Abstract
The present report describes our efforts to identify new structural classes of compounds having promising antibacterial activity using previously published double-reporter system pDualrep2. This semi-automated high-throughput screening (HTS) platform has been applied to perform a large-scale screen of a diverse small-molecule compound library. We have selected a set of more than 125,000 molecules and evaluated them for their antibacterial activity. On the basis of HTS results, eight compounds containing 2-pyrazol-1-yl-thiazole scaffold exhibited moderate-to-high activity against ΔTolC Escherichia coli. Minimum inhibitory concentration (MIC) values for these molecules were in the range of 0.037–8 μg ml−1. The most active compound 8 demonstrated high antibacterial potency (MIC = 0.037 μg ml−1), that significantly exceed that measured for erythromycin (MIC = 2.5 μg ml−1) and was comparable with the activity of levofloxacin (MIC = 0.016 μg ml−1). Unfortunately, this compound showed only moderate selectivity toward HEK293 eukaryotic cell line. On the contrary, compound 7 was less potent (MIC = 0.8 μg ml−1) but displayed only slight cytotoxicity. Thus, 2-pyrazol-1-yl-thiazoles can be considered as a valuable starting point for subsequent optimization and morphing.
Similar content being viewed by others
Introduction
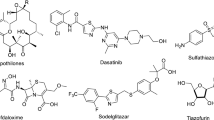
Multiple reasons, including antibiotic resistance and difficulties of licensing, limit the development of new effective antibacterial compounds. Moreover, it takes more than 10 years and costs approximately US$1.6 billion to achieve a clinically approved candidate [1]. Under the circumstances, the discovery of novel antibacterial agents is a question of low interest for many large pharmaceutical companies [2]. However, there is a perpetual need for new antibiotics [3]. Today nearly 25,000 lethal cases per year in both European Union and United States are caused by new strains of resistant bacteria [4, 5]. The golden era of antibiotics lasted from 1940 to 1970, since many new molecules with antibacterial activity have been reported. It should be noted, that most of them contain previously known scaffolds. Over the past decades, only few drug candidates of new structural classes have been launched. For instance, bedaquiline and delamanid were launched in 2013 and 2014, respectively [6, 7]. Bedaquiline was the first drug candidate for the treatment of tuberculosis approved by FDA for the last 50 years. In fact, the majority of antibiotics in clinical practice are no longer efficient against new bacteria strains such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, β-lactam-resistant Klebsiella pneumoniae, norfloxacin-resistant S. aureus, and colistin-resistant Escherichia coli. Moreover, infectious diseases caused by multidrug-resistant bacteria are the main challenge, which is associated with high mortality rates. The expansion of chemical space of antibacterial compounds may be a promising strategy to address the issues above. For the moment, a half of antibacterials are β-lactams and quinolones [3]. Many small-molecule antibacterial compounds are being developed for the last couple years. Several examples are represented in Fig. 1. These include, 4-hydroxy-2-pyridones (I) [8], 4-azabenzimidazoles (II) [9], benzoxazoles (III) [10], thienopyrimidines (IV) [11], isoxazole-substituted 2-aminothiazoles (V) [12], and isoquinoline ethyl ureas (VI) [13].
The common strategy in drug discovery for hit hunting is high-throughput screening (HTS). We have recently developed and validated a multiplex reporter system for the identification of antibacterial activity that can distinguish between antibiotics, which induce SOS response due to DNA damage via RecA/LexA/SulA pathway or block translation [14]. We have recently applied this approach to discover new translation inhibitors [14, 15]. This convenient screening method is a relatively simple, cost-effective, robust, well-automated, and has high sensitivity and specificity. In the following report, the antibacterial activity of almost 125,000 compounds against ΔTolC E. coli has been evaluated. The mutant ΔtolC E. coli strain lacks TolC, which is a cell surface component of several drug efflux pumps that are responsible for antibiotic resistance. As a result, a series of 2-pyrazol-1-yl-thiazoles has been discovered. Preliminary SAR investigation was performed and described. We have also determined the selectivity of the discovered hit compounds against three mammalian cell lines. Noteworthy, most of the compounds have demonstrated moderate or low cytotoxicity.
