Abstract
Endothelial dysfunction is closely linked to the development of atherosclerosis. This systematic review and meta-analysis reviewed the evidence on the effect of weight loss, achieved by dietary-based interventions, on biomarkers of endothelial function (EF). Two databases (Medline, Embase) were searched from inception until November 2022 for studies that met the following criteria: 1) adult subjects (≥ 18 years) without exclusion for health status, 2) dietary interventions for weight loss, and 3) measurements of changes in EF biomarkers. Random-effect meta-analysis and meta-regression were performed. Thirty-seven articles including 1449 participants were included in the systematic review. Study duration ranged from 3-52 weeks. Overall, weight loss significantly improved biomarkers of EF [standardised mean difference (SMD):0.65; 95%CI:0.49,0.81; P < 0.001;I2 = 91.9%]. Subgroup analyses showed weight loss significantly improved levels of E-selectin (P < 0.001), intercellular adhesion molecule-1 (ICAM-1) (P < 0.001), vascular cell adhesion molecule-1 (VCAM-1) (P < 0.001), nitrite/nitrate (NOx) (P < 0.001) and vascular endothelial growth factor (VEGF) (P < 0.001). Conversely, there was no significant improvement for von Willebrand Factor (vWF). Meta-regression analysis revealed that changes in EF biomarkers were not affected by age, BMI, quality of the studies or the amount of weight lost. A significant heterogeneity was observed for the effects of weight loss on changes in EF biomarkers. Dietary-induced weight loss may be associated with biomarkers changes indicating an improvement of EF, and it may represent a potential strategy to reduce atherosclerotic risk.
Similar content being viewed by others
Introduction
The endothelium plays a crucial role in maintaining vascular tone and promoting an atheroprotective environment via the synthesis and release of a multitude of vasoactive factors including for example nitric oxide (NO) or endothelin [1]. Endothelial function is typically assessed via measurement of flow-mediated dilation using ultrasound [2]. However, this technique is highly dependent upon the skill of the operator and it can be influenced by physiological variations such as shear stimulus or health status of the participant [2]. As an alternative, endothelial function can also be assessed by measuring the circulating concentrations of specific molecules which can give an indication of the integrity of the endothelium [3]. A loss of endothelial integrity is linked to an increased permeability, modification and trapping of circulating lipoprotein particles and inflammatory cells in the sub-endothelial space favouring the initiation of the atherosclerosis process [4]. Hence, endothelial dysfunction represents one of the earliest detectable changes in the development of atherosclerotic plaques and a significant risk factor for cardiovascular diseases [5].
Endothelial dysfunction is linked to increased levels of reactive oxygen species (ROS), pro-inflammatory factors and a reduction in NO bioavailability [6]. Increased generation of ROS has been linked to increased expression of intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin [7], which are involved in the formation of atherosclerotic plaques [8]. Under inflammatory conditions, endothelial cells are activated and release E-selectin and ICAM-1 [9]. Unlike ICAM-1 and VCAM-1, E-selectin is expressed only on endothelial cells. E-selectin attracts leukocytes to the site of injury where they are able to exert their effects against the infection [10]. A reduction in NO production, which occurs in endothelial dysfunction, leads to the increased expression of ICAM-1, VCAM-1, and E-selectin. One of the functions of VCAM-1 is to allow for the attachment of monocytes and lymphocytes to the endothelium and increased levels of VCAM-1 is thought to be an indicator of endothelial dysfunction [11]. von Willebrand Factor (vWF) has a key role in haemostasis and most plasma vWF is produced from endothelial cells [3]. vWF binds to factor VIII which is an essential blood clotting protein and upon injury to blood vessels, it interacts with factor IXa in the coagulation cascade, which eventually leads to thrombin cleaving fibrinogen into fibrin to form the blood clot [12].
Obesity is a growing problem with worldwide obesity rates almost tripling in the last few decades (WHO) [13]. Individuals living with obesity are more likely to be at risk of developing co-morbidities such as hypertension and type 2 diabetes mellitus [14]. Specifically, patients with excess visceral adipose tissue, are at a greater risk of developing endothelial dysfunction which is considered as a key early step in the pathogenesis of atherosclerosis, metabolic and vascular disorders [14, 15]. Weight loss has been shown to have a beneficial impact on endothelium-dependant vasodilation [16] and consequent reduction of several co-morbidities including cardiovascular disease [17], type 2 diabetes mellitus [18, 19].
A previous systematic review and meta-analysis conducted in 2020 [20] looked at the effects of weight loss achieved by bariatric surgery on biomarkers of endothelial function; the review found reduced ICAM-1 and E-selectin but not VCAM-1 concentrations after weight loss. It is possible that similar effects may occur with more conservative weight reduction strategies, such as via dietary intervention. However, this has not been systematically evaluated to date. Therefore, this systematic review aimed to evaluate for the first time the evidence on the effect of weight loss, achieved by dietary-based weight loss interventions, on biomarkers of endothelial function.
Materials and methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) guidelines [21]. The review is registered with PROSPERO: CRD42021284762.
Literature Search
Two databases (Medline and Embase) were searched to identify all relevant studies from inception through to November 2022. The primary search was carried out by the principal investigator (MS) and restricted for English language, type of study and study population (i.e., animals). Manual searching for further relevant studies was also performed to identify any articles missed from the initial search. Predefined search terms included ((Weight los* or Calori* Restrict* or CR or fasting or dieting a or low calorie diet or LCD or VLCD or time restricted eating) and (nitrate or nitrite or endoglin or endocan or endothelial microparticles or angiopoietin or von Willebrand factor or selectin or tissue plasminogen activator or tPA or vWF or EMPs or endothelin or ET-1 or endothelial progenitor cells or EPCs or vascular endothelial growth factor or VEGF or Thromboglobulin or V-CAM or I-CAM or PECAM or cadherin or nectin or endosialin or endomucin)).mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kf, fx, dq, nm, ox, px, rx, an, ui, sy]. The search algorithm is provided in the Online Supplementary Material.
