Abstract
Herein we describe the alcoholysis of super engineering plastics under mild conditions. Treatment of polysulfone (PSU) with methanol mediated by sodium hydroxide in 1,3-dimethyl-2-imidazolidinone (DMI) at 80 °C resulted in facile depolymerization to form bis(4-methoxyphenyl)sulfone and 4,4’-(propane-2,2-diyl)diphenol (bisphenol A) in high yields. These products were readily isolated by simple filtration. The DMI solvent effectively promoted depolymerization and allowed insoluble resins such as polyetheretherketone (PEEK) to undergo the reaction. This method was applicable to other alcohols, such as ethanol and isopropyl alcohol.
Similar content being viewed by others
Introduction
As the importance of the circular economy grows, the development of recycling technology for petrochemical products such as plastics has attracted much attention. Various chemical recycling methods have been developed for commodity plastics and various engineering plastics [1,2,3,4,5,6,7]. In the cases of ester-type and related polymers such as polyethylene terephthalate (PET) [8,9,10,11,12,13] and polycarbonate (PC) [14,15,16,17], various depolymerization strategies such as hydrolysis and alcoholysis have been developed. These methods provide depolymerization under mild conditions to form usable low-molecular-weight products such as dimethyl terephthalate from PET and dimethyl carbonate from PC. Therefore, this methodology offers the possibility of degrading other molecules and polymers under moderate conditions.
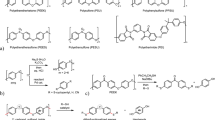
In recent years, degradation methods have been developed for persistent resins such as polyamides [18,19,20,21,22], polyureas [23, 24], and epoxy resins [25,26,27]. Among these persistent resins, super engineering plastics (Fig. 1a) are widely used and indispensable materials in industrial society. While some degradation reactions from these materials have been reported [28,29,30,31,32,33], these methods have not been sufficiently developed for the wide variety of known super engineering plastics. For example, hydroxylation-depolymerization of polyethersulfone (PESU) in subcritical water containing NaOH above 250 °C was reported (Fig. 1b upper). However, the low yields and selectivities of the products were issues [33]. Thus, their distinctive structures and stabilities, resulting in high heat and chemical resistance, make them challenging targets for chemical recycling and the development of organic reactions. Given this background, we have begun to investigate the depolymerization of super engineering plastics. Initially, we found that insoluble polyetheretherketone (PEEK) underwent depolymerization with thiols and bases in N,N-dimethylacetamide at 150 °C (Fig. 1b middle) [34]. This method selectively cleaved the main chain carbon–oxygen bonds to form dithiofunctionalized monomers and hydroquinone. We also discovered that alkali hydroxides were applicable to depolymerization with calcium hydride as a dehydrating reagent in 1,3-dimethyl-2-imidazolidinone (DMI) at 150 °C to provide bisphenol products (Fig. 1b bottom) [35]. These two methods were attributed to highly nucleophilic reagents, an alkylthiolate and a hydroxide, in a stable and polar solvent. In contrast, most reported depolymerization methods for super engineering plastics were performed at relatively high temperatures (e.g., more than 150 °C) for organic reactions. This perspective suggested a strategic implementation of depolymerization under more moderate conditions. Based on this consideration, we expected that the use of alkoxides as reactive nucleophiles would provide depolymerization of super engineering plastics under mild conditions.
Herein, we report depolymerization of super engineering plastics by alcohols, represented by methanol, and easily available bases such as sodium hydroxide under mild conditions (Fig. 1c). As a typical super engineering plastic, we focused on polysulfone (PSU), which contains a repeat structure comprising an electron-deficient diphenylsulfone and bisphenol A. The electron-withdrawing groups promote aromatic nucleophilic substitution [36, 37] such that selective cleavage of the carbon‒oxygen main chain is expected. The depolymerization of PSU with methanol proceeded quickly in the polar DMI solvent to form low-molecular-weight compounds. The final depolymerization products generated in situ were separated based on solubility differences. The influence of the solvent medium was important in promoting this depolymerization. In addition, the present method did not necessarily dissolve the targeted resins and was applicable to insoluble resins such as PEEK.
