Abstract
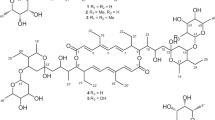
Three new 22-membered polyol macrolides, dactylides A−C (1−3), were isolated from Dactylosporangium aurantiacum ATCC 23491 employing repeated chromatographic separations, and their structures were established based on detailed analysis of NMR and MS data. The relative configurations at the stereocenters were established via vicinal 1H–1H coupling constants, NOE correlations, and by application of Kishi’s universal NMR database. In order to get insights into the biosynthetic pathway of 1−3, the genome sequence of the producer strain D. aurantiacum was obtained and the putative biosynthetic gene cluster encoding their biosynthesis was identified through bioinformatic analysis using antiSMASH. Compounds 1−3 showed significant in-vitro antimycobacterial and cytotoxic activity.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data will be made available on request.
References
Al-Fadhli AA, et al. Macrolides from rare actinomycetes: Structures and bioactivities. Int J Antimicrob Agents. 2022;59:106523. https://doi.org/10.1016/j.ijantimicag.2022.106523.
Elshahawi SI, Shaaban KA, Kharel MK, Thorson JS. A comprehensive review of glycosylated bacterial natural products. Chem Soc Rev. 2015;44:7591–697. https://doi.org/10.1039/C4CS00426D.
Onodera KI, Nakamura H, Oba Y, Ohizumi Y, Ojika M. Zooxanthellamide Cs: vasoconstrictive polyhydroxylated macrolides with the largest lactone ring size from a marine dinoflagellate of Symbiodinium sp. J Am Chem Soc. 2005;127:10406–11. https://doi.org/10.1021/ja050810g.
Cavalleri B, Arnone A, Di Modugno E, Nasini G, Goldstein BP. Structure and biological activity of lipiarmycin B. J Antibiot. 1988;41:308–15. https://doi.org/10.7164/antibiotics.41.308.
Kurabachew M, et al. Lipiarmycin targets RNA polymerase and has good activity against multidrug-resistant strains of Mycobacterium tuberculosis. J Antimicrob Chemother. 2008;62:713–9. https://doi.org/10.1093/jac/dkn269.
Zhanel GG, Walkty AJ, Karlowsky JA. Fidaxomicin: A novel agent for the treatment of Clostridium difficile infection. Can J Infect Dis Med Microbiol. 2015;26:305–12. https://doi.org/10.1155/2015/934594.
Thiemann JE. A new species of the genus Amorphosporangium isolated from Italian soil. Mycopathol Mycol Appl. 1967;33:233–40. https://doi.org/10.1007/BF02088915.
Kobayashi Y, Tan CH, Kishi Y. Toward creation of a universal NMR database for stereochemical assignment: Complete structure of the desertomycin/oasomycin class of natural products. J Am Chem Soc. 2001;123:2076–8. https://doi.org/10.1021/ja004154q.
Blin K, et al. AntiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021;49:W29–W35. https://doi.org/10.1093/nar/gkab335.
Keatinge-Clay AT. A tylosin ketoreductase reveals how chirality is determined in polyketides. Chem Biol. 2007;14:898–908. https://doi.org/10.1016/j.chembiol.2007.07.009c.
Heinrichs MT, et al. Mycobacterium tuberculosis Strains H37Ra and H37Rv have equivalent minimum inhibitory concentrations to most antituberculosis drugs. Int J Mycobacteriol. 2018;7:156–61. https://doi.org/10.4103/ijmy.ijmy_33_18.
Paramita P, et al. Evaluation of potential anti‐cancer activity of cationic liposomal nanoformulated Lycopodium clavatum in colon cancer cells. IET Nanobiotech. 2018;12:727–32. https://doi.org/10.1049/iet-nbt.2017.0106.
Shinde PB, et al. A non-immunosuppressive FK506 analogue with neuroregenerative activity produced from a genetically engineered Streptomyces strain. RSC Adv. 2015;5:6823–8. https://doi.org/10.1039/C4RA11907J.
Helaly SE, et al. Langkolide, a 32-membered macrolactone antibiotic produced by Streptomyces sp. acta 3062. J Nat Prod. 2012;75:1018–24.