Study selection
Studies were eligible for inclusion based on the following criteria: 1) the study design was a clinical intervention (randomised and non-randomised) involving human subjects. No further exclusion criteria were applied regarding whether the studies were placebo controlled, double-blinded or crossover. Studies that had an observational design were excluded; 2) the studies involved an adult population aged ≥18 years with no further exclusion criteria applied regarding health, smoking status or body size; 3) the intervention in the studies involved weight loss achieved by caloric restriction or caloric restriction combined with exercise only with an appropriate control group. Studies involving weight loss achieved by surgical or pharmacological methods were excluded. No further exclusion criteria were applied regarding the type of caloric restriction (e.g., intermittent fasting, very low-calorie diet (VLCD) etc.) and 4) the outcome of the studies reported changes in measurements of biomarkers of endothelial function. If measurements of biomarkers were missing from either the baseline or at the end of the study the article was excluded as an effect size could not be calculated.
Two reviewers independently (RM, ZA) screened the titles and abstracts of the retrieved articles to assess eligibility for inclusion. If consensus was reached between the two reviewers, articles were moved to the next stage (full text screening). Full texts of the selected articles were then critically appraised according to the inclusion/exclusion criteria to develop the final list of articles to be included in the systematic review and meta-analysis. If consensus was not reached, differences were resolved by a third reviewer (MS) at each stage.
Data extraction and quality assessment
Data extraction was completed by two independent reviewers (RM, ZA) with a third reviewer (MS) checking for inaccuracies. Information extracted included author, year of publication, country, population (health status, age, baseline weight and baseline BMI), study design, type of weight loss intervention, duration of weight loss, amount of weight loss, biomarkers of endothelial function that were measured and the change in measurement of the endothelial biomarker from the start of the study to the end. Information on any conflicts of interest was also extracted. The quality of the included studies was assessed using the modified Jadad scale with a total of 8 questions [22]. For every study, for each of the 8 questions, one point was scored if the answer to the question was yes and 0 points were scored if the answer was no. For the question about whether the study had blinding, 0.5 points were scored if the study was single blinded, and 1 point was scored if the study was double blinded. Studies were described as poor quality if they scored less than 3 points and a score greater ≥ 3 was regarded as a high quality trial [23].
Meta-analysis
The primary outcomes of the meta-analysis were changes in concentrations of endothelial function biomarkers after weight loss. Random effect models were applied to address the heterogeneity related to differences in study design and application of different biomarkers of endothelial function. In addition, some studies used several biomarkers to assess changes in endothelial function as reported in the summary tables. This may lead to reduced independence of measurements and over-estimation of the effect size derived from the meta-analysis. These methodological aspects were considered in the analysis by averaging the standardised effect sizes for each trial with the aim of providing a more conservative estimate of the effect size. The paired study design of cross-over studies was taken into account for the calculation of the effect size. Forest plots were created to summarise and illustrate the overall effects of weight loss on changes in biomarkers of endothelial function. The meta-analysis was conducted using Comprehensive Meta-Analysis software (Version 2, Biostat, Engelwood, New Jersey). Results are described as standardized mean differences (SMDs) and 95% confidence intervals (95%CI). If data were not available in the main text or in tables, figures were used to extract the information.
Sensitivity analyses were performed to investigate whether weight loss was associated with specific changes of single biomarkers if reported in at least five independent studies. Data was entered as original, non-standardised raw values to provide a more meaningful assessment of the changes associated with weight loss. A random-effect meta-regression model was applied to examine the associations between effect sizes for overall standardised endothelial function and age, BMI, Jadad quality score and amount of weight lost. Funnel plots and - Egger’s regression tests were performed to evaluate the publication bias [24]. Heterogeneity was assessed by using Cochrane Q statistics; P > 0.1 indicates significant heterogeneity. The I2 test was utilised to assess heterogeneity across trials where a value <25% indicates low risk, 25-75% indicates moderate risk, and >75% indicates a high risk [25]. Cohen’s kappa (κ) coefficients were calculated to evaluate the inter-rater reliability between the two independent reviewers during the titles and abstracts selection phase.
Results
Search results
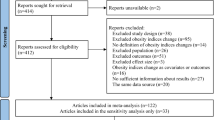
Figure 1 outlines the procedure for study selection. As shown, the initial databases search identified 16,183 studies after removal of 1375 duplicates. Title and abstract screening identified 69 articles for full-text review of which 32 articles were excluded (See Fig. 1 for the list of reasons). No additional studies were identified which met the inclusion criteria. Therefore, 37 studies were included in the review. Five of the 37 studies only had an abstract available [26,27,28,29,30] and therefore these studies were not included the meta-analysis which therefore was based on 32 studies. The two independent reviewers showed a high degree of agreement during the titles and abstracts selection (κ = 0.79, p < 0.001).
Study characteristics
Of the 37 studies included in the qualitative synthesis, there were a total of 1449 participants with a median of 26.5 participants and a range of 7–131 participants per study. In total, 21 of the studies were randomised trials with 3 studies had a crossover design [27, 31,32,33,34,35]. Three studies did not state the mean age of the participants [27,28,29] and 1 study only reported the age range of its participants, which was 35–63 years [36]. From the remaining studies, the overall median age was 45.1 years with a range of 31–58 years. The duration of the interventions ranged from 3 weeks to 52 weeks (Table 1). From the 37 studies, 27 looked at the impact of caloric restriction on obese patients [26, 27, 29, 31, 33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58], 4 in patients with type 2 diabetes mellitus (T2DM) [40, 41, 45, 53], 3 in patients with metabolic syndrome [46, 48, 59] and 1 in postmenopausal women [44]. Multiple variations of calorie restriction diets were used in the included papers ranging from very low-calorie diets (VLCD) to the Mediterranean diet and low-fat diets. Sixteen studies involved a LCD to achieve weight loss [29, 30, 34, 36, 40,41,42, 44, 47, 49, 51, 52, 55, 59,60,61] while six studies utilised a VLCD [26, 33, 35, 38, 45, 62] and another 4 studies used either a low carbohydrate or low fat diet (or a combination of both) [32, 53, 54, 57]. The majority of the studies measured the effects of weight loss on the biomarker’s ICAM-1 and the selectins (E-selectin and P-selectin) [26, 28, 29, 32,33,34, 37,38,39,40, 42, 45,46,47, 51,52,53,54, 57, 58] with 12 studies measuring VCAM-1 [26, 33, 35, 37, 40, 42, 45,46,47, 54, 57, 58] and 6 studies looking at NOx (nitrate+nitrite) [39, 55, 56, 59, 61, 62]. The number of studies investigating the effects of weight loss on each individual biomarker of endothelial function is reported in Fig. S1 of the online supplementary material. There was a high amount of heterogeneity regarding the method used to measure specific biomarkers of endothelial function. The most common method was the use of enzyme-linked immunosorbent assays (ELISAs) with a total of 16 studies using this procedure [32,33,34,35,36,37, 40, 42, 44,45,46, 51,52,53, 57, 58].
