Abstract
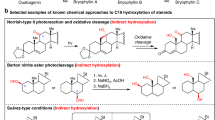
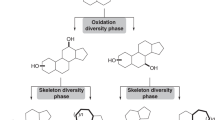
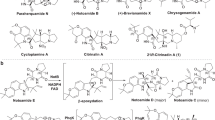
C14-functionalized steroids display biological activity and have been used as medicines. The lack of synthetic methods to access C14-functionalized steroids has, however, impeded steroidal drug discovery. In the present article, we report a modular chemoenzymatic synthesis of a diverse range of C14-functionalized steroids. This method was enabled through identification of a C14α-hydroxylase (CYP14A) from Cochliobolus lunatus, which displays high catalytic efficiency and substrate promiscuity. Protein engineering of CYP14A generated two variants, I111L–M115K and I111L–V124W, with greatly improved C14-hydroxy regiocontrol. Using this biocatalytic method, a range of C14α-hydroxy steroids with a C17 side chain were prepared in good yields, and were transformed into ∆14 olefins through a superficial elimination. The ∆14 olefin served as a versatile handle to install a variety of functional groups at the C14 position through hydro- or difunctionalization. The utility of this method was further demonstrated through application to concise semisynthesis of cardenolide periplogenin, (+)-digitoxigenin and its three diastereomers.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Crystallographic data for the structure reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition nos. CCDC 2116723 (2b), 2116721 (4cc), 2116722 (4af), 2211826 (4cg), 2116724 (4ch), 2213082 (4ai) and 2213084 (4ci). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures. All other characterization data and detailed experimental procedures are available in the supplementary materials. The sequence of the P450 genes reported in the present article has been deposited in GenBank under accession nos. ON605617 (CYP14A from CGMCC 3.3589 and JTU2.406) and ON605618 (P450lun, from CGMCC 3.4381).
References
Peng, H. et al. A dual role reductase from phytosterols catabolism enables the efficient production of valuable steroid precursors. Angew. Chem. Int. Ed. 60, 5414–5420 (2021).
El-Seedi, H. R. et al. Cardenolides: insights from chemical structure and pharmacological utility. Pharm. Res. 141, 123–175 (2019).
Gao, H., Popescu, R., Koppb, B. & Wang, Z. Bufadienolides and their antitumor activity. Nat. Prod. Rep. 28, 953–969 (2011).
Zhong, Y. et al. Total synthesis, chemical modification and structure-activity relationship of bufadienolides. Eur. J. Med. Chem. 189, 112038 (2020).
Vanos, J. L. & Oldenkamp, E. P. Oestrus control in bitches with proligestone, a new progestational steroid. J. Small. Anim. Pract. 19, 521–529 (1978).
Eichhorn, E. J. & Gheorghiade, M. Digoxin. Prog. Cardiovasc. Dis. 44, 251–266 (2002).
Lullmann, H. & Mohr, K. C14-aminosteroid LNF 209, an agent with positive inotropic and antimuscarinic activity. J. Cardiovasc. Pharmacol. 20, 807–812 (1992).
Hogg, J. A. Steroids, the steroid community, and upjohn in perspective: a profile of innovation. Steroids 57, 593–616 (1992).
Michalak, M., Michalak, K. & Wicha, J. The synthesis of cardenolide and bufadienolide aglycones, and related steroids bearing a heterocyclic subunit. Nat. Prod. Rep. 34, 361–410 (2017).
Heasley, B. Chemical synthesis of the cardiotonic steroid glycosides and related natural products. Chem. Eur. J. 18, 3092–3120 (2012).
Breslow, R., Corcoran, R., Dale, J. A., Liu, S. & Kalicky, P. Selective steroid halogenations directed by proximity and substituent effects. J. Am. Chem. Soc. 96, 1973–1974 (1974).
Welzel, P. & Stein, H. 14β-Hxdroxy steroids—III. Synthesis of digoxigenin from deoxycholic acid. Tetrahedron Lett. 22, 3385–3388 (1981).
Kirsch, G., Golde, R. & Neef, G. A cycloaddition route to 14-hydroxysteroids. Tetrahedron Lett. 30, 4497–4500 (1989).
Renata, H., Zhou, Q. & Baran, P. S. Strategic redox relay enables a scalable synthesis of ouabagenin, a bioactive cardenolide. Science 339, 59–63 (2013).