Results and discussion
Initially, PSU (1) pellets (0.2 mmol relative to the molecular weight of the monomer) with an average Mw of 35,000 determined by light scattering and an average Mn of 16,000 determined by membrane osmometry (as per the catalog specifications) were dissolved in 1,3-dimethyl-2-imidazolidinone (DMI) (0.4 mL) in the presence of 6 equiv. of methanol and 2.2 equiv. of NaOH at 60 °C. After 17–18 h, methanolysis depolymerization formed the desired products bis(4-methoxyphenyl)sulfone (BPS-Me2, 2) and 4,4’-(propane-2,2-diyl)diphenol (bisphenol A (BPA)) (3) in good yields along with the intermediate MeO-BPS-BPA-OH type dimer 4 and demethylated MeO-BPS-OH 5 after treatment with aq. HCl (Table 1, Entry 1). After examining the results for different reaction temperatures, we found that the reaction run at 80 °C afforded excellent yields of 2 and 3 (Entries 2–4). The use of KOH and CsOH·H2O instead of NaOH decreased the yields of 2 and 3 due to the formation of some unidentified byproducts (Entries 5 and 6, see Supplementary Table S1). Weak inorganic bases such as Na2CO3 and K3PO4 and a strong organic base, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), did not promote depolymerization (Entries 7–9). Thus, the typical and cost-effective NaOH was suitable for this depolymerization. Higher amounts of NaOH (4 equiv.) slightly increased the amount of 5 (Entry 10), indicating that excess NaOH enhanced the demethylation reaction. Finally, we screened the amount of methanol (Entries 11 and 12) and found that 12 equiv. of methanol was effective in producing 2 and 3 while suppressing the generation of 5 (Entry 12). Depolymerization at room temperature provided 2 and 3, albeit in low yields (Entry 13), demonstrating that this method is highly effective for degrading such robust super engineering plastics. We also examined various solvents for the depolymerization of PSU (1) pellets, such as N-methylpyrrolidone (NMP), N,N-dimethylacetamide (DMAc), and toluene (Entries 14–16, see Supplementary Table S2), but DMI was the most suitable, probably due to its high polarity and stability. Of note, a slight amount of water decreased the depolymerization reactivity (see Supplementary Scheme S1). To avoid this inhibitory effect, the sodium hydroxide, potassium hydroxide, and cesium hydroxide hydrate were dried at 150 °C under reduced pressure (9 mmHg) for 5 h before use in the depolymerization.
We analyzed the depolymerization of PSU under the conditions shown in Table 1, Entry 12 with high-temperature gel permeation chromatography (GPC). Even after 5 min, the original PSU components were fully consumed, and oligomeric compounds were obtained (Fig. 2a, b). The GPC analysis also suggested that the main chain cleavage process started from the inside of the polymer. The GPC traces after 1 h and 3 h showed that small molecules were formed. In addition, due to the high nucleophilicity of methoxide, depolymerization at room temperature (Table 1, Entry 13) afforded light oligomers while consuming the original PSU, although the yields of BPS-Me2 (2) and BPA (3) were low. We used 1H NMR to monitor the yields of 2, 3, intermediate 4, and demethylated 5 during depolymerization at 80 °C (Fig. 2c). The methanolysis depolymerization proceeded rapidly and was nearly completed after 1 h, affording 2, 3, and 4 in 30%, 33%, and 57% yields, respectively (see Supplementary Tables S3 and S4, Fig. S1 for details) [38,39,40]. This result was consistent with the GPC analysis above. Intermediate 4 was more stable than PSU but was gradually converted into 2 and 3. Visual observation of the reaction mixture supported this trend. The PSU pellets disappeared in less than 1 h, and a yellowish suspension was formed (Fig. 2d). After this, the amount of precipitate increased. We also confirmed that the PSU pellets dissolved in a mixture of DMI and methanol (0.5 mL, 4:1 (v/v)) in 1 h (see Supplementary Fig. S2a), indicating that the dissolved PSU smoothly reacted with sodium methoxide. We expected that this precipitate was the BPA disodium salt, sodium 4,4’-(propane-2,2-diyl)diphenolate, which seemed to be the most poorly soluble product after depolymerization. Depolymerization with KOH and CsOH·H2O produced less precipitate, suggesting that larger counter cations improved the solubility (Fig. 2e, f). These observations suggested that the NaOH activator was effective in accelerating the reaction by excluding one product from the reaction system and facilitating separation of the products by simple filtration.