Meta-analysis
Overall, after meta-analysis of the 32 studies, weight loss significantly improved biomarkers of endothelial function [standardised mean difference (SMD): 0.65; 95%CI:0.49,0.81; P < 0.001; Fig. 2A]. There was significant heterogeneity among the studies (I2 = 91.9%, P < 0.001).
Overall results are showed as standardised differences in means (SMDs) and 95% confidence intervals (CI) and positive SMDs indicate an improvement in endothelial function (A). Data in the forest-plots for the individual biomarkers were reported as difference in means and the direction of the effect indicates the changes observed in the raw values for each biomarker following weight loss (B–F). ICAM-1, intercellular adhesion molecule-1; VCAM-1,vascular cell adhesion molecule-1; NOx, nitrite/nitrate; vWF, von Willebrand Factor. The overall effect size of weight loss on biomarkers of endothelial function (EF) was standardised to account for differences in units of measurements between biomarkers and combined in studies that measured multiple biomarkers of EF (see methods for more details).
Seventeen studies described the effect of weight loss on the biomarker intercellular adhesion molecule-1 (ICAM-1) [32,33,34,35, 37,38,39,40, 42, 45,46,47, 51, 53, 54, 57, 58]. Meta-analysis of these studies indicated a significant decreased in levels of ICAM-1 (mean difference: -18.9 ng/mL; 95% CI: −23.8, −13.9; P < 0.001; Fig. 2B and Table S1 of the online supplementary material). A total of 11 studies examined the effect of weight loss on E-selectin [37,38,39,40, 42, 46, 47, 52,53,54, 57]. Meta-analysis of these studies revealed a significant decrease in levels of E-selectin (mean difference: −7.9 ng/mL; 95% CI: -5.9, −10.0; P < 0.001; Fig. 2C and Table S1 of the online supplementary material).
The meta-analysis also found a significant change in levels of VCAM-1 (mean difference: −23.4 ng/mL; 95% CI: −0.1, −46.5; P = 0.04, Fig. 2D and Table S1 of the online supplementary material), nitrite/nitrate (NOx) (mean difference: 5.21 µM/L; 95% CI: 2.68, 7.74; P < 0.001 Fig. 2F and Table S1 of the online supplementary material) and vascular endothelial growth factor (VEGF) (mean difference: −22.90 pg/mL; 95% CI: −28.19, −17.63; P < 0.001, Table S1 of the online supplementary material). Weight loss did not have a significant effect on the levels of vWF (mean difference: −7.7%; 95% CI: −18.4, +2.8; P = 0.15; Fig. 2E and Table S1).
Study quality and publication bias
Overall, the quality of the included studies was moderate with risk of bias scores ranging from 1-7 with a median of 3 (Table S2 of the online supplementary material). Nealy half (47%) of the studies had a total score of <3 [26,27,28,29,30, 32, 36,37,38, 43, 51, 55, 58,59,60, 62], indicating poor quality and a high risk of bias. Fifteen studies were rating as having high quality with scores >3 [31, 33,34,35, 41, 44,45,46,47,48,49, 53, 54, 57, 61]. Of the 37 studies, 23 reported a randomisation procedure [27,28,29, 31,32,33,34,35, 40,41,42, 44,45,46,47,48,49,50, 52,53,54, 56, 61]. Only 9% of reported the approach used to assess adverse effects [45, 53, 57]. Upon visual inspection of the funnel plot (Fig. S2 of the online supplementary material), there were 15 studies with wider effect estimates and publication bias was subsequently confirmed by the Egger’s regression test (P = 0.02).
Meta-regression analysis
The meta-regression analysis showed that there was no significant association between the overall effect size with age (P = 0.31), BMI (P = 0.10), Jadad quality score (P = 0.74) or the amount of weight lost (P = 0.54).
Discussion
This review assessed the effect of weight loss, achieved by dietary-based interventions, on biomarkers of endothelial function. Overall, weight loss significantly improved levels of E-selectin, ICAM-1, NOx, VCAM-1 and VEGF. However, there was no significant improvement in levels of vWF.
The effect of weight loss on endothelial function were previously investigated in a previous meta-analysis which found a significant increase in flow mediated dilation by 3.29% after an average weight loss of 8.6 kg [63]. Another systematic review and meta-analysis [20] observed an improvement in levels of ICAM-1 and E-selectin following weight loss achieved by bariatric surgery. These results agree with changes in ICAM-1 and E-selectin observed in this review. Here we found that weight loss significantly improved levels of VCAM-1. However previous studies testing the effect of weight loss on VCAM-1 have conflicting results. While Seyyedi et al. [20] reported no improvement in VCAM-1 levels after weight loss, other studies have reported a decrease in levels of VCAM-1 [64, 65]. However, the study by Seyyedi et al. used bariatric surgery as a weight loss strategy and therefore results may be not comparable to the effects reported in this meta-analysis due to the potential effect of the surgical procedures on circulating levels of VCAM-1.
