in low- and middle-income countries [14]. Levels of many essential minerals decrease during pregnancy if they are not supplemented, including Ca, Fe, Mg, Se, Zn, and possibly chromium and iodine [13]. Iron deficiency results in microcytic anaemia, fatigue, and impaired immune and endocrine function; iodine deficiency causes goiter, mental retardation, or impaired cognitive function; zinc deficiency can alter immune function and increase the risk of acute respiratory infections; folic acid supplementation in pregnancy can prevent neural tube defects and is also essential for DNA synthesis and repair, methyl group synthesis by one carbon cycle and its deficiency causes macrocytic anaemia [13].
Furthermore, micronutrient deficiencies have an emerging role in pregnancy, not only for the health of pregnant women but for the programming of the metabolic health of their children. Indeed, new evidence supports the role of micronutrient deficiencies on perinatal health and child growth and in conditioning the risk of metabolic disorders later in life [14, 15].
In 2004 Venu et al. carried out one of the first studies on the effects of maternal micronutrient inadequate intake on insulin resistance in rat offspring and found that maternal multimineral (Fe, Zn, Mg and Ca) or multivitamin restriction led to early growth retardation, altered body composition and insulin resistance in the offspring [16].
Afterwards, numerous animal and clinical studies have been conducted on the effects of individual micronutrients, including minerals (Zn, Cr, Ca, Fe, Mg, Se, Zn) and vitamins (vitamins A, D, B12, and folic acid), on the metabolic status of the offspring. Among the plausible mechanisms behind this phenomenon are epigenetic ones and, to date, the most studied is DNA methylation [17]. In 2007, Sinclair et al. conducted a study in a sheep model, in which restriction of dietary methyl donor (i.e., methionine and vitamin B12) until day 6 after insemination altered DNA methylation and led to hypertensive and insulin-resistant offspring [17]. In 2020, the first results of the EMPHASIS study reported that, in response to micronutrient supplementation provided to pregnant Gambian women, their children at the age of 7–9 years showed a series of DNA methylation alterations at multiple loci [18].
This narrative review collects and summarizes studies linking maternal micronutrient malnutrition and its negative impact on the future metabolic health of the offspring and the plausible mechanisms involved in this process, with the aim of highlighting the available evidence supporting the role of proper intake of micronutrients in pregnancy for NCDs prevention.
Methods
A search for relevant literature published in English was undertaken in October 2022 using the PUBMED, Web of Science and Scopus scientific databases. An update of the literature search was performed in June 2023. The following search including MeSH terms were used: (Micronutrients OR Dietary supplements OR Minerals OR Vitamins) AND (Early life OR Pregnancy) AND (fetal programming OR Epigenetics) AND (Noncommunicable disease OR Diabetes OR Metabolic health).
This narrative review includes observational studies, clinical trials, reviews, and guidelines written in English about the effect of micronutrient deprivation or excess in early life to highlight potential targets for preventing non-communicable diseases.
Minerals
Calcium (Ca)
Altered Ca homeostasis was correlated with abnormalities in glucose metabolism. A maternal Ca deficiency increased insulin resistance and altered glucocorticoid metabolism in adult rat offspring, especially in males, due to a possible alteration of the expression of hepatic hydroxysteroid 11-beta dehydrogenase 1 and osteocalcin, genes that could act directly on β-cells, modulating insulin secretion [19]. Other animal studies sustain that dietary calcium status during pregnancy and lactation affects lipid metabolism in mouse offspring. Ping Li et al. found that maternal insufficient or excessive calcium status (diet calcium concentration of 0.25% and 1.2%, respectively) programmed an abnormal expression of several genes implicated in lipid metabolism in liver or adipose tissue (i.e., PPAR-γ, C/EBP-α, FABP4, Fasn, UCP2, PPAR-α, HMG-Red1, Acc1, and SREBP-1c) in the offspring leading to increased body weight, dyslipidaemia and accumulation of hepatic triglycerides and total cholesterol later in life [20]. This finding was confirmed in the cohort of about 300 subjects from the Tasmanian Infant Health Study in which children of Ca-supplemented mothers had lower values of triglycerides, total cholesterol and LDL-cholesterol and this positive effect persists also in children with higher body mass index [21]. Unfortunately, there was no consistent information available on the dosage and period of supplement intake. A recent meta-analysis supported that high-dose calcium supplementation during pregnancy, consisting in most studies of dosages up to 2000 mg/day starting at 21 weeks of gestation at the latest and stopped at delivery, was associated with a decreased risk of offspring high systolic blood pressure at 5–7 years of age (risk ratio = 0.59; 95% confidence interval: 0.39–0.90), despite the results for other metabolic outcomes analysed remain unknown because of conflicting or insufficient data [22].
