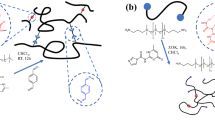
Abstract
In this study, we utilized tetra-armed polyethylene glycol (Tetra-PEG) slimes, which are model transient networks with well-controlled structures, to predict the effects of long-term degradation on the viscoelastic properties of liquids. Viscoelastic liquids, such as sodium hyaluronate, are frequently used in biomedical applications within the human body. However, precisely controlling the viscoelastic properties of these liquids in the long-term is challenging, as the main chains of the liquids undergo stochastic degradation. To establish a predictable model for studying long-term degradation effects, we employed Tetra-PEG slime, and modifications were performed to introduce specific cleavage sites in areas with connections. The Tetra-PEG slimes were characterized by single relaxation modes, and these modes were independent from the degree of degradation, which was determined by hydrolyzing the cleavage sites. Overall, this work provides a universal design for viscoelastic liquids with precisely-controllable degradation.
Similar content being viewed by others
Introduction
Viscoelastic liquids, including entangled polymer solutions, suspensions, and emulsions, exhibit intermediate properties between elasticity and viscosity, such as time-dependent mechanical characteristics. As the mechanical properties of these compounds and soft tissues are similar, these compounds are used for biomaterial applications within the human body, including mucous membrane protuberance agents, filler materials for cosmetic surgery, and surgical aids [1,2,3,4,5]. Natural polymer solutions are commercially manufactured for this purpose; one example is sodium hyaluronate, an entangled polymer solution. Inside the body, these biomaterials undergo chemical and biological degradation processes, including hydrolysis and enzymatic reactions. Therefore, it is necessary to examine how biomaterial degradation impacts viscoelasticity. However, this task remains challenging because degradation stochastically causes the scission of main chains, leading to a broader molecular weight distribution and disentanglement; as a result, qualitative predictions of viscoelastic properties are hindered [6,7,8,9,10].
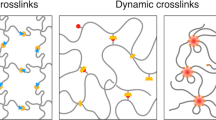
To overcome the difficulties related to controlling viscoelasticity over time, we developed tetra-armed polyethylene glycol (Tetra-PEG) slime, a model viscoelastic liquid system with a regular network structure [11, 12]. Tetra-PEG slime is formed by coupling phenylboronic acid-terminated PEG (tetra-PEG–FPBA) with glucono-delta-lactone-terminated PEG (tetra-PEG–GDL) through reversible boronic ester bonds, which produce transient reconfigurable networks; these networks are generally characterized by static and dynamic heterogeneities. Static heterogeneities include structural defects, such as dangling chains, loop structures, and branch number distributions [13,14,15,16], whereas dynamic heterogeneities represent mixed components with slow and fast dynamics, such as micelles, unimers, and low-molecular-weight crosslinkers. The heterogeneities of transient networks increases the difficulty in elucidating molecular processes that occur during viscoelastic relaxation. In contrast, Tetra-PEG slimes synthesized from symmetric four-armed precursors with a narrow distribution minimize the number of structural heterogeneities.
These methods are advantageous because the network structure and viscoelastic properties are directly correlated, which enables highly controllable and predictable material characteristics. Tetra-PEG slime exhibits single relaxation modes with distinct relaxation times, and these times are uniquely determined by network parameters, such as the polymer concentration, network strand length, and network connectivity [17, 18].
The aim of this study was to construct a predictable model for describing the long-term viscoelastic properties of transient networks. For this purpose, Tetra-PEG slimes were modified to integrate specific cleavage sites within the glucoamide groups of tetra-PEG–GDL units, which undergo hydrolysis to gluconolactone in aqueous solution [18]. We accurately measured the degradation rates of glucoamide in the prepared Tetra-PEG slime and aqueous solution and investigated the degradation mechanism (Fig. 1). The findings of this work can facilitate the design of biomaterials that must maintain viscoelastic properties over long periods for internal body applications.
Experimental
Tetra-PEG–FPBA and tetra-PEG–GDL (Mw = 4.0 × 104 g mol−1) were purchased from Xiamen Sinopeg Biotech Co., Ltd. (China). Tetra-PEG slimes were prepared by mixing equal amounts of tetra-PEG–FPBA and tetra-PEG–GDL, which were dissolved in a phosphate buffer solution (pH = 8.0, 200 mM). The polymer concentration (cPEG) was maintained at 20, 40, or 90 g L−1 over 24 h. For comparison, we also prepared a tetra-PEG–GDL solution in the aforementioned phosphate buffer. To ensure that the samples remained undisturbed at specific temperatures (T = 30, 40, and 50 °C), the thermal environment was precisely regulated through a thermostatic chamber (NCB 1210B; Eyela).
