Abstract
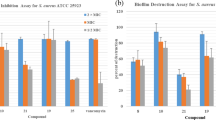
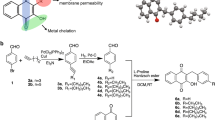
Methicillin-resistant Staphylococcus aureus (MRSA) infection is a major threat to human health due to its resistance to almost all classes of antibiotics. Discovery of novel antibacterial agents with new structures which combat the pathogens responsible for MRSA is urgent. In this study, three series of benzyl phenyl sulfide derivatives were designed and synthesized, and their antibacterial activity against eleven MRSA strains were evaluated. The results showed that two series of the synthetic compounds (5a-5l and 12p-12u) exhibit potent antibacterial activity against S. aureus and MRSA, with minimum inhibitory concentrations of 2–64 μg/mL. The structure-activity relationships are discussed and the mechanism of the antibacterial activity was shown to involve the destruction of the bacterial cell membrane. Finally, the MTT assay results suggest that the toxicity of compounds 5f and 5h is selective between bacteria and mammalian cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Guidos RJ, et al. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52:S397–S428.
Klevens RM, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA-J Am Med Assoc. 2007;298:1763–71.
Talbot GH, et al. Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability task force of the infectious diseases society of America. Clin Infect Dis. 2006;42:657–68.
Lin Z, et al. Total synthesis and antimicrobial evaluation of natural albomycins against clinical pathogens. Nat Commun. 2018;9:3445.
Wu CC, Chung JG, Tsai SJ, Yang JH, Sheen LY. Differential effects of allyl sulfides from garlic essential oil on cell cycle regulation in human liver tumor cells. Food Chem Toxicol. 2004;42:1937–47.
Rattanachaikunsopon P, Phumkhachorn P. Diallyl sulfide content and antimicrobial activity against food-borne pathogenic bacteria of chives (Allium schoenoprasum). Biosci Biotechnol Biochem. 2008;72:2987–91.
Hatano A, Nishimura M, Souta I. Impact of unnatural nucleosides on the control of microbial growth. Biocontrol Sci. 2009;14:55–60.
Mostofi M, Mohammadi Ziarani G, Lashgari N. Design, synthesis and biological evaluation of benzofuran appended benzothiazepine derivatives as inhibitors of butyrylcholinesterase and antimicrobial agents. Bioorg Med Chem. 2018;26:3076–95.
Lee MK, Senius JD, Grossman MJ. Sulfur-specific microbial desulfurization of sterically hindered analogs of dibenzothiophene. Appl Environ Microbiol. 1995;61:4362–6.
Wang N, Saidhareddy P, Jiang X. Construction of sulfur-containing moieties in the total synthesis of natural products. Nat Prod Rep. 2019; https://doi.org/10.1039/c8np00093j.
Feng M, Tang B, Liang SH, Jiang X. Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr Top Med Chem. 2016;16:1200–16.
Hatano A, et al. Inhibitory effects on microbial growth using the derivatives of benzyl phenyl sulfide. Biocontrol Sci. 2011;16:63–7.
Asgharpour Z, Farzaneh F, Ghiasi M, Azarkish M. Synthesis, characterization, density functional theory studies and antibacterial activity of a new Schiff base dioxomolybdenum(VI) complex with tryptophan as epoxidation catalyst. Appl Organomet Chem. 2017;31 (e3782).
Wan MS, et al. Synthesis and properties of novel stilbene-twelve alkyl quaternary ammonium salts as antibacterial optical whitening agents. Cellulose. 2017;24:3209–18.
Geng Z-Z, et al. Novel cajaninstilbene acid derivatives as antibacterial agents. Eur J Med Chem. 2015;100:235–45.
Huang MY, et al. Anti-inflammatory effects of cajaninstilbene acid and its derivatives. J Agric Food Chem. 2016;64:2893–900.
Wilson ZE, Brimble MA. A flexible asymmetric synthesis of the tetracyclic core of berkelic acid using a Horner-Wadsworth-Emmons/oxa-Michael cascade. Org Biomol Chem. 2010;8:1284–6.
Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163–75.
Koh J-J, et al. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim Biophys Acta Biomembr. 2013;1828:834–44.
Fauvart M, Van den Bergh B, Michiels J. Stabbed while sleeping: synthetic retinoid antibiotics kill bacterial persister cells. Mol Cell. 2018;70:763–64.
Kim W, et al. NH125 kills methicillin-resistant Staphylococcus aureus persisters by lipid bilayer disruption. Future Med Chem. 2016;8:257–69.
Kim W, et al. A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature. 2018;556:103–7.
Konai MM, Ghosh C, Yarlagadda V, Samaddar S, Haldar J. Membrane active phenylalanine conjugated lipophilic norspermidine derivatives with selective antibacterial activity. J Med Chem. 2014;57:9409–23.
Ghosh C, et al. Small molecular antibacterial peptoid mimics: the simpler the better! J Med Chem. 2014;57:1428–36.
Yarlagadda V, Akkapeddi P, Manjunath GB, Haldar J. Membrane active vancomycin analogues: a strategy to combat bacterial resistance. J Med Chem. 2014;57:4558–68.
Koh JJ, et al. Nonpeptidic amphiphilic xanthone derivatives: structure-activity relationship and membrane-targeting properties. J Med Chem. 2016;59:171–93.
Seydlova G, et al. Lipophosphonoxins II: design, synthesis, and properties of novel broad spectrum antibacterial agents. J Med Chem. 2017;60:6098–118.
Nobmann P, Bourke P, Dunne J, Henehan G. In vitro antimicrobial activity and mechanism of action of novel carbohydrate fatty acid derivatives against Staphylococcus aureus and MRSA. J Appl Microbiol. 2010;108:2152–61.
Huang M-Y, et al. Design, synthesis and anti-inflammatory effects of novel 9-O-substituted-berberine derivatives. MedChemComm. 2016;7:658–66.
Acknowledgements
We sincerely thank the National Natural Science Foundation of China (81673336), Pearl River S&T Nova Program of Guangzhou (201806010116), and the Fundamental Research Funds for the Central University (21617478) for financial support of this study. We sincerely thank Lingwei Wang and Yuemei Lu from the Second Clinical Medical College of Jinan University for their donation of clinical isolated MRSA strains.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, K., Chen, Q., Xu, XF. et al. Novel benzyl phenyl sulfide derivatives as antibacterial agents against methicillin-resistant Staphylococcus aureus. J Antibiot 73, 82–90 (2020). https://doi.org/10.1038/s41429-019-0257-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-019-0257-x