Magnesium (Mg)
Magnesium deficiency in pregnancy is still frequent in both developed and developing countries and also Mg was associated with metabolic health and the risk of T2D in offspring [23]. Magnesium serves as a critical cofactor for many enzymes. Venu et al. reported that rat pups exposed to Mg restriction in pregnancy (165 mg Mg/kg diet) have an altered body composition with increased fat and plasma triglycerides in the short-medium-term [24] and increased body adiposity and decreased insulin secretion in long-term [25]. Several underlying putative molecular mechanisms have been proposed regarding insulin secretion and signalling and fatty acids metabolism. Low Mg levels result in defective tyrosine kinase activity, post-receptor impairment in insulin action, altered cellular glucose transport, and decreased cellular glucose utilization, which promotes peripheral insulin resistance and T2D [26]. In addition, an increase in the expression of fatty acids synthase (FAS) and fatty acyl transport protein-1 (FATP1) in liver and adipose tissue was reported, inducing increased fatty acid synthesis and transport that contribute to the increase in central adiposity and body fat content [25].
Many epidemiological studies have associated being born small for gestational age (SGA) with an increased risk of insulin resistance later in life [27]. Intracellular Mg of cord blood platelets was found to be lower in SGA than in appropriate gestational weight subjects, suggesting Mg deficiency could be a potential contributor to the high risk of diabetes and metabolic complications that characterize low-birth-weight children later in life [28]. In addition, lower serum Mg concentrations were found to be associated with insulin resistance and other cardiometabolic risk factors both in children and adults [29, 30] and a recent systematic review sustain that Mg supplementation has beneficial effects on serum glucose, lipids, and blood pressure controls, especially in subjects with hypomagnesemia, supporting the role of adequate dietary magnesium intake in promoting cardiometabolic health throughout the lifespan [31].
Chromium (Cr)
Chromium plays an important role in the regulation of glucose metabolism. It enhances the glucose/insulin system in subjects with hyperglycaemia, diabetes, and hyperlipemia by improving insulin binding, internalization, and beta-cell sensitivity, with an overall increase in insulin sensitivity [32]. Chromium deficiency could contribute to dyslipidaemia and atherosclerosis [33]. Zhang et al. found that maternal low Cr irreversibly increased mice offspring body weight, adiposity, serum triglyceride and TNF-α and these changes may be attributable to expression changes of genes of the PPAR signalling pathway in adipose tissues [12] and long-term programming on various specific miRNA and MAPK signalling pathway [34]. PPAR and MAPK signalling pathways have a crucial role in adipocyte differentiation through the activation of PPARγ an adipogenic transcription factor which can induce the expression of other lipogenic transcription factors, activate lipoprotein lipase and fatty acid synthase leading to adipocyte differentiation, intracellular triglycerides hydrolysis and fatty acid release from adipocytes [12, 34]. Zhang et al. also sustain modifications of gene expression in the insulin signalling pathway accounting for late onset hyperglycaemia, hyperinsulinemia, and insulin resistance in mice offspring [35]. A meta-analysis of 22 randomized controlled trials confirmed that Cr supplementation improved glycemic control, reduced triglycerides, and increased HDL-C (high density lipoprotein-cholesterol), especially if administrating Cr picolinate at the dosage over 200 μg/day, in subjects with diabetes [36].
Zinc (Zn)
Zinc, like Cr, is an important regulatory element for glucose metabolism. It acts as an insulin-mimetic through its direct effect on the insulin signalling pathway, as it stimulates lipogenesis and glucose uptake in isolated adipocytes [37]. Zinc also acts on the regulation of inflammation through the reduction of inflammatory cytokines, oxidative stress status by participating in the synthesis of antioxidant enzymes, and as a catalyser of enzymes, taking part in lipid, carbohydrate, and protein metabolism [38]. Chronic low zinc intake is associated with an increased risk of T2D; however, the effectiveness of zinc supplementation for the improvement/treatment of obesity and diabetes status has not been demonstrated in large-scale studies, especially in adults [37].
Inadequate Zn intake is quite common at the population level, especially among socio-economically disadvantaged groups, vegetarians, the elderly and pregnant and lactating women, reflecting a general state of poor nutrition [15]. Low serum Zn levels in pregnant women are related to several pregnancy complications such as preeclampsia [39], while a meta-analysis debunked that zinc supplementation in pregnancy has a concrete effect on perinatal outcomes such as perinatal death or low birth weight [40]. Instead, as regards long term outcomes, several animal studies demonstrated a link between low Zn maternal intake and insulin resistance/diabetes in offspring. A selective maternal restriction of Zn (10 mg/kg diet) increased the percentage of body fat and decreased fat-free mass and fasting plasma insulin levels in both male and female rat offspring at 6 months of age [41]. Other animal studies confirmed these findings, although highlighting sex differences: Zn restriction during prenatal and postnatal life induced an increase in systolic blood pressure, hyperglycaemia, hypertriglyceridemia, and insulin resistance indexes in male rats, showing females less sensitive to Zn early restrictions [42].
