Abstract
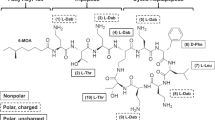
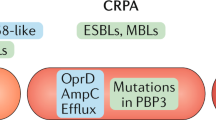
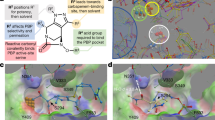
Cephalosporins comprise a β-lactam antibiotic class whose first members were discovered in 1945 from the fungus Cephalosporium acremonium. Their clinical use for Gram-negative bacterial infections is widespread due to their ability to traverse outer membranes through porins to gain access to the periplasm and disrupt peptidoglycan synthesis. More recent members of the cephalosporin class are administered as last resort treatments for complicated urinary tract infections, MRSA, and other multi-drug resistant pathogens, such as Neisseria gonorrhoeae. Unfortunately, there has been a global increase in cephalosporin-resistant strains, heteroresistance to this drug class has been a topic of increasing concern, and tolerance and persistence are recognized as potential causes of cephalosporin treatment failure. In this review, we summarize the cephalosporin antibiotic class from discovery to their mechanisms of action, and discuss the causes of cephalosporin treatment failure, which include resistance, tolerance, and phenomena when those qualities are exhibited by only small subpopulations of bacterial cultures (heteroresistance and persistence). Further, we discuss how recent efforts with cephalosporin conjugates and combination treatments aim to reinvigorate this antibiotic class.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Marshall WF, Blair JE. The cephalosporins. Mayo Clin Proc. 1999;74:187–95.
Brotzu G. Research on a new antibiotic. Cagliari Inst Hyg. 1948;1:5–15.
Newton GG, Abraham EP. Cephalosporin C, a new antibiotic containing sulphur and D-alpha-aminoadipic acid. Nature. 1955;175:548.
Abraham EP. Cephalosporins 1945–1986. Drugs. 1987;34:1–14.
Chauvette RR, et al. Chemistry of Cephalosporin Antibiotics .2. Preparation of a new class of antibiotics and relation of structure to activity. J Am Chem Soc. 1962;84:3401.
Godzeski CW, Brier G, Pavey DE. Cephalothin, a new cephalosporin with a broad antibacterial spectrum. I. In vitro studies employing the gradient plate technique. Appl Microbiol. 1963;11:122–7.
Griffith RS, Black HR. Cephalothin-a new antibiotic. Preliminary clinical and laboratory studies. JAMA. 1964;189:823–8.
Chang TW, Weinstein L. In vitro biological activity of cephalothin. J Bacteriol. 1963;85:1022–7.
WHO model list of essential medicines - 22nd list. World Health Organization, Document: WHO/MHP/HPS/EML/2021.02 (2021).
The WHO AWaRe (Access, Watch, Reserve) antibiotic book. World Health Organization, Document: ISBN 978-92-4-006238-2 (2022).
Goldstein E. Rise in the prevalence of resistance to extended-spectrum cephalosporins in the USA, nursing homes and antibiotic prescribing in outpatient and inpatient settings. J Antimicrob Chemother. 2021;76:2745–7.
Penalva G, et al. Decreasing and stabilising trends of antimicrobial consumption and resistance in Escherichia coli and Klebsiella pneumoniae in segmented regression analysis, European Union/European Economic Area, 2001 to 2018. Euro Surveill. 2019;24:1900656.
Choby JE, Ozturk T, Satola SW, Jacob JT, Weiss DS. Does cefiderocol heteroresistance explain the discrepancy between the APEKS-NP and CREDIBLE-CR clinical trial results? Comment. Lancet Microbe. 2021;2:E648–E649.
Choby JE, Ozturk T, Satola SW, Jacob JT, Weiss DS. Widespread cefiderocol heteroresistance in carbapenem-resistant Gram-negative pathogens. Lancet Infect Dis. 2021;21:597–8.
Jia X, et al. Heteroresistance to cefepime in Pseudomonas aeruginosa bacteraemia. Int J Antimicrob Agents. 2020;55:105832.
Kishii K, Ito T, Watanabe S, Okuzumi K, Hiramatsu K. Recurrence of heterogeneous methicillin-resistant Staphylococcus aureus (MRSA) among the MRSA clinical isolates in a Japanese university hospital. J Antimicrob Chemother. 2008;62:324–8.
