Abstract
Simpotentin, a new potentiator of amphotericin B activity against Candida albicans and Cryptococcus neoformans, was isolated from the culture broth of Simplicillium minatense FKI-4981 by Diaion HP-20 column chromatography, centrifugal partition chromatography, and preparative HPLC. The structure of simpotentin was elucidated by spectroscopic analyses including NMR and MS. The compound has a mannose core to which two medium-chain fatty acids are linked. Simpotentin was found to potentiate amphotericin B activity against C. albicans by the microdilution method.
Similar content being viewed by others
Introduction
The number of patients with mycoses has continued to increase over the past 30 years, and this has primarily been attributed to the application of organ transplantation, immunosuppressive drugs, radiotherapy, and antitumoral chemotherapy as highly advanced medical treatments. Candida albicans, Aspergillus fumigatus, Cryptococcus neoformans, and Rhizopus oryzae are pathogenic fungi that cause invasive infections [1]. C. albicans has been implicated in ~50% of candidiasis cases [2]. Several antifungal antibiotics are currently available for the treatment of mycoses. Amphotericin B (AmB), a polyene macrolide antifungal antibiotic produced by Streptomyces nodosus [3], has been the ‘gold standard’ since its introduction into clinical practice in the 1950s. Furthermore, liposomal AmB was developed in the 1990s to improve the tolerability profile of AmB deoxycholate. However, AmB is associated with severe side effects, such as nephrotoxicity, hypokalemia, fever, chills, and vomiting, which result in the withdrawal of drug therapy. The development of potentiators of AmB activity may result in reductions in the dose of AmB administered, which may prevent these side effects. We established a convenient screening system using a microdilution method to search for microbial potentiators of AmB activity against C. albicans. During our continuous screening program, a culture broth of the fungal strain S. minatense FKI-4981, also known as a producer of the antifungal compound simplifungin [4], was found to exhibit AmB-potentiating activity. Activity-guided purification led to the discovery of a novel compound designated simpotentin (1, Fig. 1). We herein describe the fermentation, isolation, physicochemical properties, structural elucidation, and AmB-potentiating activity of 1.
Results
Isolation
A 6-day-old culture broth (40 l) was centrifuged to separate mycelia and the supernatant. After mycelia had been extracted with acetone (9 l), acetone extracts were filtered and concentrated to remove acetone. The aqueous solution was mixed with the supernatant, applied to a Diaion HP-20 column (2 l), and eluted stepwise with 0, 30, 60, and 100% acetone (4 l for each solvent). The 60% acetone fraction exhibiting activity was concentrated to give a dark brown material (2.38 g). Crude materials (0.59 g) were subjected to preparative centrifugal partition chromatography (CPC) under the following conditions: a solvent system, with upper and lower layers of chloroform–MeOH–H2O (2:2:1) as stationary and mobile phases, respectively; flow rate, 3.0 ml min−1; rotation speed, 700 rpm; fraction, 18 ml per test tube. CPC-derived fractions (fr. 8-15) were concentrated to give a brown material (161.8 mg). This material was purified by preparative HPLC (column, PEGASIL ODS (Senshu Scientific Co., Tokyo, Japan, i.d. 20 × 250 mm); mobile phase, a 30-min linear gradient from 30 to 70% CH3CN (aq) containing 0.05% TFA; detection, evaporative light scattering detector (ELSD); flow rate, 8.0 ml min−1). Under these conditions, 1 was eluted as a peak with a retention time of 22 min (Fig. 2). The fraction was concentrated in vacuo and lyophilized to dryness to yield pure 1 (19.9 mg) as a white powder.