As regards human studies, in Nepal, maternal Zn supplementation (30 mg Zn sulfate) compared with supplementation of combinations of other minerals and vitamins during pregnancy resulted in a slight increase in stature and a reduction in adiposity at 6–8 years of age [43]. In parallel, a double-blind randomized controlled trial conducted in Peru confirmed an increase in lean mass indices at 1 year of age among infants whose mothers had received daily Zn supplementation (i.e., 15 mg Zn sulfate) during pregnancy [44].
Zinc appears to play an important role in the functioning of epigenetic enzymes such as methyltransferases, methyl-binding proteins, acetyltransferases, and deacetylases. Zinc deficiency would directly affect the biological activity of zinc-dependent epigenetic enzymes and zinc finger proteins, which are essential for DNA methylation and histone modification, resulting in a reduction of DNA methyltransferases (DNMTs) and S-adenosylmethionine in offspring [45].
Selenium (Se)
Selenium is a trace element with important physiological activity; it binds to proteins to create Se-dependent glutathione peroxidases and other seleno-protein complexes to defend against oxidative stress [46]. Thanks to its modulation activity against reactive oxygen species (ROS), Se influences glucose metabolism and its deficiency has been associated with the progression of T2D and its complications [47]. Nevertheless, also excessively high Se concentrations in non-endemically deficient populations have been associated with diabetes, leading to the exclusion of routine Se supplementation in countries with a diversified and balanced diet and adequate selenium intake [48]. Animal studies also reach the same conclusion about the dual effect of Se on glucose metabolism. Melo et al. found that in rats maternal Se supplementation, at the dosage of 1 mg/kg of sodium selenite, increases insulin secretion and glucose tolerance at 80 days of age in the offspring and reduces triglycerides in the liver and in white adipose tissue [49]. Maternal Se supplementation upregulates the antioxidative capacity of the thymus and spleen and suppresses the activation of the MAPK/NF-κB and NF-κB and ERK/Beclin-1 pathways, endoplasmic reticulum stress and autophagy induced by the lipopolysaccharide (LPS) challenge in weaning piglets [50]. In contrast, Zeng et al. found that an excess of Se (3 mg/kg) in the mother’s diet leads to insulin resistance in the offspring by increasing Gpx1 mRNA or glutathione peroxidase-1 (GPX1) activity in the pancreas, liver, and erythrocytes of dams [51]. The overproduction of GPX1 activity, which decreases intracellular ROS, alters the oxidoreductive balance and results in the dysregulation of proteins important for insulin synthesis, secretion, and signalling [52].
Iron
Iron is one of the most prescribed supplements during pregnancy to prevent pregnancy anaemia and fetal consequences like intrauterine growth retardation and prematurity [48]. Iron needs in pregnancy increase but this does not make indiscriminate supplementation necessary for all pregnant women. In fact, excessive concentrations of iron could induce oxidative stress and the formation of ROS favouring cardiovascular risk, pregnancy diabetes, and fetal complications [53]. Thus, high iron intake increases the risk of glucose metabolism disorder through interaction at multiple levels with adipocyte differentiation, lipid metabolism, and insulin secretion [54]. In particular, excess iron, once stored in the liver, interferes with glucose metabolism, causing hyperinsulinemia via both decreased insulin extraction and impaired insulin signalling [55]. However, a recent systematic review struggled to demonstrate consistent results of iron overload in pregnancy on children´s later outcomes, concluding that more research on long-term effects is needed [53]. The recommended dietary allowance (RDA) and supplementation for iron during pregnancy are reported in Table 1.
Vitamins
Vitamin B12 and folate
Group B Vitamins are extremely important for epigenetic regulation, as they are involved in the one-carbon metabolism cycle. This suggests that a maternal diet that is unbalanced in terms of B vitamins could also affect offspring DNA methylation patterns and result in altered metabolic fetal programming [56]. Several studies demonstrated that maternal vitamin B12 deficiency is related to a high risk of obesity, insulin resistance and T2D in offspring [57]. Low maternal vitamin B12 concentrations predicted higher insulin resistance in children estimated by Homeostatic model assessment (HOMA)-IR which is a gross index of insulin resistance based on fasting glucose and fasting insulin, widely used in population studies [58, 59]. In an Indian recent systematic review, low maternal vitamin B12 status was associated with adverse maternal and child health outcomes (i.e., high adiposity, insulin resistance, and low offspring B12 levels) [60].
Folic acid and folate are forms of B9 vitamin. Diet fortification with folic acid is widely used in several countries given the association with reduced incidence of neural tube defects [61]. The RDA for folate is listed as micrograms (mcg) of dietary folate equivalents (DFE) and reported in Table 1.
Epigenetic regulation, involving DNA methylation, could be the mechanism behind nutritional programming mediated by B vitamins. Maternal B12 restriction alters DNA methylation of genes involved in important metabolic processes, in particular, fatty acid metabolism (e.g., hydroxyacyl coenzyme A dehydrogenase, medium-chain specific acylCoA dehydrogenase, 3 keto-acyl-CoA thiolase) and mitochondrial transport/metabolism [62].