Ma W, Sun J, Yang S, Zhang L. Epidemiological and clinical features for cefepime heteroresistant Escherichia coli infections in Southwest China. Eur J Clin Microbiol Infect Dis. 2016;35:571–8.
Bryson D, Hettle AG, Boraston AB, Hobbs JK. Clinical mutations that partially activate the stringent response confer multidrug tolerance in Staphylococcus aureus. Antimicrob Agents Ch. 2020;64:e02103–19.
Hamad MA, Austin CR, Stewart AL, Higgins M, Vazquez-Torres A, Voskuil MI. Adaptation and Antibiotic tolerance of anaerobic Burkholderia pseudomallei. Antimicrob Agents Ch. 2011;55:3313–23.
Hemsley CM, Luo JX, Andreae CA, Butler CS, Soyer OS, Titball RW. Bacterial drug tolerance under clinical conditions is governed by anaerobic adaptation but not anaerobic respiration. Antimicrob Agents Ch. 2014;58:5775–83.
Zhang SS, Liu S, Wu N, Yuan YH, Zhang WH, Zhang Y. Small non-coding RNA RyhB mediates persistence to multiple antibiotics and stresses in Uropathogenic Escherichia coli by reducing cellular metabolism. Front Microbiol. 2018;9:1–10.
Abraham EP. A glimpse of the early history of the cephalosporins. Rev Infect Dis. 1979;1:99–105.
Bo G. Giuseppe Brotzu and the discovery of cephalosporins. Clin Microbiol Infect. 2000;6:6–9.
Brakhage AA. Molecular regulation of beta-lactam biosynthesis in filamentous fungi. Microbiol Mol Biol Rev. 1998;62:547–85.
Hu Y, Zhu B. Study on genetic engineering of Acremonium chrysogenum, the cephalosporin C producer. Synth Syst Biotechnol. 2016;1:143–9.
Abraham E. Selective reminiscences of beta-lactam antibiotics: early research on penicillin and cephalosporins. Bioessays. 1990;12:601–6.
Abraham EP, Newton GGF, Hale CW. Purification and some properties of Cephalosporin-N, a new Penicillin. Biochem J. 1954;58:94–102.
Hamilton-Miller JMT. Sir Edward Abraham’s contribution to the development of the cephalosporins: a reassessment. Int J Antimicrob Ag. 2000;15:179–84.
Hamilton-Miller JMT. The cephalosporins and Sir Edward Abraham: Recollections about a great scientist and his part in the discovery of these antibiotics. J Antibiot. 2000;53:1003–7.
Hodgkin DC, Maslen EN. The x-ray analysis of the structure of cephalosporin C. Biochem J. 1961;79:393–402.
Elks J. Structural formulae and nomenclature of the cephalosporin antibiotics. Drugs. 1987;34:240–6.
Lin XM, Kuck U. Cephalosporins as key lead generation beta-lactam antibiotics. Appl Microbiol Biot. 2022;106:8007–20.
Byford MF, Baldwin JE, Shiau CY, Schofield CJ. The mechanism of ACV Synthetase. Chem Rev. 1997;97:2631–50.
Baldwin JE, Gagnon J, Ting HH. N-Terminal Amino-acid sequence and some properties of Isopenicillin-N Synthetase from Cephalosporium-Acremonium. Febs Lett. 1985;188:253–6.
Pang CP, et al. Purification of isopenicillin N synthetase. Biochem J. 1984;222:789–95.
Randall CR, et al. X-ray-absorption studies of the ferrous active-site of Isopenicillin N-Synthase and related model complexes. Biochem-Us. 1993;32:6664–73.
Roach PL, et al. Structure of isopenicillin N synthase complexed with substrate and the mechanism of penicillin formation. Nature. 1997;387:827–30.
Ullan RV, Casqueiro J, Banuelos O, Fernandez FJ, Gutierrez S, Martin JF. A novel epimerization system in fungal secondary metabolism involved in the conversion of isopenicillin N into penicillin N in Acremonium chrysogenum. J Biol Chem. 2002;277:46216–25.
Brewer SJ, Farthing JE, Turner MK. The oxygenation of the 3-methyl group of 7beta-(5-D-aminoadipamido)-3-methylceph-3-em-4-carboxylic acid (desacetoxycephalosporin C) by extracts of Acremonium chrysogenum [proceedings]. Biochem Soc Trans. 1977;5:1024–6.