To measure the dynamic viscoelasticity of the fabricated slimes, a stress-controlled rheometer (MCR302; Anton Paar, Graz, Austria) equipped with a cone-plate fixture (diameter: 25 mm, cone angle: 4°) was utilized. The angular frequency dependences (0.1–100 rad s–1) of the storage (G′) and loss (G″) moduli were established at 25 °C. Before the measurements, the oscillatory shear strain amplitudes were confirmed to be within the linear viscoelasticity range.
Results and discussion
The loss and storage moduli of Tetra-PEG slime (PEG concentration (cPEG) = 40 g·L−1) depended on the angular frequency, which underwent time-dependent changes, as shown in Fig. 2. With increasing incubation time, the absolute values of G′ and G″ decreased, and the terminal relaxation process accelerated. The observed decrease was more pronounced at higher temperatures. The dashed lines in the plots represent the fitting results obtained using the Maxwellian model, which is expressed as follows:
Here, ΔG, ω, and τvisco are the plateau modulus, angular frequency, and viscoelastic relaxation time, respectively. The predictions of Eqs. (1a) and (1b) corresponded well with the experimental data, suggesting that the relaxation process, not the degree of degradation, was consistent. The experimental and theoretical values corresponded under all the experimental conditions, as shown in Section “Experimental” of the Supplementary Information. Notably, the deviation from the predicted value at higher frequencies was pronounced at longer degradation times, which was attributed to the Rouse modes of the dangling chains. The relaxation time is a “terminal relaxation time” and was estimated using the data at the low-frequency limit.
As the incubation time (t) increased, the terminal relaxation time (τvisco) decreased, indicating a reduction in network connectivity (p) [18]. According to our previous study [18], the relationship between τvisco and p follows a power law. To evaluate the change in p due to degradation, the relationship between τvisco and p was determined for Tetra-PEG slime with controlled network connectivity, which was produced by mixing two polymer solutions at various stoichiometric proportions (s). p can be estimated from the ratio of the FPBA concentration to the GDL concentration using the following equation:
where [X]0 represents the initial molar concentration of Compound X (FPBA or GDL) and Keq is the equilibrium constant of boronic ester (Keq = 5.67 × 102 at pH = 8.0 and 25 °C and Keq = 6.08 × 102 at pH = 8.0 and 30 °C) [12]. Figure 3 shows the relationship between τvisco and p, which was determined for various polymer concentrations. The obtained relationships follow the power law τvisco = ApB. Using these curves, we experimentally determined p from the viscoelasticity.
The top panel of Fig. 4 shows the time evolution of τvisco for Tetra-PEG slime incubated with different PEG concentrations at 30 °C. With the p value estimated from τvisco, the fraction of remaining glucoamide in slime (Rslime), which is the ratio of the molar concentration of glucoamide groups to that in the initial state, can be quantitatively estimated via the following formula:
where Nend represents the molar concentration of all end groups of tetra-PEG–FPBA and tetra-PEG–GDL and Keq denotes the equilibrium constant for the reaction involving boronic ester bonds. The bottom panel of Fig. 4 shows that Rslime consistently decreased during incubation, while the degradation rate increased with decreasing polymer concentration. On a semilogarithmic plot, the linear relationships became more evident, particularly over shorter time spans. Assuming that the degradation reaction involved pseudo-first-order kinetics, the apparent degradation rate constant for the glucoamide cleavage reaction (kdeg,app) was estimated at each temperature using the following fitting function:
Figure 5 shows the estimated kdeg, app plotted as a function of the inverse absolute temperature (T−1). The degradation data generated for the tetra-PEG–GDL precursor solution obtained via high-performance liquid chromatography (HPLC) are also presented; details are provided in Section “Introduction” of the Supplementary Information. As T−1 increased, kdeg,app linearly decreased according to the semilogarithmic plot, indicating that the reaction followed an Arrhenius-type activation process described by the following equation:
where A and Ea represent the frequency and activation energy of the degradation reaction, respectively. R is the gas constant, and T is the temperature. The dashed lines in Fig. 5 are the fitting lines obtained using Eq. (5). The slopes of these lines are consistent across the solution and network states at different polymer concentrations, suggesting that the hydrolysis of amide bonds in Tetra-PEG slime changes the viscoelasticity of the material. Moreover, the absolute values were more than ten times lower in the network state than in the solution state, and their difference increased with increasing PEG concentration. These results indicate that the formation of boronic ester bonds reduces the frequency of glucoamide cleavage.