As regards folate and folic acid deficiency, the evidence about metabolic outcomes in offspring is rather conflicting. Sinclair et al. found an association between maternal dietary folate reduction and alterations in DNA methylation and increased insulin resistance in sheep offspring [17]. Clinical studies sustained these findings and the Pune Maternal Nutrition Study reported that higher maternal erythrocyte folate concentrations at 28 weeks predicted higher offspring adiposity and higher HOMA-IR at 6 years of age [58]. Ghattu et al. confirmed that higher maternal folate concentrations at 30 gestational weeks were associated with insulin resistance in children at 9.5 and 13.5 years in a cohort of about 650 Indian children [63]. Yajnik et al. showed that children of mothers with a combination of high folate and low vitamin B12 concentrations were the most insulin resistant and sustained that low maternal vitamin B12 and high folate may contribute to the epidemic of adiposity and T2D in India [58].
While diet fortification/supplementation with folic acid is widely used in pregnancy in several countries given the association with reduced incidence of neural tube defects [61], inadequate attention is paid to vitamin B12 deficiency, which is widespread, especially in low-income countries and associated to the increase of vegetarianism and dairy-free diets worldwide. All these factors, in addition to the medical practice of prescribing routinely high doses of folic acid in early pregnancy, could contribute to creating an imbalance in these two related vitamins [63].
Vitamin D
Vitamin D deficiency during pregnancy can impair the foetus bone metabolism and immune function but it is also critical for fetal programming, which may influence susceptibility to NCDs soon after birth and later in life [64]. A recent metanalysis showed that vitamin D supplementation during pregnancy improves maternal and infant 25(OH)D concentrations that correlated inversely with maternal insulin resistance and fetal growth [65]. Moreover, maternal vitamin D deficiency has been linked with increased insulin resistance and body composition in offspring [66].
Animal models sustained the role of vitamin D deficiency during pregnancy in influencing insulin resistance in rat offspring [67]. Vitamin D levels have been correlated with levels of several inflammation molecules such as cytokines, and with methylation of the hepatic Iκbα gene, which plays an important role in persistent inflammation by decreasing Iκbα expression [67]. Another possible mechanism through which Vitamin D level in pregnancy could influence the long-term metabolic health of adult offspring is mediated by alterations of intestinal permeability and consequent increasing inflammation and circulating levels of LPS, indicating nutritional programming of the intestinal barrier function [68].
Vitamin A
In addition to antioxidant and anti-inflammatory functions, Vitamin A has a determinant role in pancreas functionality [69]. Established evidence supports the importance of retinoids in insulin and beta cell metabolism and vitamin A-dependent proteins are present in pancreatic islet progenitor cells. Moreover, the role of vitamin A in modifying the expression of sonic hedgehog and fibroblast growth factor, which may influence the neogenesis and replication of α and β cells, has already been demonstrated [70]. Vitamin A deficiency during pregnancy and the postweaning period caused important reductions in the area and number of β cells per islet and a reduction in β-cell replication in the Sprague-Dawley rats’ offspring [71]. In a retrospective cohort study, Keller et al. demonstrated that fetal exposure to small, extra amounts of vitamin A from food fortification of margarin in 1962 Denmark may reduce the long-term risk of T2D in offspring (OR 0.88; 95% CI 0·81, 0·95, P = 0·001) [69].
The role of diet
Micronutrient deficiencies constitute an important global health issue especially in low and middle-income countries [14]. Many of these deficiencies are preventable through nutrition education and consumption of a healthy diet containing diverse foods, as well as food fortification and supplementation, where needed [72]. The WHO 2016–2025 nutrition strategy includes iron and folic acid supplementation, high dose vitamin A supplementation (restricted only to areas where vitamin A deficiency is a substantial public health problem), the promotion of breastfeeding, fortification of foods with micronutrients, and healthy, diverse diets containing foods naturally rich in vitamins and minerals [73]. Indeed, diet plays a crucial role during pregnancy since it is the first source not only of micronutrients but also of macronutrients and other bioactive compounds that might affect the later health of offspring [10]. An adequate and balanced Indo-Mediterranean diet, rich in fruits, vegetables and legumes is recommended throughout the whole course of life and in particular in pregnancy and in early life [72]. Diets that exclude meat or other foods of animal origin are not recommended at these key stages of life. It is important to note that plant-based diets, such as vegetarian and vegan diets, are linked to vitamin and mineral deficiencies. The most critically deficient micronutrient is vitamin B12, but deficiencies in Ca, iron, iodine, Zn, and Se, essential amino acids, ω-3 long-chain polyunsaturated fatty acids and vitamins such as riboflavin and vitamin D, may also occur in diets free of meat, fish, and meat products [74]. The widespread Western dietary pattern must also warrant close attention. In addition to being characterized by excessive consumption of refined sugars, salt, and saturated fats, it is rich in ultra-processed foods that, while cheaper and quicker to prepare, have high energy density and low nutritional value. Moreover, by inducing a high degree of satiety, their consumption results in reduced intake of other nutrients with higher nutritional value, such as fresh fruits and vegetables, and meat/eggs, increasing the risk of developing not only obesity and NCDs but also micronutrient deficiencies [75]. Nutritional counselling and diet intervention are cornerstones of prenatal care during pregnancy, and high food quality and diversity, which are the basis of healthy nutrition, should be promoted [76].
