Abstract
Andrastins are meroterpenes isolated from Penicillium sp. FO-3929 that display highly potent inhibitory activities toward protein farnesyltransferase. Structurally, they possess a unique steroidal tetracyclic skeleton (the ABCD-ring) with three contiguous quaternary stereocenters on the C-ring. Herein, we describe our nitrile cyclization-based approach to the stereoselective construction of the BCD-ring system of andrastins, which contains three contiguous quaternary stereocenters on the C-ring and the correct oxidation states of the D-ring.
Similar content being viewed by others
Introduction
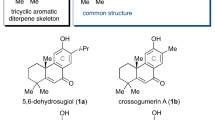
Andrastins A–D (1–4) are fungal meroterpenes that were isolated from the cultured broth of Penicillium sp. FO-3929 by Ōmura et al. in 1996 [1,2,3,4]. These compounds exhibit potent inhibitory activities toward protein farnesyltransferase (IC50, 13.3–47.1 μM) [2, 4]. Since protein farnesyltransferase is essential for maturation of the Ras oncogene protein, andrastins are considered promising anti-cancer drug candidates [5]. Structurally, andrastins A–D (1–4) share a unique steroidal 6-6-6-5 tetracyclic skeleton (the ABCD-ring), differing only in the substituent at the angular C10 position and the oxidation state at C3 of the A-ring (Scheme 1a). Their distinctive biological and pharmacological properties, coupled with their complex chemical structures, have made andrastins attractive targets for chemical synthesis. As a result, a number of research groups have been involved in synthetic studies of andrastins [6,7,8,9,10]. Recently, the first total synthesis of ( ± )-andrastin D (4) was achieved by Newhouse and co-workers via a biomimetic polyene cyclization and a diketene annulation as the key steps [6]. Additionally, Toyota and co-workers constructed the tetracyclic framework of andrastins that featured a stereoselective intramolecular Diels–Alder reaction and a carbonyl ene reaction [7, 8]. Matsuya and co-workers reported an alternative intramolecular Diels–Alder approach to the tetracyclic skeleton of andrastins [9, 10]. In order to ultimately understand the detailed mode of action of andrastins at the molecular level, we have engaged in synthetic studies of them. Herein, we report a novel nitrile cyclization-based approach for the stereoselective construction of the BCD-ring system with three contiguous quaternary asymmetric stereocenters, the common substructure in andrastins.
Results and discussion
From a synthetic perspective, the major challenges posed by andrastins 1–4 are (1) construction of the stereochemically dense C-ring, which has three contiguous quaternary stereocenters at the C8, C13, and C14 positions, and (2) construction of the five-membered D-ring, which has a sensitive 1,3-diketone moiety. To overcome these challenges, we designed a synthetic strategy based on nitrile cyclizations (Scheme 1b). To confirm our strategy, tricyclic model compound 10 was chosen as the initial target. We planned to synthesize 10 from cyclic nitrile 5 (the B-ring model), whereupon a quaternary stereocenter at the C8 position would be constructed by the intramolecular conjugate addition of α,β-unsaturated esters bearing an alkanenitrile side chain [11]. An intramolecular ene reaction of nitrile 6 would construct the C-ring structure 8 after isomerization of the olefin in the ene product 7 and the subsequent hydrolysis of the resulting imine moiety. In contrast to carbonyl ene reactions that have been widely used in organic synthesis [12], cyano ene reactions have been less frequently explored due to the poor reactivity of the cyano group as an enophile [13,14,15]. However, we anticipated that the cyano ene reaction of the simple non-activated alkanenitrile 6 would proceed upon activation by a Brønsted or Lewis acid because of an entropically favored intramolecular process. Lastly, formation of the D-ring with the desired oxidation states would be achieved through a similar intramolecular cyano ene reaction of β-ketonitrile 9 to afford tricyclic compound 10.
Our synthesis commenced with the preparation of cyano alkene 6 (Scheme 2). Alkylation of ethyl isobutyrate (11) with 5-bromo-1-pentene followed by reduction with LiAlH4 gave primary alcohol 12, which underwent cobalt-catalyzed hydrocyanation [16] to furnish secondary nitrile 14 with complete regioselectivity. The product 14 was then converted to α,β-unsaturated ester 15 through a Swern oxidation and subsequent Horner–Wadsworth–Emmons olefination. Treatment of 15 with potassium bis(trimethylsilyl)amide (KHMDS) in THF–toluene at –78 °C induced an intramolecular conjugate addition [11] to give cyclization product 5 (the B-ring) with the quaternary stereocenter at the C8 position (96% yield) with high diastereoselectivity (dr = 95:5). The high stereoselectivity of this cyclization can be explained through the chelated transition state model, wherein the keteniminate and α,β-unsaturated ester are both oriented equatorially in an antiparallel dipolar arrangement (Supplementary Information, Scheme S1). The stereochemistry of 5 was confirmed by NOESY experiments. Chemoselective reduction of the ester over the cyano group in 5 with diisobutylaluminum hydride (DIBAL-H) followed by Swern oxidation produced aldehyde 16. This compound was converted to cyano alkene 6 as inseparable geometric isomers (68:32) in three steps, i.e., addition of methyl Grignard reagent, tetrapropylammonium perruthenate (TPAP) oxidation [17] of the resulting alcohol, and Wittig olefination of the resulting methyl ketone.