Materials and methods
Screening compounds
For all the HTS rounds as well as rescreen procedure small-molecule compounds were selected from available in-house collections by ChemDiv [16], InterBioScreen [17], and Enamine [18]. The purity of tested compounds was more than 90%. In silico mining was performed in ChemoSoft software. All the selected compounds were officially purchased and delivered in separate Greiner #651201 microplates sealed with capmats (low-profile shallow-well block plates, 300 μl volume) with no empty wells (all 96-wells were filled by samples and accompanied with the list of unique ID numbers). The most part of the samples were solid. All the molecules were supplied with 1H nuclear magnetic resonance spectra and with the date of synthesis.
Reporter vector and approach
A double-reporter system pDualrep2 was used to distinguish between molecules able to block translational machinery or induce SOS response in a model E. coli system. The reporter ensemble was developed as described previously [19]. The expression of the product of Katushka2S gene (λ = 588/633 nm) increases greatly in the presence of antibiotics. They inhibit translation since the regulatory region encoded the leader peptide of trp operon is located prior to the gene of fluorescent protein. Expression of the second reporter gene, RFP that codes red fluorescent protein (RFP) (λ = 553/574 nm), increases in the presence of antibiotics that cause DNA damage or interfere with replication. This effect is clearly observed within the areas of sublethal concentration of an antibacterial molecule where it does not kill bacteria but substantially inhibit translation. The lethal concentration of a molecule is not known beforehand. Therefore, the assay was performed using the upper dose tolerant for the agar plate. Then, a concentration gradient was formed around the central drop due to diffusion. As a result, the area of sublethal concentration can be seen at some distance from the application point.
High-throughput screening
E. coli strain JW5503 [20], lacking the TolC gene, was grown at 37 °C in Luria-Bertani (LB minimal) medium, supplied with 50 μg ml−1 ampicillin if required. An overnight culture of cells was transformed with the pDualrep2 reporter plasmid. Then, the resulting culture was diluted to 0.05–0.1 optical density (OD, 590 nm) units with fresh LB medium (lysogeny broth) supplied with 50 μg ml−1 ampicillin and plated on the surface of a LB agar large square 23 × 23 cm plate with 50 μg ml−1 ampicillin. The Janus robotic station (Perkin Elmer) available at the Center of Collective Use, Moscow State University, was used to perform HTS of the compounds. Molecules were dissolved in 100% dimethyl sulfoxide (DMSO) up to concentration of 17 mg ml−1. Compounds were then added in agar plates with the reporter strain using a 96-channel pipetting head in a volume of 2 μl for each sample. The concentration gradient (from 17 to 0 mg ml−1) per each sample was achieved. After 12 h incubation at 37 °C, the petri dishes were scanned by ChemiDoc (Bio-Rad) in the Cy3 (RFP) and Cy5 (Katushka2S) channels to detect fluorescence intensity of the reporter protein. Fluorescence signals were quantified with ImageLab software. Images scanned separately in RFP and Katushka2S channels were merged as green and red pseudocolors, respectively. After incubation with the tested sample, plates were subjected to visual inspection for the areas of growth attenuation. Subinhibitory concentration of a compound induces the fluorescence of the reporter protein within the periphery of inhibitory region.
Minimum inhibitory concentration (MIC)
MICs in LB and M9 medium were determined using a broth microdilution assay [21]. The cell concentration was adjusted to approximately 5 × 105 cells/ml. The tested compounds were serially diluted twofold in a 96-well microplate (100 μl per well). Microplates were covered and incubated at 37 °C with shaking. The OD600 of each well was measured, and the lowest concentration of the tested compound that resulted in no growth after 16–20 h was assigned to MIC value.