Discussion
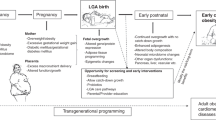
Individualized nutritional intervention strategies to achieve recommended serum micronutrients values throughout life and particularly during vulnerable phases, such as pregnancy and early life, are necessary for the maintenance of the overall metabolic health status of the population and in particular, the prevention of long-term NCDs, Fig. 2. A recent study sustained this concept using secondary data sources from 132 low- and middle-income countries and estimated a substantial impact on NCDs prevention by scaling up prenatal supplementation with iron and folic acid, multiple micronutrients, or calcium [14]. A large proportion of micronutrients also act as antioxidants or are essential cofactors for antioxidant enzymes, so they have a dual role in determining the health of pregnant women and their offspring in the short and long-term, as antioxidant deficiency in pregnancy may induce organ damage and impair embryonic development due to increased levels of ROS [77]. Prenatal supplementation with mixed antioxidant vitamins and minerals (Se, folic acid, vitamin C, and vitamin E) protected rat offspring from long-term cardiovascular damage [78]. Unfortunately, longitudinal data on humans are not currently available to confirm these promising findings and there is no strong evidence for recommending multivitamin supplementation in pregnancy nowadays [48].
The RDA for the micronutrients discussed in this narrative review and the recommendations for supplementation in the antenatal period are reported in Table 1.
Maternal micronutrient malnutrition continues to be an underestimated and threatening problem worldwide. Although adequate intakes in pregnancy of individual minerals are known, epidemiological data on pregnancy deficiencies of these minerals are largely unknown. In fact, the frequency and degree of mineral restriction in pregnancy remain currently unclear and this emphasizes how this issue is still underestimated [79]. Rarely a single micronutrient deficiency occurs alone; often more than one coexists [70, 80]. The long-term consequences of micronutrient deficiencies are not only seen at the individual level throughout the life span but also have a deleterious impact on social and economic development at the national level. In fact, it is important to consider the consequences of the micronutrient nutritional deficiencies perpetuated across generations [13]. Therefore, intervention in the first 1000 days of life is critical to breaking the cycle of malnutrition [13, 80].
Prevention is critical and needs of coordinated and sustainable efforts to implement nutrition intervention strategies to apply globally through the improvement of nutrition, supplementation, and fortification plans, diversified according to different geographic and economic areas [13, 80]. Nutritional counselling should always be included in gynaecological/obstetrical care for women from the periconceptional period onwards. In cases of increased risk of micronutrient deficiency, supplementation is indeed an effective and cost-efficient strategy to reduce both maternal and fetal adverse outcomes. A correct assessment of dietary intake and nutritional status will also substantially reduce the risk of micronutrient overload among these women, optimising dietary advice/supplementation with a view to personalising nutritional counselling. Although most micronutrients in pregnancy appear to have beneficial effects, caution and prior assessment of nutritional status are still necessary, given that adverse effects are reported in some cases of excess supplementation.
Conclusion
Suboptimal mineral intake from preconception to pregnancy increases the risk of pregnancy complications and metabolic health problems in the offspring, likely through an epigenetic link. Nevertheless, further investigations are needed to assess the prevalence of micronutrient deficiencies in different populations and to implement the assessment in pregnancy of micronutrient intake, to provide dietary changing and eventually supplementations. These findings would have extreme public health relevance because prevention by adequate nutritional counselling, supplementation or fortification of foods would consist of a simple and relatively inexpensive nutritional intervention that could affect the enormous economic burden of chronic NCDs.
References
Weihrauch-Blüher S, Schwarz P, Klusmann JH. Childhood obesity: increased risk for cardiometabolic disease and cancer in adulthood. Metabolism. 2019;92:147–52.
Wasniewska M, Pepe G, Aversa T, Bellone S, de Sanctis L, Di Bonito P, et al. Skeptical Look at the Clinical Implication of Metabolic Syndrome in Childhood Obesity. Children. 2023;10:735.
Noncommunicable diseases [Internet]. [cited 2022 Dec 25]. Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases.
Balbus JM, Barouki R, Birnbaum LS, Etzel RA, Gluckman PD, Grandjean P, et al. Early-life prevention of non-communicable diseases. Lancet. 2013;381:3–4.
Oestreich AK, Moley KH. Developmental and transmittable origins of obesity-associated health disorders. Trends Genet. 2017;33:399–407.
Bansal A, Simmons RA. Epigenetics and developmental origins of diabetes: correlation or causation? Am J Physiol Endocrinol Metab. 2018;315:E15–28.