Dotzlaf JE, Yeh WK. Copurification and characterization of deacetoxycephalosporin C synthetase/hydroxylase from Cephalosporium acremonium. J Bacteriol. 1987;169:1611–8.
Higgens CE, Hamill RL, Sands TH, Hoehn MM, Davis NE. Letter: The occurrence of deacetoxycephalosporin C in fungi and streptomycetes. J Antibiot. 1974;27:298–300.
Rabe P, Kamps J, Schofield CJ, Lohans CT. Roles of 2-oxoglutarate oxygenases and isopenicillin N synthase in beta-lactam biosynthesis. Nat Prod Rep. 2018;35:735–56.
Samson SM, et al. Cloning and expression of the fungal expandase hydroxylase gene involved in cephalosporin biosynthesis. Bio-Technol. 1987;5:1207. +
Kupka J, Shen YQ, Wolfe S, Demain AL. Studies on the ring-cyclization and ring-expansion enzymes of beta-lactam biosynthesis in Cephalosporium acremonium. Can J Microbiol. 1983;29:488–96.
Lejon S, Ellis J, Valegard K. The last step in cephalosporin C formation revealed: crystal structures of deacetylcephalosporin C acetyltransferase from Acremonium chrysogenum in complexes with reaction intermediates. J Mol Biol. 2008;377:935–44.
Schmitt EK, Hoff B, Kuck U. Regulation of cephalosporin biosynthesis. Adv Biochem Eng Biotechnol. 2004;88:1–43.
Gutierrez S, Diez B, Montenegro E, Martin JF. Characterization of the Cephalosporium acremonium pcbAB gene encoding alpha-aminoadipyl-cysteinyl-valine synthetase, a large multidomain peptide synthetase: linkage to the pcbC gene as a cluster of early cephalosporin biosynthetic genes and evidence of multiple functional domains. J Bacteriol. 1991;173:2354–65.
Martin JF, Ullan RV, Casqueiro J. Novel genes involved in cephalosporin biosynthesis: the three-component isopenicillin N epimerase system. Adv Biochem Eng Biotechnol. 2004;88:91–109.
Gutierrez S, Velasco J, Fernandez FJ, Martin JF. The Cefg gene of Cephalosporium-Acremonium is linked to the Cefef gene and encodes a Deacetylcephalosporin-C Acetyltransferase closely related to Homoserine O-Acetyltransferase. J Bacteriol. 1992;174:3056–64.
Teijeira F, Ullan RV, Guerra SM, Garcia-Estrada C, Vaca I, Martin JF. The transporter CefM involved in translocation of biosynthetic intermediates is essential for cephalosporin production. Biochem J. 2009;418:113–24.
Ullan RV, Teijeira F, Guerra SM, Vaca I, Martin JF. Characterization of a novel peroxisome membrane protein essential for conversion of isopenicillin N into cephalosporin C. Biochem J. 2010;432:227–36.
Ullan RV, Liu G, Casqueiro J, Gutierrez S, Banuelos O, Martin JF. The cefT gene of Acremonium chrysogenum C10 encodes a putative multidrug efflux pump protein that significantly increases cephalosporin C production. Mol Genet Genom. 2002;267:673–83.
Gutierrez S, Fierro F, Casqueiro J, Martin JF. Gene organization and plasticity of the beta-lactam genes in different filamentous fungi. Anton Leeuw Int J G 1999;75:81–94.
Liu L, Chen Z, Liu W, Ke X, Tian X, Chu J. Cephalosporin C biosynthesis and fermentation in Acremonium chrysogenum. Appl Microbiol Biotechnol. 2022;106:6413–26.
Skatrud PL, Queener SW. An electrophoretic molecular karyotype for an industrial strain of Cephalosporium acremonium. Gene. 1989;78:331–8.
Smith DJ, et al. Beta-Lactam antibiotic biosynthetic genes have been conserved in clusters in Prokaryotes and Eukaryotes. Embo J. 1990;9:741–7.
Pollegioni L, Rosini E, Molla G. Cephalosporin C acylase: dream and(/or) reality. Appl Microbiol Biotechnol. 2013;97:2341–55.
Bianchi D, Bortolo R, Golini P, Cesti P. Enzymatic transformation of cephalosporin C to 7-ACA by simultaneous action of immobilized d-amino acid oxidase and glutaryl-7-ACA acylase. Appl Biochem Biotech. 1998;73:257–68.