Groszek, G., Kurektyrlik, A. & Wicha, J. Synthesis of a cardenolide, 3-O-methyl uzarigenin, with 14-beta-hydroxyandrost-16-ene as the key intermediate. Tetrahedron 45, 2223–2236 (1989).
Hu, S., Genain, G. & Azerad, R. Microbial transformation of steroids: contribution to 14α-hydroxylations. Steroids 60, 337–352 (1995).
Nassiri-Koopaei, N. & Faramarzi, M. A. Recent developments in the fungal transformation of steroids. Biocatal. Biotransfor. 33, 1–28 (2015).
Kolet, S. P., Haldar, S., Niloferjahan, S. & Thulasiram, H. V. Mucor hiemalis mediated 14α-hydroxylation on steroids: in vivo and in vitro investigations of 14α-hydroxylase activity. Steroids 85, 6–12 (2014).
Kollerov, V., Shutov, A., Kazantsev, A. & Donova, M. Biotransformation of androstenedione and androstadienedione by selected Ascomycota and Zygomycota fungal strains. Phytochemistry 169, 1–9 (2020).
Andryushina, V. A. et al. 14α-Hydroxylation of steroids by mycelium of the mold fungus Curvularia lunata (VKPM F-981) to produce precursors for synthesizing new steroidal drugs. Pharmaceut. Chem. J. 47, 103–108 (2013).
Felpeto-Santero, C., Galan, B. & Garcia, J. L. Engineering the steroid hydroxylating system from Cochliobolus lunatus in Mycolicibacterium smegmatis. Microorganisms 9, 113 (2021).
Chen, J. et al. Production of 14α-hydroxysteroids by a recombinant Saccharomyces cerevisiae biocatalyst expressing of a fungal steroid 14α-hydroxylation system. Appl. Microbiol. Biot. 103, 8363–8374 (2019).
Permana, D., Niesel, K., Ford, M. J. & Ichinose, H. Latent functions and applications of cytochrome P450 monooxygenases from Thamnidium elegans: a novel biocatalyst for 14α-hydroxylation of testosterone. ACS Omega 7, 13932–13941 (2022).
Reetz, M. T. Biocatalysis in organic chemistry and biotechnology: past, present, and future. J. Am. Chem. Soc. 135, 12480–12496 (2013).
Honig, M., Sondermann, P., Turner, N. J. & Carreira, E. M. Enantioselective chemo- and biocatalysis: partners in retrosynthesis. Angew. Chem. Int. Ed. 56, 8942–8973 (2017).
Rudroff, F. et al. Opportunities and challenges for combining chemo- and biocatalysis. Nat. Catal. 1, 12–22 (2018).
King-Smith, E., Zwick, C. R. III & Renata, H. Applications of oxygenases in the chemo-enzymatic total synthesis of complex natural products. Biochemistry 57, 403–412 (2018).
Chakrabarty, S., Romero, E. O., Pyser, J. B., Yazarians, J. A. & Narayan, A. R. H. Chemo-enzymatic total synthesis of natural products. Acc. Chem. Res. 54, 1374–1384 (2021).
Bering, L., Thompson, J. & Micklefield, J. New reaction pathways by integrating chemo- and biocatalysis. Trends Chem. 4, 392–408 (2022).
Zawodny, W. et al. Chemoenzymatic synthesis of substituted azepanes by sequential biocatalytic reduction and organolithium-mediated rearrangement. J. Am. Chem. Soc. 140, 17872–17877 (2018).
Zhang, X. et al. Divergent synthesis of complex diterpenes through a hybrid oxidative approach. Science 369, 799–806 (2020).
Craven, E. J. et al. Programmable late-stage C−H bond functionalization enabled by integration of enzymes with chemocatalysis. Nat. Catal. 4, 385–394 (2021).
Peng, Y. et al. A chemoenzymatic strategy for the synthesis of steroid drugs enabled by P450 monooxygenase-mediated steroidal core modification. ACS Catal. 12, 2907–2914 (2022).
Crossley, S. W. M., Obradors, C., Martinez, R. M. & Shenvi, R. A. Mn-, Fe-, and Co-catalyzed radical hydrofunctionalizations of olefins. Chem. Rev. 116, 8912–9000 (2016).