Goyal D, Limesand SW, Goyal R. Epigenetic responses and the developmental origins of health and disease. J Endocrinol. 2019;242:T105–19.
Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–63.
Ling C, Rönn T. Epigenetics in human obesity and Type 2 diabetes. Cell Metab. 2019;29:1028–44.
Ford ND, Behrman JR, Hoddinott JF, Maluccio JA, Martorell R, Ramirez-Zea M, et al. Exposure to improved nutrition from conception to age 2 years and adult cardiometabolic disease risk: a modelling study. Lancet Glob Health. 2018;6:e875–84.
Lumey LH, Khalangot MD, Vaiserman AM. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932-33: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3:787–94.
Zhang Q, Sun X, Xiao X, Zheng J, Li M, Yu M, et al. The effect of maternal chromium status on lipid metabolism in female elderly mice offspring and involved molecular mechanism. Biosci Rep. 2017;37:BSR20160362.
Bailey RL, West KP Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66:22–33.
Blakstad MM, Fawzi WW, Castro MC, Thompson A, Arabi M, Danaei G. Scaling up prenatal nutrition could reduce the global burden of noncommunicable diseases in the next generation: a modeling analysis. Am J Clin Nutr. 2022;116:1291–302.
Wu Y, Zhang Q, Xiao X. The effect and potential mechanism of maternal micronutrient intake on offspring glucose metabolism: an emerging field. Front Nutr. 2021;8:813.
Venu L, Harishankar N, Krishna TP, Raghunath M. Maternal dietary vitamin restriction increases body fat content but not insulin resistance in WNIN rat offspring up to 6 months of age. Diabetologia. 2004;47:1493–501.
Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA. 2007;104:19351–6.
Saffari A, Shrestha S, Issarapu P, Sajjadi S, Betts M, Sahariah SA, et al. Effect of maternal preconceptional and pregnancy micronutrient interventions on children’s DNA methylation: findings from the EMPHASIS study. Am J Clin Nutr. 2020;112:1099–113.
Takaya J, Yamanouchi S, Kino J, Tanabe Y, Kaneko K. A calcium-deficient diet in dams during gestation increases insulin resistance in male offspring. Nutrients. 2018;10:1745.
Li P, Tang T, Chang X, Fan X, Chen X, Wang R, et al. Abnormality in maternal dietary calcium intake during pregnancy and lactation promotes body weight gain by affecting the gut microbiota in mouse offspring. Mol Nutr Food Res. 2019;63:e1800399.
Morley R, Carlin JB, Dwyer T. Maternal calcium supplementation and cardiovascular risk factors in twin offspring. Int J Epidemiol. 2004;33:1304–9.
Korhonen P, Tihtonen K, Isojärvi J, Ojala R, Ashorn U, Ashorn P, et al. Calcium supplementation during pregnancy and long-term offspring outcome: a systematic literature review and meta-analysis. Ann N. Y Acad Sci. 2022;1510:36–51.
Volpe SL. Magnesium, the metabolic syndrome, insulin resistance, and type 2 diabetes mellitus. Crit Rev Food Sci Nutr. 2008;48:293–300.
Venu L, Kishore YD, Raghunath M. Maternal and perinatal magnesium restriction predisposes rat pups to insulin resistance and glucose intolerance. J Nutr. 2005;135:1353–8.
Venu L, Padmavathi IJN, Kishore YD, Bhanu NV, Rao KR, Sainath PB, et al. Long-term effects of maternal magnesium restriction on adiposity and insulin resistance in rat pups. Obes (Silver Spring). 2008;16:1270–6.
Kostov K. Effects of magnesium deficiency on mechanisms of insulin resistance in Type 2 Diabetes: focusing on the processes of insulin secretion and signaling. Int J Mol Sci. 2019;20:1351.
Martín-Calvo N, Goni L, Tur JA, Martínez JA. Low birth weight and small for gestational age are associated with complications of childhood and adolescence obesity: systematic review and meta-analysis. Obes Rev. 2022;23:e13380.
Takaya J, Kaneko K. Small for gestational age and magnesium in cord blood platelets: intrauterine magnesium deficiency may induce metabolic syndrome in later life. J Pregnancy. 2011;2011:270474.
Huerta MG, Roemmich JN, Kington ML, Bovbjerg VE, Weltman AL, Holmes VF, et al. Magnesium Deficiency Is Associated With Insulin Resistance in Obese Children. Diabetes Care. 2005;28:1175–81.
Nadler JL, Buchanan T, Natarajan R, Antonipillai I, Bergman R, Rude R. Magnesium deficiency produces insulin resistance and increased thromboxane synthesis. Hypertension. 1993;21:1024–9.
Xu L, Li X, Wang X, Xu M. Effects of magnesium supplementation on improving hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes: a pooled analysis of 24 randomized controlled trials. Front Nutr. 2023;9:1020327.