Rix U, Fischer C, Remsing LL, Rohr J. Modification of post-PKS tailoring steps through combinatorial biosynthesis. Nat Prod Rep. 2002;19:542–80. https://doi.org/10.1039/B103920M.
Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyltransferases: structures, functions, and mechanisms. Annu Rev Biochem. 2008;77:521–55. https://doi.org/10.1146/annurev.biochem.76.061005.092322.
Kobayashi Y, Lee J, Tezuka K, Kishi Y. Toward creation of a universal NMR database for the stereochemical assignment of acyclic compounds: the case of two contiguous propionate units. Org Lett. 1999;1:2177–80. https://doi.org/10.1021/ol9903786.
Caradec T, et al. Dactylosporolides: Glycosylated macrolides from Dactylosporangium fulvum. J Nat Prod. 2022;85:2714–22. https://doi.org/10.1021/acs.jnatprod.2c00484.
Son S, et al. Catenulisporolides, glycosylated triene macrolides from the chemically underexploited actinomycete Catenulispora species. Org Lett. 2018;20:7234–8. https://doi.org/10.1021/acs.orglett.8b03160.
Fleury E, et al. Advances in the universal NMR database: toward the determination of the relative configurations of large polypropionates. Eur J Org Chem. 2009;2009:4992–5001. https://doi.org/10.1002/ejoc.200900616.
Kobayashi Y, Tan CH, Kishi Y. Stereochemical assignment of the C21–C38 portion of the desertomycin/oasomycin class of natural products by using universal NMR databases: prediction. Angew Chem. 2000;112:4449–51. https://doi.org/10.1002/1521-3757(20001201)112:23<4449::AID-ANGE4449>3.0.CO;2-0.
Kobayashi Y, Czechtizky W, Kishi Y. Complete stereochemistry of Tetrafibricin. Org Lett. 2003;5:93–96. https://doi.org/10.1021/ol0272895.
Seike H, Ghosh I, Kishi Y. Attempts to assemble a universal NMR database without synthesis of NMR database compounds. Org Lett. 2006;8:3861–4. https://doi.org/10.1021/ol061580t.
Zheng J, Taylor CA, Piasecki SK, Keatinge-Clay AT. Structural and functional analysis of A-type ketoreductases from the amphotericin modular polyketide synthase. Structure. 2010;18:913–22. https://doi.org/10.1016/j.str.2010.04.015.
Kwan DH, Schulz F. The stereochemistry of complex polyketide biosynthesis by modular polyketide synthases. Molecules. 2011;16:6092–115. https://doi.org/10.3390/molecules16076092.
Zhang P, Zhang Z, Zhang L, Wang J, Wu C. Glycosyltransferase GT1 family: Phylogenetic distribution, substrates coverage, and representative structural features. Comput Struct Biotechnol J. 2020;18:1383–90. https://doi.org/10.1016/j.csbj.2020.06.003.
Acknowledgements
This work was supported by Scientific and Engineering Research Board (SERB), Department of Science and Technology [ECRA/2016/000788 and EEQ/2016/000268]; and Council of Scientific and Industrial Research (CSIR), New Delhi [MLP/0027]. Yedukondalu Nalli acknowledges Scientific and Engineering Research Board (SERB) for providing National Postdoctoral Fellowship (N-PDF). Sanju Singh acknowledges Council of Scientific and Industrial Research (CSIR) for JRF and SRF fellowship. Padmaja D. Wakchaure acknowledges CSIR, New Delhi, India, for the GATE-SRF fellowship. Pankaj Kumar acknowledges Department of Biotechnology (DBT) for providing JRF and SRF fellowship. Central Instrumentation facility CSIR-CSMCRI is acknowledged for acquiring MS, FT-IR, CD, and NMR spectral data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, P., Nalli, Y., Singh, S. et al. Dactylides A−C, three new bioactive 22-membered macrolides produced by Dactylosporangium aurantiacum. J Antibiot 76, 503–510 (2023). https://doi.org/10.1038/s41429-023-00632-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00632-z