Adhesion molecules such as ICAM-1, VCAM-1 and E-selectin play a significant role in inflammation [66]. While their usual function is to bind to leukocytes and play a part in the process of leukocyte extravasation [67], they can also play a crucial role in endothelial dysfunction and the pathogenesis of atherosclerosis [68]. During inflammation, these adhesion molecules become upregulated leading to increased leukocyte migration across the vessel wall [69]. The process of leukocyte migration is key in the development of atherogenesis and adhesion molecules expressed on the surface of endothelial cells are central to this process by causing damage to the endothelium and mediating the leukocyte migration [70]. The exact mechanism by which weight loss reduces levels of adhesion molecules is uncertain [20, 71]. One possible mechanism may be through the increase in NO availability. This review found that weight loss achieved by calorie restriction significantly increased levels of NOx (nitrate plus nitrite), which are the end products of the metabolism of nitric oxide (NO) [72]. In a study by Marfella et al. [73], the impact of increased NO availability on adhesion molecules was investigated in diabetic patients. Following L-arginine supplementation, the substrate for NO, it was found that levels of ICAM-1 in the plasma subsequently decreased. The results of this study suggest NO availability has a mechanistic link with adhesion molecules [74]. Therefore, as weight loss significantly increases NO levels, as shown here, it is possible that this accounts for one of the mechanisms through which levels of adhesion molecules decrease following weight loss. Another possible mechanism through which weight loss may decrease cell adhesion molecules could be through the effect on insulin secretion and sensitivity. Previous studies have shown the beneficial impact of weight loss on insulin secretion and sensitivity [75,76,77]. There is also a relationship between insulin resistance and cell adhesion molecules. In a study by Chen et al. [78], the link between insulin resistance and E-selectin, ICAM-1 and VCAM-1 was investigated in healthy individuals. The presence of a correlation between insulin resistance with both ICAM-1 and E-selectin was observed. Hence, it is possible that this accounts for one of the mechanisms through which adhesion molecule levels improve following weight loss.
A significant improvement in levels of VEGF following a period of weight loss was observed. VEGF plays a critical role in angiogenesis, the process in which new blood vessels are formed, beginning in utero and continuing to take place throughout life [79]. The generation, migration and formation of an endothelial cell tube structure occurs in the early steps of angiogenesis with the process kickstarting under hypoxic conditions where tissues sense the low levels of oxygen and require new blood vessel formation to meet their relevant metabolic needs [79, 80]. Tio et al. [81] showed that, following treatment with VEGF gene therapy, upregulation of the endothelial gene NO synthase was discovered, reducing endothelial dysfunction. This is likely due to the subsequent increased production of NO and its relevant vasodilatory effects. However, Inoue et al. [82] concluded that higher levels of VEGF may have a deleterious effect. Compared to normal coronary arteries, the study found that coronary arteries which contained atherosclerotic plaques contained consistently higher levels of VEGF and there was also a substantial level of VEGF mRNA present in the atherosclerotic plaques. A possible reason for this may be due to the fact that higher levels of VEGF causes increased and excessive proliferation of endothelial cells and abnormal thickening of the carotid intima-media, which itself is a predictive marker for atherosclerosis [83]. The present review found that weight loss decreased VEGF levels on average by 22.9 pg/ml. These results are in line with other studies [84, 85].
No significant improvement in vWF was found following a period of weight loss. This is in line with the findings of the studies included in the meta-analysis with only 2 out of the 6 studies observing a significant improvement in levels of vWF. One of the 6 studies in the meta-analysis had an unusually large effect size [39], initially suggesting this may be the reason for the insignificant result. However, upon removal of this study from the meta-analysis, it was found there was still no significant improvement in vWF levels (p = 0.14, data not shown), indicating that the study with the large effect size was not the sole reason for the overall result. In a study by Primrose et al. [86], the effects of weight loss, achieved by bariatric surgery, on measurements of fibrinolytic and haemostatic factors including vWF was assessed. The study noted no significant changes in levels of vWF following the intervention period. While these results agree with results obtained in this review, it is important to note the small sample size of 19 patients in the study.
Strengths and limitations
To our knowledge, this is the first systematic review and meta-analysis looking at the effects of dietary-based weight loss interventions on the biomarkers of endothelial function. This study utilised a total number of 1449 participants from 37 studies. The large sample size achieved supports the reliability and validity of the results obtained. The health status of the participants ranged from healthy to metabolically impaired (i.e., morbid obesity, type 2 diabetes mellitus and metabolic syndrome), thus increasing the representativeness of the results. Studies also applied different weight loss strategies indicating an overall consistency of the beneficial effects of weight loss on endothelial function biomarkers.
There were some limitations. Although each study used calorie restriction as the primary method of weight loss, multiple studies included exercise as a secondary method of achieving weight loss. This is likely to have affected the results obtained for these studies as it is difficult to estimate how much of the improvements in the biomarkers were due to calorie restriction only and not exercise. Nonetheless, certain studies included two participant groups: a group undergoing calorie restriction only and a group doing calorie restriction combined with exercise. For these studies, results were only extracted from the group undergoing calorie restriction exclusively. There was also significant heterogeneity across the studies likely due to the differences in study design, baseline weight and BMI, study duration, health status of the participants and gender distributions. Further, studies with a short duration [26, 43, 58, 61] are likely to have not occurred for long enough to notice a significant change in biomarker levels whereas studies with a very long duration [37, 44, 54] are likely to have experienced a greater change in results compared to studies of an average duration. The calculation of the standardised means of the changes in endothelial function biomarkers allowed the evaluation of the effect of weight loss on a combined estimate of different markers of endothelial function. This approach may have limitations related to the different functional roles that biomarkers may have within the endothelium. However, a similar approach has been also used in other studies, which have showed a significant association of the compositive scores of endothelial function markers with measures of cardiovascular risk and mortality [87,88,89]. There was inconsistent reporting across studies on macronutrient composition of the diets and composition of weight lost (i.e., proportion of body mass lost as fat and lean) which did not allow for an investigation of their association with effect size. Changes in insulin and inflammatory markers could be causal mediators of the links between weight loss and changes in endothelial markers; however, the lack of consistent reporting across studies of data on these markers did not allow for an investigation of their potential mediating roles. Overall, the quality of the included articles was moderate. However, it should be noted that blinding is often difficult to achieve in studies that deal with a dietary intervention as it is often impractical to conceal from the participants. The high heterogeneity and significant publication bias may demand for a cautious interpretation of the findings, which could be attributed to differences in study design and populations, weight loss approaches and methods to measure EF biomarkers.