Structural elucidation of 1
The physicochemical properties of 1 are summarized in Table 1. IR absorption at 3392, 2929–2863, and 1722 cm−1 suggested the presence of an alcohol O–H stretch, alkyl C–H stretch, and carboxylic acid C=O stretch, respectively, in the structure. The molecular formula was elucidated as C24H44O11 based on HR-ESI-MS measurements. The 13C-NMR spectrum (in pyridine-d5) showed 24 signals, which were classified into two methyl carbons, 12 sp3 methylene carbons, eight oxygenated sp3 methine carbons, and two carbonyl carbons by an analysis of DEPT spectra. Of these, one anomeric carbon (δ 102.3), four oxygenated methine carbons (δ 69.5, 73.1, 76.2, and 79.2), and one oxygenated methylene carbon (δ 63.5) were observed, indicating the presence of a sugar moiety. The 1H-NMR spectrum (in pyridine-d5) showed 38 proton signals. The connectivity of proton and carbon atoms was established by the HSQC spectrum (Table 2). As shown by the bold lines in Fig. 3, three partial structures I to III including that of the sugar moiety were elucidated from 1H-1H COSY spectra. The 1H-13C long-range couplings of 2J and 3J observed in the HMBC spectrum are also shown in Fig. 3, leading to the following results. The cross peaks from the sp3 methylene protons H2-2 (δ 2.99, 3.34) and sp3 oxymethine proton H-3 (δ 4.88) to the carboxyl carbon C-1 (δ 174.9) indicated the presence of a β,δ-dihydroxydecanoic acid moiety containing partial structure I. Furthermore, cross peaks from the sp3 methylene protons H2-12 (δ 2.78 and 2.82) to the ester carbonyl carbon C-11 (δ 172.7) indicated the presence of a β-hydroxyoctanoic acid moiety containing partial structure II. This fatty acid was connected to the δ position of β,δ-dihydroxydecanoic acid via an ester bond by observations of the cross peak from the sp3 oxymethine proton H-5 (δ 5.56) to C-11 in the HMBC experiment, elucidating the aglycone of 1. In the sugar unit, the cross peaks from anomeric proton H-1′ (δ 5.21) to C-5′ (δ 79.2) and from H-5′ (δ 3.90) to anomeric carbon C-1′ (δ 102.3) indicated the presence of an aldohexose containing partial structure III. This sugar unit was connected to the β position of β,δ-dihydroxydecanoic acid via a glucosidic bond by the cross peaks from H-3 to C-1′ and from H-1′ to C-3 in the HMBC experiment. Based on these results, the planar structure of 1 was elucidated as shown in Fig. 3.
The relative stereochemistry for the aldohexose was evaluated by the ROESY experiment and coupling constants (Fig. 4). NOEs were observed between H-1′ and H-3′ (δ 4.15), between H-3′ and H-5′, and between H-1′ and H-5′ in addition to the coupling constants between H-3′ and H-4′ (δ 4.54, JH3′–H4′ = 9.5 Hz) and between H-4′ and H-5′ (JH4′–H5′ = 9.5 Hz), indicating that they were axially oriented. The anomeric configuration for the aldohexose was elucidated as the β-form from the one-bond coupling constant between C-1′ and H-1′ (1JC–H = 156.6 Hz observed for 1; literature values for the α-anomer, ~170 Hz; β-anomer, ~160 Hz) [5]. Based on these results, the sugar moiety in 1 was identified as β-mannose (Fig. 4). The planar structure of 1 was elucidated as shown in Fig. 1.
Antifungal drug-potentiating activity
Compound 1 showed no antifungal activity against C. albicans, A. fumigatus, C. neoformans, or R. oryzae, even at 512 µg ml−1, in the liquid microdilution method. On the other hand, the MIC values of AmB, miconazole (MCZ), and micafungin (MCFG) alone against C. albicans were 0.500, 0.00390, and 0.0625 µg ml−1, respectively, which were within the permissible range [6]. Compound 1 (2.00–64.0 µg ml−1) enhanced AmB activity against C. albicans and C. neoformans in a concentration-dependent manner (Table 3), but not against A. fumigatus or R. oryzae. The MIC90 values of AmB against C. albicans and C. neoformans in combination with 1 (64.0 and 16 µg ml−1, respectively) decreased from 0.50 and 1.00 µg ml−1 to 0.0625 and 0.125 µg ml−1, respectively, both yielding 8-fold potentiations of AmB activity. On the other hand, the MIC values of MCZ and MCFG against C. albicans, A. fumigatus, C. neoformans, and R. oryzae remained unchanged in combination with 1 (2.0–64.0 µg ml−1), indicating that 1 did not potentiate MCZ or MCFG activity against these fungi.