Apparent degradation rate constant of the cleavage reaction plotted as a function of the inverse temperature (T−1). The circles and squares represent the data obtained for the tetra-PEG–GDL solution and Tetra-PEG slime, respectively. The dashed lines represent the fitting lines obtained using the Arrhenius equation
To elucidate the mechanism by which boronic ester bonds inhibit glucoamide degradation, a process that combines the reversible reaction between tetra-PEG–FPBA and tetra–PEG–GDL and hydrolysis of tetra-PEG–GDL should be considered. In the equilibrium state, glucoamide, which contains amide bonds at its termini, exhibits two different conformations, one with minor cyclic structures and one with major linear structures. According to a previous study [19], the cyclic conformation is preferentially hydrolyzed, leading to its cleavage. FPBA binding primarily involves the linear structure, which inhibits the formation of the cyclic conformation; as a result, the overall degradation rate decrease. Considering the reversible reaction of boronic ester bonds and hydrolysis of cyclic tetra-PEG–GDL, the kinetic equations for each reaction can be written as follows.
Considering the law of mass conservation for each functional group, the following equation is obtained:
where NFPBA, NGDL, Ndegraded-GDL, and NFPBA-GDL are the end group concentrations of tetra-PEG–FPBA and tetra-PEG–GDL and the molar concentrations of the degraded gluconolactone and boronic ester bonds, respectively. ka (=3.5 × 105 d−1 mol−1 L at 30 °C) and kd (=5.8 × 102 d−1 at 30 °C) are the rate constants of the binding and dissociation processes measured via surface plasmon resonance in our previous study [12]. khyd (=0.129 d−1 at 30 °C) is the rate of glucosamine hydrolysis in tetra-PEG–GDL measured via HPLC.
According to Eqs. (6)–(8), NGDL and Rslime can be numerically derived as functions of t. By fitting the time dependence of the simulated Rslime via Eq. (4), a relationship between the theoretical kdeg,app and cPEG was obtained (see the solid line in Fig. 6; details of the fitting procedure are described in Section “Experimental” of the Supplementary Information). The predicted kdeg,app value decreased with increasing cPEG values and asymptotically approached the experimental curve at the low cPEG limit. Thus, the concentration dependence of kdeg,app qualitatively agreed with the experimental data represented by the squares. Compared to the experimental values, the theoretical values were approximately five times larger. Therefore, the numerical solution obtained at khyd = 0.3·khyd,HPLC is plotted as a dashed line in Fig. 6, which more accurately predicts the experimental results.
The apparent delay of the hydrolysis process indicates that the binding between boronic acid and “cyclic” glucoamide suppresses the hydrolysis reaction, as shown in Fig. 7. Although the corresponding constant is lower than that of binding reactions between boronic acid and linear glucoamide, boronic acid in the tetravalent state easily binds to sugars, such as glucose and ribose, which are widely utilized in glucose sensors [20,21,22,23,24,25]. The reaction between the hydroxyl group of cyclic glucoamide and boronic acid prevents an amino group from forming, further delaying the cleavage reaction.
Conclusion
In summary, we explored the time-dependent nature of the viscoelastic properties of Tetra-PEG slime as a model transient network. We found that the viscoelastic behavior of Tetra-PEG slime corresponds with a Maxwellian model that is characterized by a single relaxation mode independent from the degree of degradation. As the incubation time increased, the terminal relaxation time decreased, which was primarily attributed to the hydrolysis of glucoamide groups. However, compared to that in aqueous solution, the degradation rate of these groups in Tetra-PEG slime was more than ten times lower, which was influenced by the concentration of reactive groups. The obtained results imply the hydrolysis of glucoamide groups is inhibited when the groups bind to phenylboronic acid in linear and cyclic states. This study suggested that Tetra-PEG slimes can be used as biomaterials to control viscoelasticity in the long-term by tuning the degradation of associative points.
References
Yoshida T, Hirose R, Naito Y, Inoue K, Dohi O, Yoshida N, et al. Viscosity: an important factor in predicting the performance of submucosal injection materials. Mater Des. 2020;195:109008.
Hirose R, Nakaya T, Naito Y, Daidoji T, Dohi O, Yoshida N, et al. Identification of the critical viscoelastic factor in the performance of submucosal injection materials. Mater Sci Eng C Mater Biol Appl. 2019;94:909–19.
de Almeida AT, Figueredo V, da Cunha ALG, Casabona G, Costa de Faria JR, Alves EV, et al. Consensus recommendations for the use of hyperdiluted calcium hydroxyapatite (Radiesse) as a face and body biostimulatory agent. Plast Reconstr Surg Glob Open. 2019;7:e2160.
Arshinoff SA, Jafari M. New classification of ophthalmic viscosurgical devices—2005. J Cataract Refract Surg. 2005;31:2167–71.
Yayehrad AT, Siraj EA, Matsabisa M, Birhanu G. 3D printed drug loaded nanomaterials for wound healing applications. Regen Ther. 2023;24:361–76.
Carrasco F, Pérez-Maqueda LA, Sánchez-Jiménez PE, Perejón A, Santana OO, Maspoch ML. Enhanced general analytical equation for the kinetics of the thermal degradation of poly(lactic acid) driven by random scission. Polym Test. 2013;32:937–45.
