Abstract
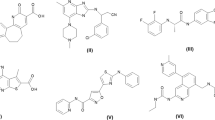
FtsZ inhibitors represent a new drug class as no drugs using this mode of action (MOA) have been approved by regulators. 3-alkoxy substituted 2,6-difluorobenzamide scaffold is one of the most studied FtsZ inhibitors among which the most promising anti-MRSA candidate TXA709 is in clinical trial. In this paper, we present the screening and evaluation of a benzamide class that is functionalized at the alkoxy fragment targeting Gram-negative bacteria. The variations in 3-alkoxy substitutions, specifically the hydroxylated alkyl residues to the secondary and stereogenic pseudo-benzylic carbon of their methyleneoxy linker, are particularly active against K. pneumoniae ATCC 10031 in marked contrast to the derivatives related to PC190723, all of which were inactive against Gram-negative bacteria. The two lead molecules TXA6101 and TXY6129 inhibit the polymerization of E. coli FtsZ in a concentration-dependent manner and induce changes in the morphology of E. coli and K. pneumoniae consistent with inhibition of cell division. These classes of compounds, however, were found to be substrates for efflux pumps in Gram-negative bacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
WHO Global antimicrobial resistance surveillance system (GLASS) report: early implementation 2016-2017. Geneva: World Health Organization; 2017.
O’Neill J. Tackling drug-resistant infections globally: final report and recommendations: Government of the United Kingdom; 2016.
Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13:1057–98.
Luepke KH, Suda KJ, Boucher H, Russo RL, Bonney MW, Hunt TD, et al. Past, present, and future of antibacterial economics: increasing bacterial resistance, limited antibiotic pipeline, and societal implications. Pharmacotherapy. 2017;37:71–84.
Perez F, Van Duin D. Carbapenem-resistant Enterobacteriaceae: a menace to our most vulnerable patients. Cleve Clin J Med. 2013;80:225–33.
Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25:682–707.
Adams DW, Errington J. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol. 2009;7:642–53.
Bi EF, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–4.
Erickson HP, Anderson DE, Osawa M. FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiol Mol Biol Rev. 2010;74:504–28.
Awasthi D, Kumar K, Ojima I. Therapeutic potential of FtsZ inhibition: a patent perspective. Expert Opin Ther Pat. 2011;21:657–79.
Hurley KA, Santos TM, Nepomuceno GM, Huynh V, Shaw JT, Weibel DB. Targeting the bacterial division protein FtsZ. J Med Chem. 2016;59:6975–98.
Carro L. Recent progress in the development of small-molecule ftsz inhibitors as chemical tools for the development of novel antibiotics. Antibiotics. 2019; 8.
Kusuma KD, Payne M, Ung AT, Bottomley AL, Harry EJ. FtsZ as an antibacterial target: status and guidelines for progressing this avenue. ACS Infect Dis. 2019;5:1279–94.
Schaffner-Barbero C, Martin-Fontecha M, Chacon P, Andreu JM. Targeting the assembly of bacterial cell division protein FtsZ with small molecules. ACS Chem Biol. 2012;7:269–77.
Artola M, Ruiz-Avila LB, Vergonos A, Huecas S, Araujo-Bazan L, Martin-Fontecha M, et al. Effective GTP-replacing FtsZ inhibitors and antibacterial mechanism of action. ACS Chem Biol. 2015;10:834–43.
Singh D, Bhattacharya A, Rai A, Dhaked HP, Awasthi D, Ojima I, et al. SB-RA-2001 inhibits bacterial proliferation by targeting FtsZ assembly. Biochemistry. 2014;53:2979–92.
Haydon DJ, Stokes NR, Ure R, Galbraith G, Bennett JM, Brown DR, et al. An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science. 2008;321:1673–5.
Kaul M, Mark L, Zhang Y, Parhi AK, LaVoie EJ, Pilch DS. Pharmacokinetics and in vivo antistaphylococcal efficacy of TXY541, a 1-methylpiperidine-4-carboxamide prodrug of PC190723. Biochem Pharm. 2013;86:1699–707.