Conclusions
The present systematic review and meta-analysis revealed that diet-induced weight loss may improve biomarkers of endothelial function, but the effects may require further verification given the high heterogeneity and bias present across studies. More research is required into recently discovered endothelial function biomarkers such as endothelial microparticles and endothelial progenitor cells.
References
Galley HF, Webster NR. Physiology of the endothelium. Br J Anaesth. 2004;93:105–13. https://doi.org/10.1093/bja/aeh163.
Chia PY, Teo A, Yeo TW Overview of the Assessment of Endothelial Function in Humans. Front Med. 2020; 7. https://doi.org/10.3389/fmed.2020.542567.
Goncharov NV, Nadeev AD, Jenkins RO, Avdonin PV. Markers and Biomarkers of Endothelium: When Something Is Rotten in the State. Oxid Med Cell Longev. 2017;2017:9759735. https://doi.org/10.1155/2017/9759735.
Gimbrone MA,Jr., García-Cardeña G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circulation Res. 2016;118:620–36. https://doi.org/10.1161/CIRCRESAHA.115.306301.
Mudau M, Genis A, Lochner A, Strijdom H. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovascular J Afr. 2012;23:222–31. https://doi.org/10.5830/cvja-2011-068.
Sun H-J, Wu Z-Y, Nie X-W, Bian J-S Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front Pharmacol. 2020; 10. https://doi.org/10.3389/fphar.2019.01568.
Yuyun MF, Ng LL, Ng GA. Endothelial dysfunction, endothelial nitric oxide bioavailability, tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular disease. Where are we with therapy? Microvasc Res. 2018;119:7–12. https://doi.org/10.1016/j.mvr.2018.03.012.
Varona JF, Ortiz-Regalón R, Sánchez-Vera I, López-Melgar B, García-Durango C, Castellano Vázquez JM, et al. Soluble ICAM 1 and VCAM 1 Blood Levels Alert on Subclinical Atherosclerosis in Non Smokers with Asymptomatic Metabolic Syndrome. Arch Med Res. 2019;50:20–28. https://doi.org/10.1016/j.arcmed.2019.05.003.
Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, et al. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology. 1992;77:543–9.
Roldán V, Marín F, Lip GY, Blann AD. Soluble E-selectin in cardiovascular disease and its risk factors. A review of the literature. Thromb Haemost. 2003;90:1007–20. https://doi.org/10.1160/th02-09-0083.
Schram MT, Stehouwer CD. Endothelial dysfunction, cellular adhesion molecules and the metabolic syndrome. Horm Metab Res. 2005;37:49–55. https://doi.org/10.1055/s-2005-861363.
Chavin SI. Factor VIII: structure and function in blood clotting. Am J Hematol. 1984;16:297–306. https://doi.org/10.1002/ajh.2830160312.
World Health Organisation. Obesity and overweight. In: WHO, 2021.
Piché ME, Tchernof A, Després JP. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ Res. 2020;126:1477–1500. https://doi.org/10.1161/circresaha.120.316101.
Sanches E, Topal B, Proczko M, Stepaniak PS, Severin R, Philips SA, et al. Endothelial function in obesity and effects of bariatric and metabolic surgery. Expert Rev Cardiovasc Ther. 2020;18:343–53. https://doi.org/10.1080/14779072.2020.1767594.
Sasaki S, Higashi Y, Nakagawa K, Kimura M, Noma K, Sasaki S, et al. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension*. Am J Hypertension. 2002;15:302–9. https://doi.org/10.1016/s0895-7061(01)02322-6.
Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004;101:6659–63. https://doi.org/10.1073/pnas.0308291101.
Anderson JW, Konz EC. Obesity and disease management: effects of weight loss on comorbid conditions. Obes Res. 2001;9:326s–334s. https://doi.org/10.1038/oby.2001.138.
Ryan DH, Yockey SR. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr Obes Rep.2017;6:187–94. https://doi.org/10.1007/s13679-017-0262-y.
Seyyedi J, Alizadeh S. Effect of Surgically Induced Weight Loss on Biomarkers of Endothelial Dysfunction: a Systematic Review and Meta-Analysis. Obes Surg. 2020;30:3549–60. https://doi.org/10.1007/s11695-020-04710-1.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. https://doi.org/10.1136/bmj.b2700.
Clark HD, Wells GA, Huët C, McAlister FA, Salmi LR, Fergusson D, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials. 1999;20:448–52. https://doi.org/10.1016/s0197-2456(99)00026-4.
Cioffi I, Farella M. Quality of randomised controlled trials in dentistry. Int Dent J. 2011;61:37–42. https://doi.org/10.1111/j.1875-595X.2011.00007.x.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. https://doi.org/10.1136/bmj.315.7109.629.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. https://doi.org/10.1136/bmj.327.7414.557.
Bosanská L, Lacinová Z, Roubícek T, Mráz M, Bártlová M, Dolezalová R, et al. The influence ofvery-low-calorie diet on soluble adhesion molecules and their gene expression in adiposetissue of obese women. Cas Lek Cesk. 2008;147:32–37.
Darakhshan F, Pelloux V, Rouault C, Laromiguiere M, Debrus G, Massiera F et al. Effect of a high-protein-low-glycaemic-index hypocaloric diet on adiposity markers, cardiovascular and metabolic risk factors: a randomised controlled trial. Diabetes 2010;1772–P.
Ewa Kwiecinska ES-G, Borkowska A, Saryusz-Wolska M, Pawlowski M, Loba J, Czupryniak L. Integrated Physiology/Obesity. Diabetes. 2012;61:A688–A719. https://doi.org/10.2337/db12-2763-2907.
Kitabchi AE SFB, McDaniel K, et al. Effect of dietary macronutrients on oxidative stress, cardiovascular risk factors, and insulin sensitivity in obese, non-diabetic, premenopausal women. Diabetologia 2011; https://doi.org/10.1007/s00125-011-2276-4.
Lee PSS, Naseer F, Lim SL, Khoo EY, Yeo TC, Richards AM, et al. Abstract 15421: Effect of Weight Loss Intervention Program on Cardiac Function, Endothelium Progenitor Cells and Microparticles in Mildly Obese Asians. Circulation. 2016;134:A15421–A15421. https://doi.org/10.1161/circ.134.suppl_1.15421.