Antimicrobial and cytotoxic activities
In the paper disk method, 1 showed no antimicrobial activity at 10 µg per 6-mm disk against the following 9 test microorganisms: Bacillus subtilis, Staphylococcus aureus, Micrococcus luteus, Mycobacterium smegmatis, Escherichia coli, Pseudomonas aeruginosa, Xanthomonas campestris pv. oryzae, Bacteroides fragilis, and Acholeplasma laidlawii.
The cytotoxic activity of AmB in combination with 1 against HEK293 cells (human embryonic kidney-derived cell line) was measured. The respective IC50 values of AmB and 1 were 15 and >128 µg ml−1. The IC50 values of AmB were similar (15 µg ml−1) in combination with 1 (0–128 µg ml−1), indicating that the combination did not enhance AmB cytotoxicity.
Discussion
In the present study, we screened for microbial potentiators of AmB activity against C. albicans and isolated the new compound, simpotentin (1), from the culture broth of S. minatense FKI-4981. The genus Simplicillium was segregated from the former Verticillium section, Prostrata besides Lecanicillium, Haptocillium, and Rotiferophthora [7]. Of these, V. lamellicola has been reported as a fungus that produces two secondary metabolites, lamellicolic anhydride [8] and verlamelin [9]. We also recently isolated simplifungin, an antifungal antibiotic, from the simpotentin-producing fungus [4] and aogacillins A and B, arbekacin potentiators, from Simplicillium sp. FKI-5985 [10].
Based on its structure, 1 belongs to a class of glycolipids composed of a mannosyl group with two medium-chain fatty acids, β,δ-dihydroxydecanoic acid and β-hydroxyoctanoic acid. The configuration at the anomeric center of mannose was found to be the β position by ROESY experiments and 1H–1H and 1H–13C coupling constants, whereas the stereochemistries of the chiral center carbons at C-3, C-5, and C-13 in the aglycone were not elucidated by ROESY experiments or 1H–1H coupling constants. To clarify absolute stereochemistries, the total synthesis of 1 is ongoing.
As structurally related compounds to 1, rhamnolipids (RLs) consisting of one or two rhamnose units linked to one or two fatty acids with a saturated or unsaturated 3-hydroxyalkyl chain between C8 and C12 were isolated from P. aeruginosa [11, 12]. The structural difference between 1 and RLs is that the second fatty acid and a sugar are linked to the 5- and 3-OH groups of the first fatty acid in 1, while a mono/disaccharide is linked to the 3-OH group of the second fatty acid in RLs, which is linked to the 3-OH group of the first fatty acid. RLs are expected to be a promising class of biosurfactants [13]. RLs were previously reported to exhibit antimicrobial activity against zoosporic plant pathogens, mycoplasmas, some non-zoosporic fungi, except for yeast, and certain bacteria [12, 14] as well as cytotoxic activity [15]. Takemoto et al. reported that the antifungal activity of the cyclolipodepsipeptide syringomycin E (SR-E) produced by a plant bacterium (P. syringae pv. syringae) [16] was enhanced when mixed with RLs [17]. Furthermore, SR-E acted on the fungal membrane by forming voltage-dependent and non-selective ion channels [18] and the authors speculated that RLs may perturb membrane function in fungi. Structurally, 1 may exhibit a surfactant characteristic, but not antimicrobial activity.