Vincent JB. Elucidating a biological role for chromium at a molecular level. Acc Chem Res. 2000;33:503–10.
Mahdi GS. Chromium deficiency might contribute to insulin resistance, Type 2 diabetes mellitus, dyslipidaemia, and atherosclerosis. Diabetic Medicine, (1996). Wiley Online Library. Available from: https://onlinelibrary.wiley.com/doi/10.1002/(SICI)1096-9136(199604)13:4%3C389::AID-DIA65%3E3.0.CO;2-J.
Zhang Q, Xiao X, Zheng J, Li M, Yu M, Ping F, et al. Maternal chromium restriction modulates miRNA profiles related to lipid metabolism disorder in mice offspring. Exp Biol Med (Maywood). 2017;242:1444–52.
Zhang Q, Sun X, Xiao X, Zheng J, Li M, Yu M, et al. Maternal chromium restriction leads to glucose metabolism imbalance in mice offspring through insulin signaling and wnt signaling pathways. Int J Mol Sci. 2016;17:1767.
Suksomboon N, Poolsup N, Yuwanakorn A. Systematic review and meta-analysis of the efficacy and safety of chromium supplementation in diabetes. J Clin Pharm Ther. 2014;39:292–306.
Fukunaka A, Fujitani Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int J Mol Sci. 2018;19:476.
Olechnowicz J, Tinkov A, Skalny A, Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiolog Sci. 2018;68:19.
Jin S, Hu C, Zheng Y. Maternal serum zinc level is associated with risk of preeclampsia: a systematic review and meta-analysis. Front Public Health. 2022;10:968045.
Carducci B, Keats EC, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2021;3:CD000230.
Padmavathi IJN, Kishore YD, Venu L, Ganeshan M, Harishankar N, Giridharan NV, et al. Prenatal and perinatal zinc restriction: effects on body composition, glucose tolerance and insulin response in rat offspring. Exp Physiol. 2009;94:761–9.
Mendes Garrido Abregú F, Gobetto MN, Castañón A, Lucero D, Caniffi C, Elesgaray R, et al. Fetal and postnatal zinc restriction: sex differences in metabolic alterations in adult rats. Nutrition. 2019;65:18–26.
Stewart CP, Christian P, LeClerq SC, West KP, Khatry SK. Antenatal supplementation with folic acid + iron + zinc improves linear growth and reduces peripheral adiposity in school-age children in rural Nepal. Am J Clin Nutr. 2009;90:132–40.
Iannotti LL, Zavaleta N, León Z, Shankar AH, Caulfield LE. Maternal zinc supplementation and growth in Peruvian infants. Am J Clin Nutr. 2008;88:154–60.
Sanusi KO, Ibrahim KG, Abubakar B, Malami I, Bello MB, Imam MU, et al. Effect of maternal zinc deficiency on offspring health: the epigenetic impact. J Trace Elem Med Biol. 2021;65:126731.
Burk RF. Selenium, an antioxidant nutrient. Nutr Clin Care. 2002;5:75–9.
González de Vega R, Fernández-Sánchez ML, Fernández JC, Álvarez Menéndez FV, Sanz-Medel A. Selenium levels and Glutathione peroxidase activity in the plasma of patients with type II diabetes mellitus. J Trace Elem Med Biol. 2016;37:44–9.
WHO recommendations on antenatal care for a positive pregnancy experience [Internet]. [cited 2023 Jan 1]. Available from: https://www.who.int/publications/i/item/9789241549912.
Laureano-Melo R, Império GE, Kluck GEG, da Conceição RR, de Souza JS, Marinho BG, et al. Selenium supplementation during pregnancy and lactation promotes metabolic changes in Wistar rats’ offspring. Clin Exp Pharm Physiol. 2020;47:1272–82.
Ding D, Mou D, Zhao L, Jiang X, Che L, Fang Z, et al. Maternal organic selenium supplementation alleviates LPS induced inflammation, autophagy and ER stress in the thymus and spleen of offspring piglets by improving the expression of selenoproteins. Food Funct. 2021;12:11214–28.
Zeng MS, Li X, Liu Y, Zhao H, Zhou JC, Li K, et al. A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radic Biol Med. 2012;52:1335–42.
Li X, Chen H, Epstein PN. Metallothionein and catalase sensitize to diabetes in nonobese diabetic mice. Diabetes. 2006;55:1592–604.
Quezada-Pinedo HG, Cassel F, Duijts L, Muckenthaler MU, Gassmann M, Jaddoe VWV, et al. Maternal iron status in pregnancy and child health outcomes after birth: a systematic review and meta-analysis. Nutrients. 2021;13:2221.
Ferńandez-Real JM, Mcclain D, Review MM. Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of Type 2 diabetes. Diabetes Care. 2015;38:2169–76.
Ferrannini E. Insulin resistance, iron, and the liver. Lancet. 2000;355:2181–2.