Culture conditions and treatments
HEK293 (human embryonic kidney 293 cells), A549 (human lung carcinoma), MCF7 (human breast adenocarcinoma) cell lines were purchased from the Russian Cell Culture Collection (Institute of Cytology Russian Academy of Science, Saint Petersburg, Russia). HEK293, A549, and MCF7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, USA) supplemented with 2 mM l-glutamine (Sigma-Aldrich, UK), 10% fetal bovine serum (Invitrogen, USA), 50 μg ml−1 gentamicin sulfate (Invitrogen, USA) at 37 °C and 5% CO2. All compounds were dissolved in 100% DMSO (Sigma-Aldrich, UK) to 100 mM stock solutions and diluted in completed DMEM immediately before addition to the assay plates. DMSO was maintained at a final concentration of 0.1%.
Cell viability
Cells were cultured at appropriate density in 96-well plates (3 × 104 cells/well for HEK293; 1.2 × 104 cells/well for A549; 1.2 × 104 cells/well for MCF7) and allowed to grow for 24 h. Thereafter, cells were treated with compounds at a final concentration of 10 µM for 48 h and cell viability was measured by the “PrestoBlue Cell Viability Reagent” (Thermo Fisher Scientific, USA) following manufacturer’s instruction using “2300 EnSpire® Multimode Plate Reader” (Perkin Elmer, USA) at 590 nm. Experiments were repeated independently two times in triplicate and data were expressed as PrestoBlue reagent reduction relative to control (cells treated with 0.1% DMSO). The viability of control cells was set as 100%, and the viability in the experimental groups was calculated by comparing the OD reading with the control.
Statistical analysis
All results were expressed as mean values ± standard deviation. One-way analysis of variance followed by Dunnett’s post hoc test was used for all comparisons.
IP assessment
The structure of the most active molecule was investigated on its IP profile using the common sources, e.g., SciFinder database [22] and Thompson Integrity [23], as well as by the detailed analysis of patent records generally focusing on matching the Markush structure claimed and substituents listed therein.
Results and discussion
The screening library was collected in accordance with the list of principle rules: (I) low structural similarity of screening compounds to that published earlier in scientific literature or reported in specific databases, e.g., Thomson Integrity [23] and ChEMBL [24]; compounds should not exceed Tanimoto coefficient of 0.7; (II) screening library should not include structural analogues and molecules of reported scaffolds with the evaluated antibacterial activity, (III) the final chemical space was normalized (clustering procedure was performed followed by selection of the most diverse structures per cluster) and outliers were excluded via Sammon mapping [25]. The key molecular descriptors, including MW, LogP, HBA, HBD, PSA, LogSw, and RBN were calculated and used in the selection procedure and for nonlinear mapping. The final library was screened utilizing the double-reported pDualrep2 system described above. As a result, 7900 compounds were observed to have visible inhibition signature. While, several wells were cloudy by a strong inhibition response of one molecule, it was somewhat difficult to visually inspect the antibacterial activity of a specific compound. Therefore, the rescreening procedure was performed, resulting in the confirmed antibacterial potency for 65% of compounds. The in vivo activity of the hits disclosed was assigned into four classes in accordance with the diameter of inhibition zone: “±” – 4–7 mm, “+” – 7–11 mm, “++” – 11–15 mm, “+++” – 16–20 mm; “++++” – 20–25 mm. Among them, 688 molecules were classified as having moderate-to-high activity (++ and higher). The upper antibacterial potency (+++ and higher) was revealed for 117 molecules. The most promising compounds were used as templates for further searching of structural analogues within mentioned vendors’ collections. Among the 10,000 of selected compounds, a series of 38 2-pyrazol-1-yl-thiazoles was identified as new chemotype for antibacterial compounds. Thomson Integrity and SciFinder databases analysis demonstrated that antibacterial activity of the described compounds was not reported previously. For the most interesting hits the MICs have been evaluated. The obtained results are summarized in Table 1. Compounds 1–8 were found from ChemDiv commercial collection. These molecules demonstrated a visible red signature response, proving that translation inhibition is the mechanism of action (Fig. 2). The data on HTS suggests that in this series of molecules 5-hydroxyl on the pyrazole ring is necessary for good antibacterial activity. Thus, the replacement of this moiety with hydrogen, methyl or larger alkyl groups resulted in the total loss of activity. It was found that compounds bearing 4-methyl substituent on thiazol had a significantly improved activity (“++” and higher). Compound 1 was equipotent with compound 2, thus it can be speculated that the length of carbon chain between thiazol ring and amide have no influence on MIC activity. Moreover, introduction of meta-fluorine atom on the phenyl ring in compound 3 resulted in a twofold increase in activity, as compared to compound 2, containing 4-fluoro-phenyl. These observations suggest that position 5 may be considered as a diversity point for the activity optimization. Phenyl ring substitution with larger electron-withdrawing groups (Br, –CF3, –CH3CHO) has led to the highly potent compounds. The further investigation on different phenyl substituents is a clear way to achieve a promising gain in potency.