Elander RP. Industrial production of beta-lactam antibiotics. Appl Microbiol Biotechnol. 2003;61:385–92.
Bush K, Bradford PA. beta-Lactams and beta-Lactamase Inhibitors: An Overview. Cold Spring Harb Perspect Med. 2016;6:a025247.
Chaudhry SB, Veve MP, Wagner JL. Cephalosporins: A focus on side chains and beta-lactam cross-reactivity. Pharmacy. 2019;7:103.
Giamarellou H. Fourth generation cephalosporins in the antimicrobial chemotherapy of surgical infections. J Chemother. 1999;11:486–93.
Nikaido H, Liu W, Rosenberg EY. Outer membrane permeability and beta-lactamase stability of dipolar ionic cephalosporins containing methoxyimino substituents. Antimicrob Agents Chemother. 1990;34:337–42.
Hebeisen P, Heinze-Krauss I, Angehrn P, Hohl P, Page MGP, Then RL. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob Agents Ch. 2001;45:825–36.
Moisan H, Pruneau M, Malouin F. Binding of ceftaroline to penicillin-binding proteins of Staphylococcus aureus and Streptococcus pneumoniae. J Antimicrob Chemoth. 2010;65:713–6.
Pfaller MA, et al. Ceftobiprole activity against gram-positive and -negative pathogens collected from the United States in 2006 and 2016. Antimicrob Agents Chemother. 2018;63:e01566-18.
Sader HS, Fritsche TR, Kaniga K, Ge Y, Jones RN. Antimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strains. Antimicrob Agents Chemother. 2005;49:3501–12.
Moya B, Zamorano L, Juan C, Perez JL, Ge Y, Oliver A. Activity of a new cephalosporin, CXA-101 (FR264205), against beta-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patients. Antimicrob Agents Chemother. 2010;54:1213–7.
Bassetti M, Merelli M, Temperoni C, Astilean A. New antibiotics for bad bugs: where are we? Ann Clin Microbiol Antimicrob. 2013;12:22.
Spratt BG. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci USA. 1975;72:2999–3003.
Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32:234–58.
Sauvage E, Terrak M. Glycosyltransferases and Transpeptidases/Penicillin-binding proteins: valuable targets for new antibacterials. Antibiotics. 2016;5:1–27.
Tipper DJ, Strominger JL. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci USA. 1965;54:1133–41.
Nikaido H. Crossing the envelope: how cephalosporins reach their targets. Clin Microbiol Infec. 2000;6:22–26.
Delcour AH. Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta. 2009;1794:808–16.
Breijyeh Z, Jubeh B, Karaman R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25:1340.
Balaban NQ, et al. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol. 2019;17:441–8.
Kowalska-Krochmal B, Dudek-Wicher R. The minimum inhibitory concentration of antibiotics: methods, interpretation, clinical relevance. Pathogens. 2021;10:165.
Brauner A, Fridman O, Gefen O, Balaban NQ. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol. 2016;14:320–30.
Miller WR, Munita JM, Arias CA. Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti Infect Ther. 2014;12:1221–36.
Arbeloa A, et al. Role of class A penicillin-binding proteins in PBP5-mediated beta-lactam resistance in Enterococcus faecalis. J Bacteriol. 2004;186:1221–8.
Lazzaro LM, Cassisi M, Stefani S, Campanile F. Impact of PBP4 alterations on beta-Lactam resistance and ceftobiprole non-susceptibility among Enterococcus faecalis Clinical Isolates. Front Cell Infect Mi. 2022;11:1–9.
Moon TM, et al. The structures of penicillin-binding protein 4 (PBP4) and PBP5 from Enterococci provide structural insights into beta-lactam resistance. J Biol Chem. 2018;293:18574–84.
Rice LB, et al. Impact of specific pbp5 mutations on expression of beta-lactam resistance in Enterococcus faecium. Antimicrob Agents Chemother. 2004;48:3028–32.
Vesic D, Kristich CJ. MurAA is required for intrinsic Cephalosporin resistance of Enterococcus faecalis. Antimicrob Agents Ch. 2012;56:2443–51.
Fergestad ME, Stamsas GA, Morales Angeles D, Salehian Z, Wasteson Y, Kjos M. Penicillin-binding protein PBP2a provides variable levels of protection toward different beta-lactams in Staphylococcus aureus RN4220. Microbiologyopen. 2020;9:e1057.