With nitrile 6 in hand, we examined the key ene reaction for the C-ring formation (Scheme 3). Upon treatment of 6 with methanesulfonic acid (MsOH) (10 equiv.) in 1,2-dichloroethane at 80 °C, the intramolecular ene reaction of nitrile 6 and the subsequent isomerization of the olefin occurred smoothly. After the disappearance of 6, which was monitored by TLC, the solvent was removed by evaporation, and the resulting mixture containing α,β-unsaturated iminium 19 and MsOH was hydrolyzed at 80–100 °C to afford enone 8 (86% yield). Thus, this intramolecular cyano ene reaction proved to be a powerful method for the formation of a cyclic enone because the C–C bond forming reaction proceeded at the sterically congested neopentyl position. At present, we assume that the MsOH-mediated cyclization of 6 proceeded via a cyano ene reaction, although an alternative reaction mechanism [18] involving a cationic cyclization of a nitrilium ion cannot be ruled out.
Our next objective was the synthesis of the D-ring cyclization precursor 27 (Scheme 4). Treatment of enone 8 with trimethylsilyl cyanide (TMSCN) in the presence of a trimethylsilyl trifluoromethanesulfonate (TMSOTf) catalyst afforded a diastereomeric mixture of cyanohydrins and their trimethylsilyl (TMS) ethers 20. Dehydration of the crude products 20 with MsOH yielded the desired unsaturated nitrile 21 along with enone 8 and β-cyano ketone 22. Construction of the quaternary stereocenter at the C14 position was accomplished by deconjugative alkylation of 21. Upon treatment of 21 with lithium diisopropylamide (LDA) in THF at –78 °C followed by addition of benzyloxymethyl chloride (BOMCl), deconjugative alkylation proceeded at the less hindered face of the six-membered ring, i.e., the opposite side of the C8 methyl group, to provide target compound 23 in 82% yield (dr = 92:8) accompanied by γ-alkylation product 24 (14% yield). The stereochemistry of 23 was determined by an NOE experiment of alcohol 25 after removal of the benzyl group in 23. Alcohol 25 was successfully converted to nitrile 27 as an inseparable mixture of four isomers (dr = 55:26:12:7) by a three-step sequence: (1) partial hydrogenation of the diene in 25 using Pd/C (5%) with concomitant isomerization to afford tetrasubstituted olefin 26, wherein a minor diastereomer derived from 23 was separated, (2) Swern oxidation of the primary alcohol, and (3) addition of the deprotonated propionitrile to the resulting aldehyde.
The final task in the synthesis was the challenging cyano ene reaction for the formation of the D-ring with construction of the quaternary stereocenter at the C13 position (Scheme 5). To this end, alcohol 27 was oxidized with pyridinium chlorochromate (PCC) to β-ketonitrile 9. The intramolecular cyano ene reaction of 9 proceeded smoothly when treated with BCl3 (6 equiv.) at room temperature, affording the stable enaminone 28 as a single diastereomer (93% yield). NOESY experiments revealed that 28 possessed the 6,5-cis-fused ring system (the CD-ring) with the correct stereostructure. However, enaminone 28 resisted hydrolysis to 1,3-diketone 29 under either acidic or basic conditions. To enhance the reactivity toward hydrolysis as well as to prevent unreactive enaminone formation, we designed the new cyclization precursor 30, possessing an electron-withdrawing chlorine atom at the α-position of the cyano group. The requisite nitrile 30 was directly synthesized from 27 by a Swern oxidation using an excess amount of oxidant (3 equiv.) [19]. As expected, the cyclization of 30 with BCl3 gave α-chloro imine 31, and the subsequent hydrolysis of 31 with aqueous MsOH afforded diketone 32 (50% yield, two steps). Finally, reductive dechlorination of 32 with Zn–AcOH followed by isomerization of the exo olefin yielded the tricyclic compound 10 (84% yield, two steps), thus completing the synthesis of the andrastin BCD-ring system.
Conclusion
In conclusion, we have developed a stereoselective synthetic route to the BCD-ring system of novel protein farnesyltransferase inhibitors, andrastins. The synthesis features (1) construction of the B-ring through a stereoselective conjugate addition of an α-cyano carbanion to an α,β-unsaturated ester, and (2) intramolecular cyano ene reactions for the formation of the C and D-rings. The successful synthesis of the highly sterically congested BCD-ring component having three contiguous stereocenters on the C-ring demonstrates the synthetic utility of nitrile cyclizations (i.e., an intramolecular conjugate addition and a cyano ene reaction). Further efforts on the total syntheses of andrastins are currently underway.