Dang et al. [19] isolated three new mannosyl lipids from Simplicillium lamellicola BCP as antimicrobial compounds against phytopathogenic bacteria. Among them, (3R,5R)-3-O-β-D-mannosyl-3,5-dihydroxydecanoic acid is a partial structure of 1 (deshydroxyoctanoyl 1). It is intriguing that analogous mannosyl lipids are produced by the same genus Simplicillium [19] and also to test whether it exhibits AmB-potentiating activity. This compound has not been isolated as an AmB potentiator in the culture broth of S. minatence FKI-4981.
Compound 1 potentiated AmB activity against C. albicans, but not MCZ or MCFG activity. These clinical antifungal antibiotics have different mechanisms of action; AmB selectively and irreversibly binds to fungal cell membrane ergosterols, MCZ inhibits cytochrome P450-dependent 14α-lanosterol demethylation in the ergosterol biosynthetic pathway, and MCFG inhibits 1,3-β-glucan synthase in many fungi. Based on experimental data, 1 only potentiated AmB activity. Based on previous findings on RLs, 1 appears to directly interact with fungal cell membranes in order to increase the permeability of AmB. Moreover, the potentiation activity of 1 may be limited to Candida species. Fungal cell walls are commonly composed of glycoproteins, polysaccharides, glucan, and chitin to protect cells from environmental stresses. Furthermore, the structural components of A. fumigatus and R. oryzae are more complex than those of yeast including C. albicans and C. neoformans. Therefore, 1 may not have the ability to permeate the cell surfaces of these fungi. Further studies on the potentiation mechanisms of 1 are required to clarify these points.
Materials and methods
Microorganisms
The simpotentin-producing fungus S. mitatense FKI-4981 was isolated from soil collected at Minato-ku, Tokyo, Japan. The taxonomy of this fungus was extensively studied to establish a new genus designated Simplicillium [20]. Strain FKI-4981 was used for the production of 1.
The following fungi were used in antifungal tests: C. albicans ATCC90029, C. neoformans ATCC90113, A. fumigatus NBRC33022, and R. oryzae NBRC4705. The following microorganisms were used for antimicrobial tests: B. subtilis ATCC 6633, S. aureus ATCC 6538P (MSSA), M. luteus ATCC 9341, M. smegmatis ATCC 607, E. coli NIHJ, E. coli NIHJ JC-2 IFO 12734, P. aeruginosa IFO 3080, X. campestris pv. oryzae, B. fragilis ATCC 23745, and A. laidlawii PG 8.
General experimental procedures
Daiaion HP-20 (Mitsubishi Chemical Co., Tokyo, Japan) was used for HP-20 column chromatography. Preparative centrifugal partition chromatography (CPC) was performed using a LLB-M high performance centrifugal partition chromatograph (System Instruments Co., Ltd., Tokyo, Japan). Preparative HPLC was performed using an evaporative light scattering detector (Alltech ELSD 2000, MD, USA). UV spectra were recorded on a spectrophotometer (U-2800 UV-Visible Double Beam spectrophotometer; Hitachi High-Technologies, Tokyo, Japan). IR spectra were recorded on a Fourier transform infrared spectrometer (FT/IR-460; JASCO Co., Tokyo, Japan). Optical rotations were measured with a digital polarimeter (DIP-370; JASCO Co.). HR-ESI-MS spectra were recorded on a mass spectrometer (JMS-T100 LP, JEOL, Tokyo, Japan). Various NMR spectra were measured with a spectrometer (Inova 600; Agilent Technologies, Santa Clara, CA, USA).
Materials
AmB and MCZ were purchased from Sigma-Aldrich (St. Louis, MO, USA). MCFG was purchased from Wako Pure Chemical Industries (Osaka, Japan). RPMI1640 medium was purchased from Thermo Fisher Scientific (Waltham, MA, USA). 3-Morpholinopropanesulphonic acid (MOPS) was purchased from DOJINDO (Kumamoto, Japan). Yeast extract was purchased from Oriental Yeast (Tokyo, Japan). Polypeptone was purchased from Nihon Pharmaceutical (Tokyo, Japan). Meat extract was purchased from Kyokuto Pharmaceutical Industrial (Tokyo, Japan). Corn steep powder was purchased from Marcor Development Corporation (Carlstadt, NJ, USA). Yeast extract, polypeptone, meat extract, corn steep powder, and agar were used in the culture broth. Other materials used were special grade chemicals.