The design and development of new antibiotics are associated with high risk of unfavorable off-target activity against eukaryotes. Thus, after assessing the antibacterial activity of the presented series, we next evaluated the cytotoxicity of eight most active compounds against human embryonic kidney 293 (HEK293), adenocarcinomic human alveolar basal epithelial (A549), and Michigan cancer foundation—7 (MCF7) cell lines using PestoBlue viability test (Table 2). No correlation between MIC values and cytotoxicity against normal HEK293 cells was found. Thus the Pearson correlation coefficient value is 0.06. Therefore, we can conclude that antibacterial activity of the described compounds is not associated with the cytotoxicity.
Most of the presented compounds have shown low-to-moderate cytotoxicity, as compared to levofloxacin, demonstrating survival rate in HEK 239 of 60%. Compounds 2 and 6 with the similar structure were found to exhibit moderate cytotoxicity against HEK293, which may be associated with the enhanced length of carbon chain. The lack of selectivity of compound 4 against HEK293 may be associated with the ester group, which may result in undesired toxicity. Unfortunately, the most active compound 8 have demonstrated undesired cytotoxicity against HEK293, which may result from ketone para-substituent. Interestingly, compound 7 with meta-trifluoromethyl substituent exhibited potent antibacterial activity with low cytotoxicity against the observed cell lines. Further evaluation on phenyl substituents may result in significant increase in potency and improved cytotoxicity profile. Effort will be made to determine the target in the complex translation apparatus in the future. Further work will also be conducted on the frequency of resistance development and spectrum of activity prior to further optimization and development of the hit compound.
Conclusion
In summary, we have successfully applied our unique HTS platform for the identification of new small molecules with high antibacterial potency. In the present work the series of 2-pyrazol-1-yl-thiazoles was described as new antibacterials. Several compounds displayed the potent activity against E. coli with MIC values in the range of 0.037–8 μg ml−1. The best activity was observed for compound 8, which exhibited a MIC of 0.037 μg ml−1. However, compound 8 showed an undesired cytotoxicity in the mammalian cells. The most promising compound 7 demonstrated both high antibacterial activity and selectivity. The preliminary SAR studies were described, suggesting the key structural determinates and variability points. Future research will focus on follow-up synthesis, and in vivo studies against a panel of resistant bacterial strains.
References
DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ 2016;47:20–33.
Website. Available at: www.idsociety.org/Content.aspx?id=17577. Accessed 7 December 2018.
Singh N, et al. QSAR classification model for antibacterial compounds and its use in virtual screening. J Chem Inf Model. 2012;52:2559–69.
Gulland A. Antimicrobial resistance is now widespread, warns WHO. Br Med J. 2014;348:g3062–g3062.
Website. Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed 7 December 2018.