Kaul M, Mark L, Zhang Y, Parhi AK, LaVoie EJ, Pilch DS. An FtsZ-targeting prodrug with oral antistaphylococcal efficacy in vivo. Antimicrob Agents Chemother. 2013;57:5860–9.
Kaul M, Mark L, Zhang Y, Parhi AK, Lyu YL, Pawlak J, et al. TXA709, an FtsZ-targeting benzamide prodrug with improved pharmacokinetics and enhanced in vivo efficacy against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2015;59:4845–55.
Lepak AJ, Parhi A, Madison M, Marchillo K, VanHecker J, Andes DR. In vivo pharmacodynamic evaluation of an FtsZ inhibitor, TXA-709, and its active metabolite, TXA-707, in a murine neutropenic thigh infection model. Antimicrob Agents Chemother. 2015;59:6568–74.
Stokes NR, Baker N, Bennett JM, Berry J, Collins I, Czaplewski LG, et al. An improved small-molecule inhibitor of FtsZ with superior in vitro potency, drug-like properties, and in vivo efficacy. Antimicrob Agents Chemother. 2013;57:317–25.
Stokes NR, Baker N, Bennett JM, Chauhan PK, Collins I, Davies DT, et al. Design, synthesis and structure-activity relationships of substituted oxazole-benzamide antibacterial inhibitors of FtsZ. Bioorg Med Chem Lett. 2014;24:353–9.
Fujita J, Maeda Y, Mizohata E, Inoue T, Kaul M, Parhi AK, et al. Structural flexibility of an inhibitor overcomes drug resistance mutations in Staphylococcus aureus FtsZ. ACS Chem Biol. 2017;12:1947–55.
UniProt C. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D15.
Kaul M, Zhang Y, Parhi AK, Lavoie EJ, Pilch DS. Inhibition of RND-type efflux pumps confers the FtsZ-directed prodrug TXY436 with activity against Gram-negative bacteria. Biochem Pharm. 2014;89:321–8.
Kaul M, Zhang Y, Parhi AK, Lavoie EJ, Tuske S, Arnold E, et al. Enterococcal and streptococcal resistance to PC190723 and related compounds: molecular insights from a FtsZ mutational analysis. Biochimie 2013;95:1880–7.
CLSI Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—Tenth Edition. Wayne, PA2015.
Kaul M, Mark L, Parhi AK, LaVoie EJ, Pilch DS. Combining the FtsZ-targeting prodrug TXA709 anD the Cephalosporin Cefdinir Confers Synergy and Reduces the Frequency of Resistance in Methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2016;60:4290–6.
Ferrer-Gonzalez E, Fujita J, Yoshizawa T, Nelson JM, Pilch AJ, Hillman E, et al. Structure-guided design of a fluorescent probe for the visualization of FtsZ in clinically important gram-positive and gram-negative bacterial pathogens. Sci Rep. 2019;9:20092.
Acknowledgements
We are indebted to Prof. Edmond J. LaVoie for providing scientific inputs. We are also indebted to Prof. Daniel Pilch and Dr. Edgar Ferrer-González for providing us with the differential interference contrast (DIC) micrographs and with the E. coli W4573, N43 (acrA1), LZ2096 (∆norE), and LZ2310 (acrA1 ∆mdfA ∆norE) strains.
Author information
Authors and Affiliations
Contributions
Conceptualization, AP; methodology, AP and JR; investigation, JR; YS; AB; YC; PD; YZ, and YY; writing—original draft preparation, AP; writing—review and editing, YY; JR; supervision, AP; project administration, AP; JR; and YS; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
AP, JR, PD, and YZ are employees of TAXIS Pharmaceuticals, Inc. and, therefore, have a financial interest in the company.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rosado-Lugo, J.D., Sun, Y., Banerjee, A. et al. Evaluation of 2,6-difluoro-3-(oxazol-2-ylmethoxy)benzamide chemotypes as Gram-negative FtsZ inhibitors. J Antibiot 75, 385–395 (2022). https://doi.org/10.1038/s41429-022-00531-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-022-00531-9
This article is cited by
-
Unrealized targets in the discovery of antibiotics for Gram-negative bacterial infections
Nature Reviews Drug Discovery (2023)