Fermentation
A slant culture of the strain FKI-4981 grown on slant medium (glycerol 0.10%, KH2PO4 0.080%, K2HPO4 0.020%, MgSO4•7H2O 0.020%, KCl 0.020%, NaNO3 0.20%, yeast extract 0.020%, and agar 1.5%, pH 6.0) was used to inoculate four 500-ml Erlenmeyer flasks each containing 100 ml of the seed medium (glucose 2.0%, yeast extract 0.20%, MgSO4•7H2O 0.050%, polypeptone 0.50%, KH2PO4 0.10%, and agar 0.10%, pH 6.0). The flasks were shaken on a rotary shaker at 27 °C for 3 days. The seed culture (200 ml) was transferred into two 30-l jar fermenters containing production medium (20 l; saccharose 2.0%, glucose 1.0%, corn steep powder 0.50%, meat extract 0.50%, KH2PO4 0.10%, CaCO3 0.30%, and agar 0.10%, pH 6.0). Fermentation was performed at 27 °C for 6 days with aeration of 10 l min−1 and agitation of 250 rpm.
Assay for antifungal activity with or without antifungal drugs
Two yeasts (C. albicans and C. neoformans), one filamentous fungus (A. fumigatus), and one zygomycetous fungus (R. oryzae) were used in antifungal activity assays [4]. The broth microdilution method using 96-well microplates (Corning, New York, USA) was performed according to the guidelines of the CLSI documents M27-A3 [6] and M38-A2 [21]. Regarding yeast, five colonies with diameters of 1 mm were suspended in sterile 0.85% saline to adjust to a 0.5 McFarland standard by spectrophotometric measurements. The seed of yeast was diluted 2000-fold with RPMI1640 medium (165 mM MOPS buffer, pH 7.0). Regarding filamentous fungi and zygomycetous fungi, spores were suspended in sterile 0.85% saline. After allowing heavy particles to settle for five minutes, the supernatant was transferred to a sterile tube and adjusted to an optical density at 550 nm (OD550) of 0.0621 for filamentous fungi or 0.0765 for zygomycetous fungi. This seed was diluted 50-fold with RPMI1640 medium. The diluted seed (100 µl) and RPMI1640 medium (100 µl) were added to each well of a 96-well microplate with or without serial concentrations of test compounds (1 µl DMSO solution). These 96-well plates were incubated at 35 °C for 24 (Candida spp. and zygomycetous), 48 (filamentous fungi), or 72 h (C. neoformans). After the incubation, OD550 was measured with a microplate reader (Elx808, Bio-Tek Instruments, VT, USA) to obtain the minimum inhibitory concentration (MIC). The antifungal activities of 1 and the antifungal drugs, AmB, MCZ, and MCFG, were tested at concentrations ranging between 0.125 and 512 µg ml−1 for 1 with or without 0.0313 to 2.00 µg ml−1 for AmB, 0.000250 to 0.0156 µg ml−1 for MCZ, and 0.00390 to 0.250 µg ml−1 for MCFG. MIC90 was defined as the lowest concentration of an antifungal agent at which growth was inhibited by 90% from that of growth in drug-free control wells.
Other biological assays
The antimicrobial activity of a sample against 10 species of microorganisms was measured by the paper disk method [22]. Media for microorganisms were as follows: GAM agar (Nissui Seiyaku Ltd., Tokyo, Japan) for B. fragilis; Bacto PPLO agar (Becton, Dickinson and Co., NJ, USA) supplemented with horse serum 15%, glucose 0.10%, phenol red 0.25%, and agar 1.5% for A. laidlawii; nutrient agar (Becton, Dickinson and Co.) for the other bacteria. Bacteria, except for X. oryzae, were incubated at 37 °C for 24 h. X. oryzae was incubated at 27 °C for 24 h. A paper disk (diameter of 6 mm) containing a sample (10 µg) was placed on an agar plate. Antimicrobial activity was expressed as the diameter (mm) of the inhibitory zone.