Kosowska-Shick K, McGhee PL, Appelbaum PC. Affinity of ceftaroline and other beta-lactams for penicillin-binding proteins from Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother. 2010;54:1670–7.
Saravolatz LD, Stein GE, Johnson LB. Ceftaroline: A novel cephalosporin with activity against Methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2011;52:1156–63.
Tomberg J, et al. Alanine 501 mutations in Penicillin-binding Protein 2 from Neisseria gonorrhoeae: Structure, mechanism, and effects on cephalosporin resistance and biological fitness. Biochem-Us. 2017;56:1140–50.
Pucci MJ, Boice-Sowek J, Kessler RE, Dougherty TJ. Comparison of cefepime, cefpirome, and cefaclidine binding affinities for penicillin-binding proteins in Escherichia coli K-12 and Pseudomonas aeruginosa SC8329. Antimicrob Agents Chemother. 1991;35:2312–7.
Hedge PJ, Spratt BG. Resistance to beta-lactam antibiotics by re-modelling the active site of an E. coli penicillin-binding protein. Nature. 1985;318:478–80.
Alm RA, Johnstone MR, Lahiri SD. Characterization of Escherichia coli NDM isolates with decreased susceptibility to aztreonam/avibactam: role of a novel insertion in PBP3. J Antimicrob Chemother. 2015;70:1420–8.
Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22:161–82.
Mammeri H, Poirel L, Fortineau N, Nordmann P. Naturally occurring extended-spectrum cephalosporinases in Escherichia coli. Antimicrob Agents Chemother. 2006;50:2573–6.
Berrazeg M, et al. Mutations in beta-Lactamase AmpC increase resistance of Pseudomonas aeruginosa isolates to Antipseudomonal Cephalosporins. Antimicrob Agents Chemother. 2015;59:6248–55.
Hidri N, et al. Resistance to ceftazidime is associated with a S220Y substitution in the omega loop of the AmpC beta-lactamase of a Serratia marcescens clinical isolate. J Antimicrob Chemoth. 2005;55:496–9.
Jacobs C, Frere JM, Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible beta-lactam resistance in gram-negative bacteria. Cell. 1997;88:823–32.
Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother. 2010;54:969–76.
Castanheira M, Simner PJ, Bradford PA. Extended-spectrum beta-lactamases: an update on their characteristics, epidemiology and detection. JAC Antimicrob Resist. 2021;3:dlab092.
Bahr G, Gonzalez LJ, Vila AJ. Metallo-beta-lactamases in the age of multidrug resistance: from structure and mechanism to evolution, dissemination, and inhibitor design. Chem Rev. 2021;121:7957–8094.
Mojica MF, Rossi MA, Vila AJ, Bonomo RA. The urgent need for metallo-beta-lactamase inhibitors: an unattended global threat. Lancet Infect Dis. 2022;22:e28–e34.
Charrel RN, Pages JM, De Micco P, Mallea M. Prevalence of outer membrane porin alteration in beta-lactam-antibiotic-resistant Enterobacter aerogenes. Antimicrob Agents Chemother. 1996;40:2854–8.
Pages JM, James CE, Winterhalter M. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol. 2008;6:893–903.
Choi U, Lee CR. Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front Microbiol. 2019;10:953.
Harder KJ, Nikaido H, Matsuhashi M. Mutants of Escherichia-Coli that are resistant to certain Beta-Lactam compounds lack the Ompf Porin. Antimicrob Agents Ch. 1981;20:549–52.
Stephan J, Mailaender C, Etienne G, Daffe M, Niederweis M. Multidrug resistance of a porin deletion mutant of Mycobacterium smegmatis. Antimicrob Agents Chemother. 2004;48:4163–70.
Domenech-Sanchez A, et al. Role of Klebsiella pneumoniae OmpK35 porin in antimicrobial resistance. Antimicrob Agents Chemother. 2003;47:3332–5.
Bredin J, et al. Alteration of pore properties of Escherichia coli OmpF induced by mutation of key residues in anti-loop 3 region. Biochem J. 2002;363:521–8.
De E, et al. A new mechanism of antibiotic resistance in Enterobacteriaceae induced by a structural modification of the major porin. Mol Microbiol. 2001;41:189–98.