Kim CT, et al. Bedaquiline and delamanid for the treatment of multidrug-resistant tuberculosis: a multicentre cohort study in Korea. Eur Respir J. 2018;51:1702467.
Pontali E, et al. Combined treatment of drug-resistant tuberculosis with bedaquiline and delamanid: a systematic review. Eur Respir J. 2018;52:1800934.
Gerasyuto AI, et al. Discovery and optimization of indolyl-containing 4-hydroxy-2-pyridone type II DNA topoisomerase inhibitors active against multidrug resistant gram-negative bacteria. J Med Chem. 2018;61:4456–75.
Skepper CK, et al. Discovery and optimization of phosphopantetheine adenylyltransferase inhibitors with gram-negative antibacterial activity. J Med Chem. 2018;61:3325–49.
Chacko S, et al. Expanding benzoxazole-based inosine 5′-monophosphate dehydrogenase (IMPDH) inhibitor structure–activity as potential antituberculosis agents. J Med Chem. 2018;61:4739–56.
De Schutter JW, Morrison JP, Morrison MJ, Ciulli A, Imperiali B. Targeting bacillosamine biosynthesis in bacterial pathogens: development of inhibitors to a bacterial amino-sugar acetyltransferase from Campylobacter jejuni. J Med Chem. 2017;60:2099–118.
Azzali E, et al. Substituted N-Phenyl-5-(2-(phenylamino)thiazol-4-yl)isoxazole-3-carboxamides are valuable antitubercular candidates that evade innate efflux machinery. J Med Chem. 2017;60:7108–22.
Panchaud P, et al. Discovery and optimization of isoquinoline ethyl ureas as antibacterial agents. J Med Chem. 2017;60:3755–75.
Osterman IA, et al. Sorting out antibiotics’ mechanisms of action: a double fluorescent protein reporter for high throughput screening of ribosome and DNA biosynthesis inhibitors. Antimicrob Agents Chemother. 2016;60:7481–9.
Komarova Andreyanova ES, et al. 2-Guanidino-quinazolines as a novel class of translation inhibitors. Biochimie. 2017;133:45–55.
ChemDiv - Contract Research Organization. Chemdiv. Available at: http://www.chemdiv.com/. Accessed 7 December 2018.
InterBioScreen ltd. Compound Libraries. InterBioScreen ltd. Compound Libraries. Available at: https://www.ibscreen.com. Accessed 7 December 2018.
EnamineStore. Available at: www.enaminestore.com/. Accessed 7 December 2018.
Osterman IA, et al. Attenuation-based dual-fluorescent-protein reporter for screening translation inhibitors. Antimicrob Agents Chemother. 2012;56:1774–83.
Schumacher SD, Hannemann F, Teese MG, Bernhardt R, Jose J. Autodisplay of functional CYP106A2 in Escherichia coli. J Biotechnol. 2012;161:104–12.
Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163–75.
SciFinder - Sign In. Available at: https://scifinder.cas.org/. Accessed 7 December 2018.
Website. Available at: https://integrity.thomson-pharma.com/. Accessed 7 December 2018.
EMBL-EBI. The European Bioinformatics Institute <EMBL-EBI. Available at https://www.ebi.ac.uk/. Accessed 4 December 2018.
Sammon JW. A nonlinear mapping for data structure. Anal IEEE Trans Comput C. 1969;18:401–9.
Acknowledgements
The authors would like to kindly acknowledge the Ministry of Education and Science of the Russian Federation, government grant 20.9907.2017/VU (expert opinion, discussion, and paper preparation) and Russian Science Foundation No. 17-74-30012, IBG RAS Ufa (biological evaluation and compound selection).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ivanenkov, Y.A., Yamidanov, R.S., Osterman, I.A. et al. 2-Pyrazol-1-yl-thiazole derivatives as novel highly potent antibacterials. J Antibiot 72, 827–833 (2019). https://doi.org/10.1038/s41429-019-0211-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-019-0211-y