The cytotoxicity of a sample to HEK293 cells was assessed by the MTT (Sigma-Aldrich, MO, USA) assay [23]. HEK293 cells (5.0 × 104 cells in 100 µl) in each well of a 96-well microplate were treated with a sample (1.0 µl in DMSO to make a final concentration of 0–128 µg ml−1) at 37 °C for 24 h. After being incubated, cells were treated with 10 µl MTT solution (5.5 mg ml−1 in phosphate-buffered saline) at 37 °C for 4 h. Ninety microliters of cell lysis solution (40% N,N′-dimethylformamide, 2.0% CH3COOH, 20% SDS, and 0.03 M HCl) was added to each well, and the plate was incubated for 2 h. The absorbance at 550 nm of each well was read using a microtiter plate reader (Elx 808, Bio-Tek Instruments, VT, USA).
References
Pfaller MA, Pappas PG, Wingard JR. Invasive fungal pathogens: current epidemiological trends. Clin Infect Dis. 2006;43(Suppl 1):S3–S14.
Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, Kauffman CA, Hyslop N, Mangino JE, Chapman S, Horowitz HW, Edwards JE, Dismukes WE, NIAID Mycoses Study Group. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis. 2003;37:634–43.
Oura M, Sternberg TH, Wright ET. A new antifungal antibiotic, amphotericin B. Antibiot Annu. 1956;3:566–73.
Ishijima H, Uchida R, Ohtawa M, Kondo A, Nagai K, Shima K, Nonaka K, Masuma R, Iwamoto S, Onodera H, Nagamitsu T, Tomoda H. Simplifungin and valsafungins, antifungal antibiotics of fungal origin. J Org Chem. 2016;81:7373–83.
Bock K, Lundt I, Pedersen C. Assignment of anomer structure to carbohydrates through geminal 13C-1H coupling constants. Tetrahedron Lett. 1973;13:1037–40.
Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts. CLSI document M27-A3. 3rd edn. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
Gams W, Zare R. A revision of Verticillium sect. Prostrata. III. Generic Classif Nova Hedwig. 2001;72:329–37.
McCorkindale NJ, Hutchinson SA, McRitchie AC, Sood GR. Lamellicolic anhydride, 4-O-carbomethoxylamellicolic anhydride and monomethyl 3-chlorolamellicolate, metabolites of Verticillium lamellicola. Tetrahedron. 1983;39:2283–8.
Rowin GL, Miller JE, Albers-Schönberg G, Onishi JC, Davis D, Dulaney EL. Verlamelin, a new antifungal agent. J Antibiot. 1986;39:1772–5.
Takata K, Iwatsuki M, Yamamoto T, Shirahata T, Nonaka K, Masuma R, Hayakawa Y, Hanaki H, Kobayashi Y, Petersson GA, Ōmura S, Shiomi K. Aogacillins A and B produced by Simplicillium sp. FKI-5985: new circumventors of arbekacin resistance in MRSA. Org Lett. 2013;15:4678–81.
Itoh S, Honda H, Tomita F, Suzuki T. Rhamnolipids produced by Pseudomonas aeruginosa grown on n-paraffin. J Antibiot. 1971;24:855–9.
Haba E, Pinazo A, Jauregui O, Espuny MJ, Infante MR, Manresa A. Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47T2 NCBIM 40044. Biotechnol Bioeng. 2003;81:316–22.
Dobler L, Vilela LF, Almeida RV, Neves BC. Rhamnolipids in perspective: gene regulatory pathways, metabolic engineering, production and technological forecasting. N Biotechnol. 2016;33:123–35.