Simonet V, Mallea M, Pages JM. Substitutions in the eyelet region disrupt cefepime diffusion through the Escherichia coli OmpF channel. Antimicrob Agents Ch. 2000;44:311–5.
Pagel M, Simonet V, Li J, Lallemand M, Lauman B, Delcour AH. Phenotypic characterization of pore mutants of the Vibrio cholerae porin OmpU. J Bacteriol. 2007;189:8593–8600.
Grosjean M, et al. Reassessment of the cooperativity between efflux system MexAB-OprM and cephalosporinase AmpC in the resistance of Pseudomonas aeruginosa to β-lactams. J Antimicrob Chemoth. 2021;76:536–9.
Masuda N, Gotoh N, Ohya S, Nishino T. Quantitative correlation between susceptibility and OprJ production in NfxB mutants of Pseudomonas aeruginosa. Antimicrob Agents Ch. 1996;40:909–13.
Purssell A, Poole K. Functional characterization of the NfxB repressor of the mexCD-oprJ multidrug efflux operon of Pseudomonas aeruginosa. Microbiology. 2013;159:2058–73.
El-Halfawy OM, Valvano MA. Antimicrobial heteroresistance: an emerging field in need of clarity. Clin Microbiol Rev. 2015;28:191–207.
Band VI, Weiss DS. Heteroresistance: A cause of unexplained antibiotic treatment failure? PLoS Pathog. 2019;15:e1007726.
Band VI, et al. Antibiotic failure mediated by a resistant subpopulation in Enterobacter cloacae. Nat Microbiol. 2016;1:16053.
Nicoloff H, Hjort K, Levin BR, Andersson DI. The high prevalence of antibiotic heteroresistance in pathogenic bacteria is mainly caused by gene amplification. Nat Microbiol. 2019;4:504–14.
Hallander HO, Laurell G. Identification of cephalosporin-resistant Staphylococcus aureus with the disc diffusion method. Antimicrob Agents Chemother. 1972;1:422–6.
Hung KH, Wang MC, Huang AH, Yan JJ, Wu JJ. Heteroresistance to cephalosporins and penicillins in Acinetobacter baumannii. J Clin Microbiol. 2012;50:721–6.
Lu Y, et al. Quorum sensing regulates heteroresistance in Pseudomonas aeruginosa. Front Microbiol. 2022;13:1017707.
Potrykus K, Cashel M. p)ppGpp: still magical?. Annu Rev Microbiol. 2008;62:35–51.
Kim H, Kim JH, Cho H, Ko KS. Overexpression of a DNA Methyltransferase increases persister cell formation in Acinetobacter baumannii. Microbiol Spectr. 2022;10:e0265522.
Yu W, et al. Absence of tmRNA Increases the persistence to Cefotaxime and the Intercellular Accumulation of Metabolite GlcNAc in Aeromonas veronii. Front Cell Infect Microbiol. 2020;10:44.
Alkasir R, et al. Characterization and Transcriptome analysis of Acinetobacter baumannii persister cells. Micro Drug Resist. 2018;24:1466–74.
Advancing Health through Innovation: New Drug Approvals 2019. United States Food and Drug Administration, Document: https://www.fda.gov/media/134493/download (2020).
Sato T, Yamawaki K. Cefiderocol: Discovery, chemistry, and in vivo profiles of a novel Siderophore Cephalosporin. Clin Infect Dis. 2019;69:S538–S543.
Cook-Libin S, Sykes EME, Kornelsen V, Kumar A. Iron acquisition mechanisms and their role in the virulence of Acinetobacter baumannii. Infect Immun. 2022;90:e0022322.
Page MGP. The role of Iron and Siderophores in infection, and the development of Siderophore antibiotics. Clin Infect Dis. 2019;69:S529–S537.
Ji C, Juarez-Hernandez RE, Miller MJ. Exploiting bacterial iron acquisition: siderophore conjugates. Future Med Chem. 2012;4:297–313.
Ito A, et al. In Vitro antibacterial properties of cefiderocol, a Novel Siderophore Cephalosporin, against gram-negative bacteria. Antimicrob Agents Chemother. 2018;62:e01454-17.
Karakonstantis S, Rousaki M, Kritsotakis EI. Cefiderocol: Systematic review of mechanisms of resistance, heteroresistance and in vivo emergence of resistance. Antibiotics. 2022;11:723.