References
Shiomi K, Uchida R, Inokoshi J, Tanaka H, Iwai Y, Ōmura S. Andrastins A~C, new protein farnesyltransferase inhibitors, produced by Penicillium sp. FO-3929. Tetrahedron Lett. 1996;37:1265–8.
Ōmura S, Inokoshi J, Uchida R, Shiomi K, Masuma R, Kawakubo T, et al. Andrastins A-C, new protein farnesyltransferase inhibitors produced by Penicillium sp. FO-3929. I. Producing strain, fermentation, isolation, and biological activities. J Antibiot. 1996;49:414–7.
Uchida R, Shiomi K, Inokoshi J, Sunazuka T, Tanaka H, Iwai Y, et al. Andrastin A-C, new protein farnesyltransferase inhibitors produced by Penicillium sp. FO-3929. II. Structure elucidation and biosynthesis. J Antibiot. 1996;49:418–24.
Uchida R, Shiomi K, Inokoshi J, Tanaka H, Iwai Y, Ōmura S. Andrastin D, novel protein farnesyltransferase inhibitor produced by Penicillium sp. FO-3929. J Antibiot. 1996;49:1278–80.
Hinterding K, Alonso-Díaz D, Waldmann H. Organic synthesis and biological signal transduction. Angew Chem Int Ed. 1998;37:688–749.
Xu G, Elkin M, Tantillo DJ, Newhouse TR, Maimone TJ. Traversing biosynthetic carbocation landscapes in the total synthesis of andrastin and terretonin meroterpenes. Angew Chem Int Ed. 2017;56:12498–502.
Okamoto R, Takeda K, Tokuyama H, Ihara M, Toyota M. Toward the total synthesis of (±)-Andrastin C. J Org Chem. 2013;78:93–103.
Toyota M, Okamoto R, Ogata T, Ihara M. Ti(III)-induced radical cyclization. A stereoseletive entry to the perhydrobenzo[e]indene unit of new protein farnesyltransferase inhibitors, andrastins A–D. Tetrahedron Lett. 2004;45:9203–5.
Yin S, Sugimoto K, Nemoto H, Matsuya Y. Synthetic study towards construction of potential scaffold of antitumor agents andrastins. Heterocycles. 2017;95:187–99.
Yin S, Takai K, Minato D, Sugimoto K, Ohtsu H, Tsuge K, et al. Construction of cis-fused hydrindane skeleton with a lactone tether utilizing intramolecular Diels–Alder reaction. Heterocycles. 2016;93:783–91.
Yoshimura F, Torizuka M, Mori G, Tanino K. Intramolecular conjugate addition of α,β-unsaturated lactones having an alkanenitrile side chain: stereocontrolled construction of carbocycles with quaternary carbon atoms. Synlett. 2012;23:251–4.
Clarke ML, France MB. The carbonyl ene reaction. Tetrahedron. 2008;64:9003–31.
Hamana H, Sugasawa T. The ene reaction of trisubstituted alkenes with electron-deficient nitriles in the presence of boron trichloride. Chem Lett. 1985;14:575–8.
Shimizu H, Murakami M. Reaction of 2-alkynylbenzoyl cyanides with carboxylic acids producing functionalized indenones. Synlett 2008;1817–20.
Sakai T, Danheiser RL. Cyano Diels–Alder and cyano ene reactions. Applications in a formal [2+2+2] cycloaddition strategy for the synthesis of pyridines. J Am Chem Soc. 2010;132:13203–5.
Gasper B, Carreira EM. Mild Cobalt-Catalyzed Hydrocyanation of Olefins with Tosyl Cyanide. Angew Chem Int Ed. 2007;46:4519–22.
Ley SV, Norman J, Griffith WP, Marsden SP. Tetrapropylammonium perruthenate, Pr4N+RuO4 −, TPAP: a catalytic oxidant for organic synthesis. Synthesis. 1994;639–66.
Hill RK, Conley RT. Abnormal Beckmann rearrangement of spiroketoximes in polyphosphoric acid. J Am Chem Soc. 1960;82:645–52.
Smith AB III, Leenay TL, Liu H-J, Nelson LAK, Ball RG. A caveat on the swern oxidation. Tetrahedron Lett. 1988;29:49–52.
Acknowledgements
We acknowledge Dr. Eri Fukushi and Mr. Yusuke Takata (GC-MS & NMR Laboratory, Faculty of Agriculture, Hokkaido University) for their mass spectral measurements. TA is a Research Fellow of the Japan Society for the Promotion of Science (JSPS). This work was supported by JSPS KAKENHI Grant Numbers JP15K01795, JP18K05339, JP18H01970, and JP15H05842 in Middle Molecular Strategy.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dedication: Dedicated with respect to Professor Samuel J. Danishefsky for his outstanding contributions to natural products synthesis.
Supplementary information
Rights and permissions
About this article
Cite this article
Yoshimura, F., Abe, T., Ishioka, Y. et al. Synthetic study of andrastins: stereoselective construction of the BCD-ring system. J Antibiot 72, 384–388 (2019). https://doi.org/10.1038/s41429-018-0136-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-018-0136-x