Rizkalla SW, Prifti E, Cotillard A, Pelloux V, Rouault C, Allouche R, et al. Differential effects of macronutrient content in 2 energy-restricted diets on cardiovascular risk factors and adipose tissue cell size in moderately obese individuals: a randomized controlled trial. Am J Clin Nutr. 2012;95:49–63. https://doi.org/10.3945/ajcn.111.017277.
Sharman MJ, Volek JS. Weight loss leads to reductions in inflammatory biomarkers after a very-low-carbohydrate diet and a low-fat diet in overweight men. Clin Sci (Lond). 2004;107:365–9. https://doi.org/10.1042/cs20040111.
Joris PJ, Plat J, Kusters YH, Houben AJ, Stehouwer CD, Schalkwijk CG, et al. Diet-induced weight loss improves not only cardiometabolic risk markers but also markers of vascular function: a randomized controlled trial in abdominally obese men. Am J Clin Nutr. 2017;105:23–31. https://doi.org/10.3945/ajcn.116.143552.
Mashayekhi M, Beckman JA, Nian H, Garner EM, Mayfield D, Devin JK et al. Comparative effects of weight loss and incretin-based therapies on vascular endothelial function, fibrinolysis and inflammation in individuals with obesity and prediabetes: A randomized controlled trial. Diabetes, Obesity Metabol. 2022; n/a. https://doi.org/10.1111/dom.14903.
Sánchez E, Santos MD, Nuñez-Garcia M, Bueno M, Sajoux I, Yeramian A et al. Randomized Clinical Trial to Evaluate the Morphological Changes in the Adventitial Vasa Vasorum Density and Biological Markers of Endothelial Dysfunction in Subjects with Moderate Obesity Undergoing a Very Low-Calorie Ketogenic Diet. Nutrients 2021; 14. e-pub ahead of print 2022/01/12; https://doi.org/10.3390/nu14010033.
Maeda S, Miyaki A, Kumagai H, Eto M, So R, Tanaka K, et al. Lifestyle modification decreases arterial stiffness and plasma asymmetric dimethylarginine level in overweight and obese men. Coron Artery Dis. 2013;24:583–8. https://doi.org/10.1097/MCA.0b013e3283647a99.
Sag SJM, Strack C, Zeller J, Mohr M, Loew T, Lahmann C, et al. Successful weight loss reduces endothelial activation in individuals with severe obesity participating in a multimodal weight loss program. Obes Res Clin Pr. 2021;15:249–55. https://doi.org/10.1016/j.orcp.2021.03.013.
López-Domènech S, Martínez-Herrera M, Abad-Jiménez Z, Morillas C, Escribano-López I, Díaz-Morales N, et al. Dietary weight loss intervention improves subclinical atherosclerosis and oxidative stress markers in leukocytes of obese humans. Int J Obes. 2019;43:2200–9. https://doi.org/10.1038/s41366-018-0309-5.
Korybalska K, Luczak J, Swora-Cwynar E, Kanikowska E, Czepulis N, Kanikowska D, et al. Weight Loss-Dependent And -Independent Effects Of Moderate Calorie Restriction On Endothelial Cell Markers In Obesity. J Physiol Pharmacol. 2017;68:597–608.
Abd El-Kader SM, Al-Jiffri OH, Neamatallah ZA, AlKhateeb AM, AlFawaz SS. Weight reduction ameliorates inflammatory cytokines, adipocytokines and endothelial dysfunction biomarkers among Saudi patients with type 2 diabetes. Afr Health Sci. 2020;20:1329–36. https://doi.org/10.4314/ahs.v20i3.39.
Abd El-Kader SMA-JO. Coagulation, fibrinolytic and cytokines parameters response to weight reduction in obese subjects. Eur J Gen Med. 2018;15:27–32.
Abd El-Kader SM, Al-Jiffri OH. Impact of weight reduction on insulin resistance, adhesive molecules and adipokines dysregulation among obese type 2 diabetic patients. Afr Health Sci. 2018;18:873–83. https://doi.org/10.4314/ahs.v18i4.5.
Firszt-Adamczyk A, Ruszkowska-Ciastek B, Adamczyk P, Szafkowski R, Firszt M, Ponikowska I, et al. Effect of a 3-Week Low-Calorie Diet and Balneological Treatment on Selected Coagulation Parameters in Morbidly Obese Patients. Adv Clin Exp Med. 2016;25:755–61. https://doi.org/10.17219/acem/42414.
Duggan C, Tapsoba JDD, Wang C-Y, McTiernan A. Dietary Weight Loss and Exercise Effects on Serum Biomarkers of Angiogenesis in Overweight Postmenopausal Women: A Randomized Controlled Trial. Cancer Res. 2016;76:4226–35. https://doi.org/10.1158/0008-5472.CAN-16-0399.
Berk KA, Oudshoorn TP, Verhoeven AJM, Mulder MT, Roks AJM, Dik WA, et al. Diet-induced weight loss and markers of endothelial dysfunction and inflammation in treated patients with type 2 diabetes. Clin Nutr ESPEN. 2016;15:101–6. https://doi.org/10.1016/j.clnesp.2016.06.011.
Egert S, Baxheinrich A, Lee-Barkey YH, Tschoepe D, Wahrburg U, Stratmann B. Effects of an energy-restricted diet rich in plant-derived α-linolenic acid on systemic inflammation and endothelial function in overweight-to-obese patients with metabolic syndrome traits. Br J Nutr. 2014;112:1315–22. https://doi.org/10.1017/s0007114514002001.
Torres MRSG, Sanjuliani AF. Effects of weight loss from a high-calcium energy-reduced diet on biomarkers of inflammatory stress, fibrinolysis, and endothelial function in obese subjects. Nutrition. 2013;29:143–51. https://doi.org/10.1016/j.nut.2012.05.012.