Tsuji H, Fukui I. Enhanced thermal stability of poly(lactide)s in the melt by enantiomeric polymer blending. Polymer. 2003;44:2891–6.
Sheng S, Meiling Z, Suying Z, Wensheng H, Zhifeng Y. Evolution of physicochemical structure of waste cotton fiber (hydrochar) during hydrothermal carbonation. Autex Res J. 2020;20:319–26.
Daly PA, Bruce DA, Melik DH, Harrison GM. Thermal degradation kinetics of poly(3‐hydroxybutyrate-co-3-hydroxyhexanoate). J Appl Polym Sci. 2005;98:66–74.
Choong GYH, De Focatiis DSA. A method for the determination and correction of the effect of thermal degradation on the viscoelastic properties of degradable polymers. Polym Degrad Stab. 2016;130:182–8.
Michida S, Chung U-I, Katashima T. Probing the molecular mechanism of viscoelastic relaxation in transient networks. Gels. 2023;9:945.
Katashima T, Kudo R, Naito M, Nagatoishi S, Miyata K, Chung UI, et al. Experimental comparison of bond lifetime and viscoelastic relaxation in transient networks with well-controlled structures. ACS Macro Lett. 2022;11:753–9.
Shibayama M. Spatial inhomogeneity and dynamic fluctuations of polymer gels. Macromol Chem Phys. 1998;199:1–30.
Sakai T. Experimental verification of homogeneity in polymer gels. Polym J. 2014;46:517–23.
Katashima T. Rheological studies on polymer networks with static and dynamic crosslinks. Polym J. 2021;53:1073–82.
Katashima T. Molecular understanding of viscoelasticity in transient polymer networks based on multiple methods. Nihon Reoroji Gakkaishi. 2022;50:51–6.
Katashima T, Kobayashi R, Ishikawa S, Naito M, Miyata K, Chung UI, et al. Decoupling between translational diffusion and viscoelasticity in transient networks with controlled network connectivity. Gels. 2022;8:830.
Katashima T, Kudo R, Onishi R, Naito M, Nagatoishi S, Miyata K, et al. Effects of network connectivity on viscoelastic relaxation in transient networks using experimental approach. Front Soft Matter. 2022;2:1059156.
Wolfrom ML, Bennett RB, Crum JD. The hydrolytic instability of the aldonamides. J Am Chem Soc. 1958;80:944–6.
Shiomi Y, Saisho M, Tsukagoshi K, Shinkai S. Specific complexation of glucose with a diphenylmethane-3,3′-diboronic acid derivative: Correlation between the absolute configuration of mono- and di-saccharides and the circular dichroic activity of the complex. J Chem Soc Perkin Trans. 1993;1:2111–7.
Matsumoto A, Ikeda S, Harada A, Kataoka K. Glucose-responsive polymer bearing a novel phenylborate derivative as a glucose-sensing moiety operating at physiological pH conditions. Biomacromolecules. 2003;4:1410–6.
Park S, Boo H, Chung TD. Electrochemical non-enzymatic glucose sensors. Anal Chim Acta. 2006;556:46–57.
Shoji E, Freund MS. Potentiometric saccharide detection based on the pK(a) changes of poly(aniline boronic acid). J Am Chem Soc. 2002;124:12486–93.
James TD, Sandanayake KRAS, Shinkai S. Saccharide sensing with molecular receptors based on boronic acid. Angew Chem Int Ed. 1996;35:1910–22.
James TD, Sandanayake KRAS, Shinkai S. Chiral discrimination of monosaccharides using a fluorescent molecular sensor. Nature. 1995;374:345–7.
Acknowledgements
This study was supported by the Japan Society for the Promotion of Science (JSPS) through Scientific Research (A) (grant numbers 21H04688 to T.S. and 21H04952 to U.C.). It was also supported by the Data Creation and Utilization-Type Material Research and Development Project (grant number JPMXP1122714694 to T.S.), the Japan Science and Technology Agency (JST) Moon-shot R&D (grant number 1125941 to T.S.), and CREST (grant numbers JPMJCR1992 to T.S. and JPMJCR23L2 to T.K.).
Funding
Open Access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
TK conceptualized the project. RK, MN, and TK designed the experiments. RK and MN performed the data acquisition and analysis. RK and MN synthesized the samples. TK handled the administrative, technical, and supervisory tasks. RK, MN and TK drafted the manuscript. All the authors contributed to the data interpretation and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kobayashi, R., Naito, M., Miyata, K. et al. Predicting the effects of degradation on viscoelastic relaxation time using model transient networks. Polym J (2024). https://doi.org/10.1038/s41428-024-00902-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41428-024-00902-z