Vanhees K, Vonhögen IGC, Van Schooten FJ, Godschalk RWL. You are what you eat, and so are your children: the impact of micronutrients on the epigenetic programming of offspring. Cell Mol Life Sci. 2014;71:271–85.
Stewart CP, Christian P, Schulze KJ, Arguello M, LeClerq SC, Khatry SK, et al. Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J Nutr. 2011;141:1912–7.
Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51:29–38.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Behere RV, Deshmukh AS, Otiv S, Gupte MD, Yajnik CS. Maternal vitamin B12 status during pregnancy and its association with outcomes of pregnancy and health of the offspring: a systematic review and implications for policy in India. Front Endocrinol (Lausanne). 2021;12:619176.
Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LYC. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285:2981–6.
Tanwar VS, Ghosh S, Sati S, Ghose S, Kaur L, Kumar KA, et al. Maternal vitamin B12 deficiency in rats alters DNA methylation in metabolically important genes in their offspring. Mol Cell Biochem. 2020;468:83–96.
Krishnaveni GV, Veena SR, Karat SC, Yajnik CS, Fall CHD. Association between maternal folate concentrations during pregnancy and insulin resistance in Indian children. Diabetologia. 2014;57:110–21.
Lapillonne A. Vitamin D deficiency during pregnancy may impair maternal and fetal outcomes. Med Hypotheses. 2010;74:71–5.
Gallo S, McDermid JM, Al-Nimr RI, Hakeem R, Moreschi JM, Pari-Keener M, et al. Vitamin D supplementation during pregnancy: an evidence analysis center systematic review and meta-analysis. J Acad Nutr Diet. 2020;120:898–924.e4.
Hrudey EJ, Reynolds RM, Oostvogels AJJM, Brouwer IA, Vrijkotte TGM. The association between maternal 25-Hydroxyvitamin D concentration during gestation and early childhood cardio-metabolic outcomes: is there interaction with pre-pregnancy BMI? PLoS One. 2015;10:e0133313.
Zhang H, Chu X, Huang Y, Li G, Wang Y, Li Y, et al. Maternal vitamin D deficiency during pregnancy results in insulin resistance in rat offspring, which is associated with inflammation and Iκbα methylation. Diabetologia. 2014;57:2165–72.
Villa CR, Chen J, Wen B, Sacco SM, Taibi A, Ward WE, et al. Maternal vitamin D beneficially programs metabolic, gut and bone health of mouse male offspring in an obesogenic environment. Int J Obes (Lond). 2016;40:1875–83.
Keller A, Ängquist L, Jacobsen R, Vaag A, Heitmann BL. A retrospective analysis of a societal experiment among the Danish population suggests that exposure to extra doses of vitamin A during fetal development may lower type 2 diabetes mellitus (T2DM) risk later in life. Br J Nutr. 2017;117:731–6.
Christian P, Stewart CP. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J Nutr. 2010;140:437–45.
Matthews KA, Rhoten WB, Driscoll HK, Chertow BS. Vitamin A deficiency impairs fetal islet development and causes subsequent glucose intolerance in adult rats. J Nutr. 2004;134:1958–63.
Danielewicz H, Myszczyszyn G, Dębińska A, Myszkal A, Boznański A, Hirnle L. Diet in pregnancy—more than food. Eur J Pediatr. 2017;176:1573–9.
Ambition and Action in Nutrition 2016–2025. Geneva: World Health Organization. 2017;(Licence: CC BY-NC-SA 3.0 IGO.).
Kristensen NB, Madsen ML, Hansen TH, Allin KH, Hoppe C, Fagt S, et al. Intake of macro- and micronutrients in Danish vegans. Nutr J. 2015;14:115.
Popkin BM, Barquera S, Corvalan C, Hofman KJ, Monteiro C, Ng SW, et al. Towards unified and impactful policies to reduce ultra-processed food consumption and promote healthier eating. Lancet Diabetes Endocrinol. 2021;9:462–70.
Lopes SO, Abrantes LCS, Azevedo FM, Morais N, de S, de, Morais D, et al. Food insecurity and micronutrient deficiency in adults: a systematic review and meta-analysis. Nutrients. 2023;15:1074.
Mistry HD, Williams PJ. The importance of antioxidant micronutrients in pregnancy. Oxid Med Cell Longev. 2011;2011:841749.
Franco M, do C, Ponzio BF, Gomes GN, Gil FZ, Tostes R, et al. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009;85:327–33.
Adams JB, Sorenson JC, Pollard EL, Kirby JK, Audhya T. Evidence-based recommendations for an optimal prenatal supplement for women in the U.S., part two: minerals. Nutrients. 2021;13:1849.
Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–51.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
AM performed the literature search and wrote the manuscript; AM, RG and CM discussed and edited the manuscript. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maguolo, A., Gabbianelli, R. & Maffeis, C. Micronutrients in early life and offspring metabolic health programming: a promising target for preventing non-communicable diseases. Eur J Clin Nutr 77, 1105–1112 (2023). https://doi.org/10.1038/s41430-023-01333-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-023-01333-4