Fernández JM, Rosado-Álvarez D, Da Silva Grigoletto ME, Rangel-Zúñiga OA, Landaeta-Díaz LL, Caballero-Villarraso J, et al. Moderate-to-high-intensity training and a hypocaloric Mediterranean diet enhance endothelial progenitor cells and fitness in subjects with the metabolic syndrome. Clin Sci (Lond). 2012;123:361–73. https://doi.org/10.1042/cs20110477.
Fayh AP, Lopes AL, da Silva AM, Reischak-Oliveira A, Friedman R. Effects of 5 % weight loss through diet or diet plus exercise on cardiovascular parameters of obese: a randomized clinical trial. Eur J Nutr. 2013;52:1443–50. https://doi.org/10.1007/s00394-012-0450-1.
Cullberg K, Christiansen T, Paulsen S, Bruun J, Pedersen S, Richelsen B. Effect of weight loss and exercise on angiogenic factors in the circulation and in adipose tissue in obese subjects. Obesity. 2013;21:454–60. https://doi.org/10.1002/oby.20060.
Mavri A, Poredoš P, Suran D, Gaborit B, Juhan-Vague I, Poredoš P. Effect of diet-induced weight loss on endothelial dysfunction: early improvement after the first week of dieting. Heart Vessels. 2011;26:31–38. https://doi.org/10.1007/s00380-010-0016-1.
Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, et al. Comparing Effects of a Low‐energy Diet and a High‐protein Low‐fat Diet on Sexual and Endothelial Function, Urinary Tract Symptoms, and Inflammation in Obese Diabetic Men. J Sex Med. 2011;8:2868–75. https://doi.org/10.1111/j.1743-6109.2011.02417.x.
Davis NJ, Crandall JP, Gajavelli S, Berman JW, Tomuta N, Wylie-Rosett J, et al. Differential effects of low-carbohydrate and low-fat diets on inflammation and endothelial function in diabetes. J Diabetes its Complications. 2011;25:371–6. https://doi.org/10.1016/j.jdiacomp.2011.08.001.
Wycherley TP, Brinkworth GD, Keogh JB, Noakes M, Buckley JD, Clifton PM. Long-term effects of weight loss with a very low carbohydrate and low fat diet on vascular function in overweight and obese patients. J Intern Med. 2010;267:452–61. https://doi.org/10.1111/j.1365-2796.2009.02174.x.
Miyaki A, Maeda S, Yoshizawa M, Misono M, Saito Y, Sasai H, et al. Effect of weight reduction with dietary intervention on arterial distensibility and endothelial function in obese men. Angiology. 2008;60:351–7. https://doi.org/10.1177/0003319708325449.
Wycherley TP, Brinkworth GD, Noakes M, Buckley JD, Clifton PM. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10:1062–73. https://doi.org/10.1111/j.1463-1326.2008.00863.x.
Keogh JB, Brinkworth GD, Noakes M, Belobrajdic DP, Buckley JD, Clifton PM. Effects of weight loss from a very-low-carbohydrate diet on endothelial function and markers of cardiovascular disease risk in subjects with abdominal obesity. Am J Clin Nutr. 2008;87:567–76. https://doi.org/10.1093/ajcn/87.3.567.
Roberts CK, Won D, Pruthi S, Kurtovic S, Sindhu RK, Vaziri ND, et al. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. J Appl Physiol. 2006;100:1657–65. https://doi.org/10.1152/japplphysiol.01292.2005.
Angelico F, Loffredo L, Pignatelli P, Augelletti T, Carnevale R, Pacella A, et al. Weight loss is associated with improved endothelial dysfunction via NOX2-generated oxidative stress down-regulation in patients with the metabolic syndrome. Intern Emerg Med. 2012;7:219–27. https://doi.org/10.1007/s11739-011-0591-x.
Maeda S, Jesmin S, Iemitsu M, Otsuki T, Matsuo T, Ohkawara K, et al. Weight loss reduces plasma endothelin-1 concentration in obese men. Exp Biol Med (Maywood). 2006;231:1044–7.
Alizadeh M, Daneghian S, Ghaffari A, Ostadrahimi A, Safaeiyan A, Estakhri R, et al. The effect of hypocaloric diet enriched in legumes with or without L-arginine and selenium on anthropometric measures in central obese women. J Res Med Sci. 2010;15:331–43.
Fenster CP, Darley-Usmar VM, Landar AL, Gower BA, Weinsier RL, Hunter GR, et al. Weight loss and race modulate nitric oxide metabolism in overweight women. Free Radic Biol Med. 2004;37:695–702. https://doi.org/10.1016/j.freeradbiomed.2004.05.021.
Joris PJ, Zeegers MP, Mensink RP. Weight loss improves fasting flow-mediated vasodilation in adults: a meta-analysis of intervention studies. Atherosclerosis. 2015;239:21–30. https://doi.org/10.1016/j.atherosclerosis.2014.12.056.
Porter Starr KN, Orenduff M, McDonald SR, Mulder H, Sloane R, Pieper CF, et al. Influence of Weight Reduction and Enhanced Protein Intake on Biomarkers of Inflammation in Older Adults with Obesity. J Nutr Gerontol Geriatr. 2019;38:33–49. https://doi.org/10.1080/21551197.2018.1564200.
Bellido C, López-Miranda J, Pérez-Martínez P, Paz E, Marín C, Gómez P, et al. The Mediterranean and CHO diets decrease VCAM-1 and E-selectin expression induced by modified low-density lipoprotein in HUVECs. Nutr Metab Cardiovasc Dis. 2006;16:524–30. https://doi.org/10.1016/j.numecd.2005.09.007.
González-Amaro R, Díaz-González F, Sánchez-Madrid F. Adhesion molecules in inflammatory diseases. Drugs. 1998;56:977–88. https://doi.org/10.2165/00003495-199856060-00003.
Smith CW. Endothelial adhesion molecules and their role in inflammation. Can J Physiol Pharm. 1993;71:76–87. https://doi.org/10.1139/y93-012.
Habas K, Shang L. Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue Cell. 2018;54:139–43. https://doi.org/10.1016/j.tice.2018.09.002.
Golias C, Tsoutsi E, Matziridis A, Makridis P, Batistatou A, Charalabopoulos K. Review. Leukocyte and endothelial cell adhesion molecules in inflammation focusing on inflammatory heart disease. Vivo. 2007;21:757–69.