Stanghellini ME, Miller RM. Biosurfactants: Their identity and potential efficacy in the biological control of zoosporic plant pathogens. Plant Dis. 1997;81:4–12.
Kristoffersen V, Rämä T, Isaksson J, Andersen JH, Gerwick WH, Hansen E. Characterization of rhamnolipids produced by an arctic marine bacterium from the pseudomonas fluorescence group. Mar Drugs. 2018;16:E163.
Sorensen KN, Kim KH, Takemoto JY. In vitro antifungal and fungicidal activities and erythrocyte toxicities of cyclic lipodepsinonapeptides produced by Pseudomonas syringae pv. syringae. Antimicrob Agents Chemother. 1996;40:2710–3.
Takemoto JY, Bensaci M, De Lucca AJ, Cleveland TE, Gandhi NR, Skebba VP. Inhibition of fungi from diseased grape by syringomycin E-rhamnolipid mixture. Am J Enol Vitic. 2010;61:120–4.
Takemoto, JY, Brand JG, Kaulin YA, Malex, VV, Schabina LV, Blasko, K. The syringomycins: lipodepsipeptide pore formers from plant bacterium. In: Menestrina, G, Serra MD & Lazarovic, P, editors. Pore forming peptides and protein toxins. London: Taylor & Francis; 2003. p. 260–71.
Le Dang Q, Shin TS, Park MS, Choi YH, Choi GJ, Jang KS, Kim IS, Kim JC. Antimicrobial activities of novel mannosyl lipids isolated from the biocontrol fungus Simplicillium lamellicola BCP against phytopathogenic bacteria. J Agric Food Chem. 2014;62:3363–70.
Nonaka K, Kaifuchi S, Ōmura S, Masuma R. Five new Simplicillium species (Cordycipitaceae) from soils in Tokyo, Japan. Mycoscience. 2012;54:42–53.
Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. CLSI document M38-A2. 3rd edn. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
Koyama N, Nagahiro T, Yamaguchi Y, Masuma R, Tomoda H, Ōmura S. Stemphones, novel potentiators of imipenem activity against methicillin-resistant Staphylococcus aureus, produced by Aspergillus sp. FKI-2136. J Antibiot. 2005;58:695–703.
Kaneko M, Matsuda D, Ohtawa M, Fukuda T, Nagamitsu T, Yamori T, Tomoda H. Potentiation of bleomycin in Jurkat cells by fungal pycnidione. Biol Pharm Bull. 2012;35:18–28.
Acknowledgements
We express our thanks to Dr. Kenichiro Nagai and Ms. Noriko Sato of the School of Pharmacy, Kitasato University for the measurements of NMR and mass spectra. This work was supported by JSPS KAKENHI Grant Numbers 16H05095 (to RU) and 21310146 (to HT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Uchida, R., Kondo, A., Yagi, A. et al. Simpotentin, a new potentiator of amphotericin B activity against Candida albicans, produced by Simplicillium minatense FKI-4981. J Antibiot 72, 134–140 (2019). https://doi.org/10.1038/s41429-018-0128-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-018-0128-x
This article is cited by
-
Synthesis and biological evaluation of nectriatide derivatives, potentiators of amphotericin B activity
The Journal of Antibiotics (2024)
-
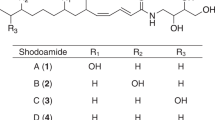
New potentiators of amphotericin B activity, shodoamides A to C produced by Pseudophialophora sp. BF-0158
The Journal of Antibiotics (2023)
-
New piericidin rhamnosides as potentiators of amphotericin B activity against Candida albicans produced by actinomycete strain TMPU-A0287
The Journal of Antibiotics (2023)
-
Taxonomic and phylogenetic characterizations reveal four new species of Simplicillium (Cordycipitaceae, Hypocreales) from Guizhou, China
Scientific Reports (2021)
-
Podogigants A and B, two new potentiators of amphotericin B activity, from Sordariomycete Podostroma giganteum
Journal of Natural Medicines (2021)