Abstract
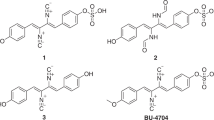
A new pluramycin-class polyketide, rausuquinone (1), and its known congener hydramycin (2) were isolated from the culture extract of the deep-sea water-derived Rhodococcus sp. RD015140. Compound 1 possesses a γ-pyrone-fused anthraquinone core with a 3-butene-1,2-diol side chain. Structures of 1 was determined by extensive analysis of 1D and 2D NMR spectroscopic data. Compound 1 showed antimicrobial activity against Gram-positive bacteria. This is the first discovery of aromatic polyketides from the genus Rhodococcus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hansen MR, Hurley LH. Pluramycins. Old drugs having modern friends in structural biology. Acc Chem Res. 1996;29:249–58.
Kondo S, Miyamoto M, Naganawa H, Takeuchi T, Umezawa H. Structures of pluramycin A and neopluramycin. J Antibiot. 1997;30:1143–5.
Yasuzawa T, Saitoh Y, Sano H. Structures of the novel anthraquinone antitumor antibiotics, DC-92B and DC92-D. J Antibiot. 1989;43:485–91.
Vértesy L, Barbone FP, Cashmen E, Decker H, Ehrlich K, Jordan B, et al. Potent antitumor antibiotics from Saccharothrix sp. DSM12931. J Antibiot. 2001;54:718–29.
Murphy BT, Narender T, Kauffman CA, Woolery M, Jensen PR, Fenical W. Saliniquinones A-F, new members of the highly cytotoxic anthraquinone- γ-pyrones from the marine actinomycete salinispora arenicola. Aust J Chem. 2010;63:929–34.
Jensen PR, Williams PG, Oh DC, Zeigler L, Fenical W. Species-specific secondary metabolite production in marine actinomycetes of the genus Salinispora. Appl Environ Microbiol. 2007;73:1146–52.
Jensen PR, Moore BS, Fenical W. The marine actinomycete genus Salinispora: A model organism for secondary metabolite discovery. Nat Prod Rep. 2015;32:738–51.
Komaki H, Sakurai K, Hosoyama A, Kimura A, Igarashi Y, Tamura T. Diversity of nonribosomal peptide synthetase and polyketide synthase gene clusters among taxonomically close Streptomyces strains. Sci Rep. 2018;8:1–11.
Komaki H, Sakurai K, Hosoyama A, Kimura A, Trujilo ME, Igarashi Y, et al. Diversity of PKS and NRPS gene clusters between Streptomyces abyssomicinicus sp. nov. and its taxonomic neighbor. J Antibiot. 2020;73:141–51.
Yang T, Yamada K, Zhou T, Harunari E, Igarashi Y, Terahara T, et al. Akazamicin, a cytotoxic aromatic polyketide from marine-derived Nonomuraea sp. J Antibiot. 2019;72:202–9.
Karim MRU, In Y, Zhou T, Harunari E, Oku N, Igarashi Y. Nyuzenamides A and B: bicyclic peptides with antifungal and cytotoxic activity from a marine-derived Streptomyces sp. Org Lett. 2021;23:2109–13.
Igarashi Y, Matsuyuki Y, Yamada M, Fujihara N, Harunari E, Oku N, et al. Structure determination, biosynthetic origin, and total synthesis of akazaoxime, an enteromycin-class metabolite from a marine-derived actinomycete of the genus Micromonospora 2021;86:6528–37.
Itoh J, Tsuyuki T, Fujita K, Sezaki M. Studies on a new antibiotic SF-2330 II. The structural elucidation. J Antibiot. 1986;39:780–3.
Schumacher RW, Davidson BS, Montenegro DA, Bernan VS. γ-Indomycinone, a new pluramycin metabolite from a deep-sea derived actinomycete. J Nat Prod. 1985;58:613–7.
Biabani MAF, Laatsch H, Helmke E, Weyland H. δ-Indomycinone: a new member of pluramycin class of antibiotics isolated from marine Streptomyces sp. J Antibiot. 1997;50:874–7.
Matsumoto M, Nagaoka K, Ishizeki S, Yokoi K, Nakajima T. Manufacture of antibiotic ss43405e by Streptomyces. Jpn Kokai Tokkyo Koho. 1986;JP 6118928.
Konishi M, Shimizu K, Ohbayashi M, Tomita K, Miyaki T, Oki T. BU-3839T antibiotic isolation and use as antibacterial agent and neoplasm inhibitor. 1990;U.S. patent 4927848.
Hanada M, kaneta K, Nishiyama Y, Hoshino Y, Konishi M, Oki T. Hydramycin, a new antitumor antibiotic. Taxonomy, isolation, physico-chemical properties, structure and biological activity. J Antibiot. 1991;44:824–31.
Uyeda M, Yokomizo K, Ito A, Nakayama K, Watanabe H, Kido Y. A new antiherpetic agent, AH-1763 IIa, produced by Streptomyces cyaneus strain No. 1763. J Antibiot. 1997;50:828–32.
Ceniceros A, Dijkhuizen L, Petrusma M, Medema MH. Genome-based exploration of the specialized metabolic capacities of the genus Rhodococcus. BMC Genomics. 2017;18:1–16.
Blin K, Shaw S, Steinke K, Villebro R, Ziemert N, Lee SY, et al. AntiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019;47:W81–7.
Nachtigall J, Schneider K, Nicholson G, Goodfellow M, Zinecker H, Imhoff JF, et al. Two new aurachins from Rhodococcus sp. Acta 2259. J Antibiot. 2010;63:567–9.
Kurosawa K, Ghiviriga I, Sambandan TG, Lessard PA, Barbara JE, Rha C, et al. Rhodostreptomycins, antibiotics biosynthesized following horizontal gene transfer from Streptomyces padanus to Rhodococcus fascians. J Am Chem Soc. 2008;130:1126–7.
Sharma AR, Harunari E, Oku N, Matsuura N, Trianto A, Igarashi Y. Two antibacterial and PPARα/γ-agonistic unsaturated keto fatty acids from a coral-associated actinomycete of the genus Micrococcus. Beilstein J Org Chem. 2020;16:297–304.
Acknowledgements
This work was partially supported by the Japan Society for the Promotion of Science (JSPS) for Young Scientists (21K14794).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Harunari, E., Bando, M. & Igarashi, Y. Rausuquinone, a non-glycosylated pluramycin-class antibiotic from Rhodococcus. J Antibiot 75, 86–91 (2022). https://doi.org/10.1038/s41429-021-00489-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-021-00489-0
This article is cited by
-
Development of a drug discovery approach from microbes with a special focus on isolation sources and taxonomy
The Journal of Antibiotics (2023)