Malik S, Kaminski M, Landman D, Quale J. Cefiderocol resistance in Acinetobacter baumannii: Roles of beta-Lactamases, Siderophore receptors, and Penicillin binding Protein 3. Antimicrob Agents Chemother. 2020;64:e01221-20.
Poirel L, Sadek M, Nordmann P. Contribution of PER-Type and NDM-Type beta-Lactamases to Cefiderocol Resistance in Acinetobacter baumannii. Antimicrob Agents Chemother. 2021;65:e0087721.
Klein S, et al. Rapid development of Cefiderocol resistance in Carbapenem-resistant Enterobacter cloacae during therapy is associated with heterogeneous mutations in the Catecholate Siderophore receptor cirA. Clin Infect Dis. 2022;74:905–8.
Lee N, Yuen KY, Kumana CR. Clinical role of beta-lactam/beta-lactamase inhibitor combinations. Drugs. 2003;63:1511–24.
van Duin D, Bonomo RA. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation beta-Lactam/beta-Lactamase inhibitor combinations. Clin Infect Dis. 2016;63:234–41.
Castanheira M, Mills JC, Costello SE, Jones RN, Sader HS. Ceftazidime-avibactam activity tested against Enterobacteriaceae isolates from U.S. hospitals (2011 to 2013) and characterization of beta-lactamase-producing strains. Antimicrob Agents Chemother. 2015;59:3509–17.
Farrell DJ, Flamm RK, Sader HS, Jones RN. Antimicrobial activity of Ceftolozane-Tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. Hospitals (2011-2012). Antimicrob Agents Ch. 2013;57:6305–10.
Flamm RK, Farrell DJ, Sader HS, Jones RN. Ceftazidime/avibactam activity tested against Gram-negative bacteria isolated from bloodstream, pneumonia, intra-abdominal and urinary tract infections in US medical centres (2012). J Antimicrob Chemother. 2014;69:1589–98.
Sader HS, Castanheira M, Mendes RE, Flamm RK, Farrell DJ, Jones RN. Ceftazidime-avibactam activity against multidrug-resistant Pseudomonas aeruginosa isolated in U.S. medical centers in 2012 and 2013. Antimicrob Agents Chemother. 2015;59:3656–9.
Farrag HA, Abdallah N, Shehata MMK, Awad EM. Natural outer membrane permeabilizers boost antibiotic action against irradiated-resistant bacteria. J Biomed Sci. 2019;26:69.
Chiu S, et al. Causes of polymyxin treatment failure and new derivatives to fill the gap. J Antibiot. 2022;75:593–609.
Gunderson BW, Ibrahim KH, Hovde LB, Fromm TL, Reed MD, Rotschafer JC. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 2003;47:905–9.
Pages JM, Peslier S, Keating TA, Lavigne JP, Nichols WW. Role of the outer membrane and Porins in susceptibility of beta-Lactamase-producing Enterobacteriaceae to Ceftazidime-Avibactam. Antimicrob Agents Chemother. 2015;60:1349–59.
Nang SC, Azad MAK, Velkov T, Zhou QT, Li J. Rescuing the last-line Polymyxins: Achievements and challenges. Pharm Rev. 2021;73:679–728.
Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother. 2017;72:1373–85.
Mushtaq S, Vickers A, Woodford N, Haldimann A, Livermore DM. Activity of nacubactam (RG6080/OP0595) combinations against MBL-producing Enterobacteriaceae. J Antimicrob Chemother. 2019;74:953–60.
Morinaka A, et al. OP0595, a new diazabicyclooctane: mode of action as a serine beta-lactamase inhibitor, antibiotic and beta-lactam ‘enhancer’. J Antimicrob Chemoth. 2015;70:2779–86.
Kim S, et al. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res. 2021;49:D1388–D1395.
Acknowledgements
This work was supported by the 250th Anniversary Fund for Innovation in Undergraduate Education, the Program for Community Engaged Scholarship, and the Council on Science and Technology at Princeton University (MPB). The content is solely the responsibility of the authors and does not necessarily represent the views of the funding agencies.
Author information
Authors and Affiliations
Contributions
AHA, RSB, SDWC, LLE, DS, EJW, GL, KJS, and MPB wrote and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Araten, A.H., Brooks, R.S., Choi, S.D.W. et al. Cephalosporin resistance, tolerance, and approaches to improve their activities. J Antibiot 77, 135–146 (2024). https://doi.org/10.1038/s41429-023-00687-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00687-y