Zhao, Y. et al. Biocatalytic C14-hydroxylation on androstenedione enabled modular synthesis of cardiotonic steroids. ACS Catal. 12, 9839–9845 (2022).
Kille, S., Zilly, F. E., Acevedo, J. P. & Reetz, M. T. Regio- and stereoselectivity of P450-catalysed hydroxylation of steroids controlled by laboratory evolution. Nat. Chem. 3, 738 (2011).
Grobe, S. et al. Engineering regioselectivity of a P450 monooxygenase enables the synthesis of ursodeoxycholic acid via 7β-hydroxylation of lithocholic acid. Angew. Chem. Int. Ed. 60, 753–757 (2021).
Li, A. et al. Regio-and stereoselective steroid hydroxylation at C7 by cytochrome|P450 monooxygenase mutants. Angew. Chem. Int. Ed. 59, 12499–12505 (2020).
Chen, W., Fisher, M. J., Leung, A., Cao, Y. & Wong, L. L. Oxidative diversification of steroids by nature-inspired scanning glycine mutagenesis of P450BM3 (CYP102A1). ACS Catal. 10, 8334–8343 (2020).
Acevedo-Rocha, C. G. et al. P450-catalyzed regio- and diastereoselective steroid hydroxylation: efficient directed evolution enabled by mutability landscaping. ACS Catal. 8, 3395–3410 (2018).
Khatri, Y. et al. Structure-based engineering of steroidogenic CYP260A1 for stereo- and regioselective hydroxylation of progesterone. ACS Chem. Biol. 13, 1021–1028 (2018).
Szaleniec, M., Wojtkiewicz, A. M., Bernhardt, R., Borowski, T. & Donova, M. Bacterial steroid hydroxylases: enzyme classes, their functions and comparison of their catalytic mechanisms. Appl. Microbiol. Biotechnol. 102, 8153–8171 (2018).
Bureik, M. & Bernhardt, R. in Modern Biooxidation: Enzymes, reactions and applications (eds. Schmid, R. D. & Urlacher, V. B.) 155–176 (Wiley-VCH, 2007).
Bracco, P. et al. CYP154C5 regioselectivity in steroid hydroxylation explored by substrate modifications and protein engineering. ChemBioChem 22, 1099–1110 (2021).
Kim, D. E., Chivian, D. & Baker, D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 32, 526–531 (2004).
Baek, M. et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 373, 871–876 (2021).
Reetz, M. T. & Carballeira, J. D. Iterative saturated mutagenesis (ISM) for rapid directed evolution of functional enzymes. Nat. Protoc. 2, 891–903 (2007).
Reetz, M. T., Carballeira, J. D. & Vogel, A. Iterative saturated mutagenesis on the basis of B factors as a strategy for increasing protein thermostability. Angew. Chem. Int. Ed. 45, 7745–7751 (2006).
Liu, B., Yang, L., Li, P., Wang, F. & Li, X. Recent advances in transition metal-catalyzed olefinic C-H functionalization. Org. Chem. Front. 8, 1085–1101 (2021).
Bhunia, A., Bergander, K., Daniliuc, C. G. & Studer, A. Fe-catalyzed anaerobic mukaiyama-type hydration of alkenes using nitroarenes. Angew. Chem. Int. Ed. 60, 2–10 (2021).
Barker, T. J. & Boger, D. L. Fe (III)/NaBH4-mediated free radical hydrofluorination of unactivated alkenes. J. Am. Chem. Soc. 134, 13588–13591 (2012).
Leggans, E. K., Barker, T. J., Duncan, K. K. & Boger, D. L. Iron (III)/NaBH4-mediated additions to unactivated alkenes: synthesis of novel 20′-vinblastine analogues. Org. Lett. 14, 1428–1431 (2012).
Ma, X. & Herzon, S. B. Non-classical selectivities in the reduction of alkenes by cobalt-mediated hydrogen atom transfer. Chem. Sci. 6, 6250–6255 (2015).
Lo, J. C., Yabe, Y. & Baran, P. S. A practical and catalytic reductive olefin coupling. J. Am. Chem. Soc. 136, 1304–1307 (2014).
Anastasia, M., Fiecchi, A. & Scala, A. Side-chain inversion of steroidal olefins promoted by hydrogen chloride. J. Org. Chem. 43, 3505–3508 (1978).