Poredos P. Endothelial dysfunction in the pathogenesis of atherosclerosis. Int Angiol. 2002;21:109–16.
Ito H, Ohshima A, Inoue M, Ohto N, Nakasuga K, Kaji Y, et al. Weight reduction decreases soluble cellular adhesion molecules in obese women. Clin Exp Pharm Physiol. 2002;29:399–404. https://doi.org/10.1046/j.1440-1681.2002.03672.x.
Lundberg JO, Weitzberg E, Gladwin MT. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. https://doi.org/10.1038/nrd2466.
Marfella R, Esposito K, Giunta R, Coppola G, De Angelis L, Farzati B, et al. Circulating adhesion molecules in humans: role of hyperglycemia and hyperinsulinemia. Circulation. 2000;101:2247–51. https://doi.org/10.1161/01.cir.101.19.2247.
Ashor AW, Chowdhury S, Oggioni C, Qadir O, Brandt K, Ishaq A, et al. Inorganic Nitrate Supplementation in Young and Old Obese Adults Does Not Affect Acute Glucose and Insulin Responses but Lowers Oxidative Stress. J Nutr. 2016;146:2224–32. https://doi.org/10.3945/jn.116.237529.
Brennan AM, Standley RA, Yi F, Carnero EA, Sparks LM, Goodpaster BH. Individual Response Variation in the Effects of Weight Loss and Exercise on Insulin Sensitivity and Cardiometabolic Risk in Older Adults. Front Endocrinol (Lausanne). 2020;11:632. https://doi.org/10.3389/fendo.2020.00632.
Escalante-Pulido M, Escalante-Herrera A, Milke-Najar ME, Alpizar-Salazar M. Effects of weight loss on insulin secretion and in vivo insulin sensitivity in obese diabetic and non-diabetic subjects. Diabetes Nutr Metab. 2003;16:277–83.
Velasquez-Mieyer PA, Cowan PA, Arheart KL, Buffington CK, Spencer KA, Connelly BE, et al. Suppression of insulin secretion is associated with weight loss and altered macronutrient intake and preference in a subset of obese adults. Int J Obes Relat Metab Disord. 2003;27:219–26. https://doi.org/10.1038/sj.ijo.802227.
Chen NG, Holmes M, Reaven GM. Relationship between insulin resistance, soluble adhesion molecules, and mononuclear cell binding in healthy volunteers. J Clin Endocrinol Metab. 1999;84:3485–9. https://doi.org/10.1210/jcem.84.10.6065.
Montani THA, Jean P Overview of Angiogenesis. In: Morgan & Claypool Life Sciences, 2010.
Kliche S, Waltenberger J. VEGF receptor signaling and endothelial function. IUBMB Life. 2001;52:61–66. https://doi.org/10.1080/15216540252774784.
Tio RA, Wijpkema J, Tan ES, Asselbergs FW, Hospers GAP, Jessurun GAJ, et al. Reduction of endothelial dysfunction following VEGF gene therapy. Neth Heart J. 2005;13:139–41.
Inoue M, Itoh H, Ueda M, Naruko T, Kojima A, Komatsu R, et al. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation. 1998;98:2108–16. https://doi.org/10.1161/01.cir.98.20.2108.
Nezu T, Hosomi N, Aoki S, Matsumoto M. Carotid Intima-Media Thickness for Atherosclerosis. J Atheroscler Thromb. 2016;23:18–31. https://doi.org/10.5551/jat.31989.
García de la Torre N, Rubio MA, Bordiú E, Cabrerizo L, Aparicio E, Hernández C, et al. Effects of Weight Loss after Bariatric Surgery for Morbid Obesity on Vascular Endothelial Growth Factor-A, Adipocytokines, and Insulin. J Clin Endocrinol Metab. 2008;93:4276–81. https://doi.org/10.1210/jc.2007-1370.
Sanft TB, Cartmel B, Harrigan M, Li F, Loftfield E, Playdon M, et al. Impact of weight loss and exercise on VEGF levels in breast cancer survivors. J Clin Oncol. 2016;34:10103. https://doi.org/10.1200/JCO.2016.34:15_suppl.10103.
Primrose JN, Davies JA, Prentice CR, Hughes R, Johnston D. Reduction in factor VII, fibrinogen and plasminogen activator inhibitor-1 activity after surgical treatment of morbid obesity. Thromb Haemost. 1992;68:396–9.
Astrup AS, Tarnow L, Pietraszek L, Schalkwijk CG, Stehouwer CD, Parving HH, et al. Markers of endothelial dysfunction and inflammation in type 1 diabetic patients with or without diabetic nephropathy followed for 10 years: association with mortality and decline of glomerular filtration rate. Diabetes Care. 2008;31:1170–6. https://doi.org/10.2337/dc07-1960.
Persson F, Rossing P, Hovind P, Stehouwer CD, Schalkwijk CG, Tarnow L, et al. Endothelial dysfunction and inflammation predict development of diabetic nephropathy in the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (IRMA 2) study. Scand J Clin Lab Invest. 2008;68:731–8. https://doi.org/10.1080/00365510802187226.
Hunt KJ, Baker NL, Cleary PA, Klein R, Virella G, Lopes-Virella MF. Longitudinal Association Between Endothelial Dysfunction, Inflammation, and Clotting Biomarkers With Subclinical Atherosclerosis in Type 1 Diabetes: An Evaluation of the DCCT/EDIC Cohort. Diabetes Care. 2015;38:1281–9. https://doi.org/10.2337/dc14-2877.
Funding
This research was funded by Nottingham University.
Author information
Authors and Affiliations
Contributions
MS conceptualized the study. RM, ZA and MS conducted the search and screened the articles. RM and MS conducted the analysis and wrote the manuscript. MS is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the discussion and interpretation of data, and reviewed / critically edited the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mathur, R., Ahmid, Z., Ashor, A.W. et al. Effects of dietary-based weight loss interventions on biomarkers of endothelial function: a systematic review and meta-analysis. Eur J Clin Nutr 77, 927–940 (2023). https://doi.org/10.1038/s41430-023-01307-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-023-01307-6