Fujimoto, Y., Morisaki, M., Ikekawa, N., Horie, Y. & Nakasone, S. Synthesis of 24,28-iminofucosterol and its inhibitory effects on growth and steroid metabolism in the silkworm, Bombyx mori. Steroids 24, 367–375 (1974).
Chen, J., Kilpatrick, B., Oliver, A. G. & Wulff, J. E. Expansion of Thiele’s acid chemistry in pursuit of a suite of conformationally constrained scaffolds. J. Org. Chem. 80, 8979–8989 (2015).
Jung, M. E. & Yoo, D. First total synthesis of rhodexin A. Org. Lett. 13, 2698–2701 (2011).
Mukaiyama, T. & Yamada, T. Recent advances in aerobic oxygenation. Bull. Chem. Soc. Jpn 68, 17–35 (1995).
Cheng, M. et al. Asymmetric total synthesis of bufospirostenin A. J. Am. Chem. Soc. 142, 12602–12607 (2020).
Engman, L. & Stem, D. Thiol/Diselenide exchange for the generation of benzeneselenolate ion. Catalytic reductive ring-opening of α,β-epoxy ketone. J. Org. Chem. 59, 5179–5183 (1994).
Fernandes, R. A. (ed.) Protecting-Group-Free Organic Synthesis: Improving Economy and Efficiency (John Wiley & Sons, 2018).
Burke, M. D. & Schreiber, S. L. A planning strategy for diversity-oriented synthesis. Angew. Chem. Int. Ed. 43, 46–58 (2004).
Galloway, W. R. J. D., Isidro-Llobet, A. & Spring, D. R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 1, 80–93 (2010).
Freitas, C. S. et al. Digitoxigenin presents an effective and selective antileishmanial action against Leishmania infantum and is a potential therapeutic agent for visceral leishmaniasis. Parasitol. Res. 120, 321–335 (2021).
Acknowledgements
We thank X.-G. Meng (CCNU) for X-ray crystallographic analysis assistance, L. Shao (Shanghai Institute of Pharmaceutical Industry) for kind gifting of C. lunatus JTU 2.406, H.-G. Cheng, T. Yang, J.-X. Ye, L. Zhou and H. Wei (WHU) for helpful discussions, and L. Cao (WHU) for assistance with the preparation of the manuscript. This work was supported by the National Key R&D Program of China (grant no. 2018YFA0900400 to X.Q.), the Fundamental Research Funds for the Central Universities (grant no. 2042021kf0214 to Q.Z.) and the start-up funding from Wuhan University. We dedicate this paper to Professor Dawei Ma (SIOC) on the occasion of his 60th birthday.
Author information
Authors and Affiliations
Contributions
Q.Z. and X.Q. conceived this project. F.S., M.Z., J.W., H.L., Z.L. and B.L. performed the experiment under the supervision of X.Q., Q.Z. and Z.D. H.C. performed the test and analysis of single crystal structures. F.S., M.Z., Q.Z. and X.Q. cowrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Thomas Bayer, Sebastian Cosgrove, Yang Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–6 and Figs. 1–7.
Supplementary Data 1
Crystallographic Data for 2b, CCDC2116723.
Supplementary Data 2
Crystallographic Data for 4af, CCDC 2116722.
Supplementary Data 3
Crystallographic Data for 4ai, CCDC 2213082.
Supplementary Data 4
Crystallographic Data for4cc, CCDC 2116721.
Supplementary Data 5
Crystallographic Data for 4cg, CCDC 2211826.
Supplementary Data 6
Crystallographic Data for 4ch, CCDC 2116724.
Supplementary Data 7
Crystallographic Data for 4ci CCDC 2213084.
Supplementary Table 8
Source data for Supplementary Figs. 4a and 5aa–5ma.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, F., Zheng, M., Wang, J. et al. Chemoenzymatic synthesis of C14-functionalized steroids. Nat. Synth 2, 729–739 (2023). https://doi.org/10.1038/s44160-023-00280-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00280-z
This article is cited by
-
Enzymes on steroids
Nature Synthesis (2023)
-
Breakthrough in the challenging P450-catalyzed chemoenzymatic synthesis of C14-functionalized steroids
Science China Chemistry (2023)