Time course for methanolysis of PSU (1) pellets in DMI. a, b High-temperature GPC trace for the depolymerization of PSU (purple) with methanol (12 equiv.) and NaOH (2.2 equiv.) at room temperature for 18 h (gray), at 80 °C for 5 min (blue), 1 h (green), and 3 h (red). c Time course for the yields of BPS-Me2 (2, blue), BPA (3, red), 4 (green), and 5 (orange) with methanol (12 equiv.) and NaOH (2.2 equiv.) at 80 °C. d-f Photographs of (d) the reaction mixture after 10 min, 1 h, and 18 h with methanol (12 equiv.) and NaOH (2.2 equiv.), and the reaction mixture after 18 h with methanol (6 equiv.) and (e) KOH (2.2 equiv.) and (f) CsOH·H2O (2.2 equiv.)
To gain more insight into this depolymerization process, we examined the reaction of PSU (1) with sodium methoxide in DMI at 80 °C (Fig. 3). The reaction with 2.2 equiv. of NaOMe proceeded to give the desired products 2 and 3 in approximately 70% yields, with the remaining 4 in 21% yield. High doses of NaOMe (12 equiv.) fully consumed 4 but generated a nonnegligible amount of 5 (44% yield), suggesting that the demethylation was caused by NaOMe. Depolymerization with NaOMe in a 29 wt% methanol solution (2.2 equiv., 0.1 mL), which is essentially the same situation as that in Table 1, Entry 12, formed 2 and 3 in excellent yields, without remaining 4. These results suggested that the presence of methanol promoted depolymerization. When a large amount of NaOMe in methanol (12 equiv., 0.58 mL) was used, the PSU pellets swelled without dissolving (see Supplementary Fig. 2b and 2c) but reacted with NaOMe to furnish 2 and 3, albeit in moderate yields, without remaining 4. After the reaction, a pale-yellow solution containing residual PSU pellets was obtained without generating a precipitate (see Supplementary Fig. S3a), showing that the excess methanol dissolved the generated sodium salts but not the PSU pellets. Furthermore, a combination of PSU pellets, NaOH (2.2 equiv.), and 0.4 mL of MeOH in the absence of the DMI solvent did not produce the products 2, 3, 4, and 5, and the PSU pellets remained intact (see Supplementary Fig. S3b). Thus, the combination of methanol, NaOH and DMI provided facile and efficient methanolysis, possibly due to solvation of the NaOMe by DMI. In addition, even if the PSU was not dissolved but swollen with an appropriate amount of DMI in methanol (e.g., DMI/methanol = 2:3 (v/v), see Supplementary Fig. 2b), this depolymerization with NaOMe proceeded.
To gain further information about the formation of 2 and 3, we examined the aryl‒O bond cleavage of 4 under various conditions at 80 °C for 4 h (Fig. 4). When approximately 2 equiv. of NaOMe was used, both 2 and 3 were produced in more than 80% yields, respectively, with a small amount of 5. With the use of 2 equiv. of NaOH and 6 equiv. of methanol, 2 and 3 were obtained in equal yields, whereas 5 was not observed. On the other hand, the reaction with 2 equiv. of NaOH in the absence of methanol hardly proceeded. We next attempted the demethylation of 2 with 1 equiv. of NaOMe for 4 h. As a result, 5 was generated in 7% yield (Fig. 5). Extending the reaction time (18 h) increased the yield of 5. On the other hand, NaOH provided hardly any demethylation. These results demonstrated that the aryl‒O bonds in PSU and 4 were cleaved by NaOMe under the present conditions at 80 °C due to its high nucleophilicity. However, NaOMe also slightly demethylated 2.
The effects of NaOH described above led us to examine the separation protocol for 2 and BPA (3). We examined gram-scale depolymerization of PSU (1) pellets under the optimized conditions with 12 equiv. of MeOH and 2.2 equiv. of NaOH (Fig. 6 and Supplementary Information Page S3). After reacting for 28 h, the generated precipitate was separated by filtration and treated with aq. HCl to obtain crude 3, which was isolated by NaOH-HCl extraction. The filtrate was washed with aq. NaOH to obtain the crude product containing mainly 2, which was purified by recrystallization. In addition, gram-scale depolymerization was attempted for a longer reaction time (41 h), which gave 2 and 3 in higher yields (see supplementary Scheme S2).
The proposed methanolysis depolymerization pathway is shown in Fig. 7. The reaction of PSU (1) with NaOMe produced by the reaction of methanol and NaOH proceeded via the cleavage of the aryl–O bonds in the main chain to give BPS-Me2 (2) and the BPA sodium salt 6 mainly through the formation of dimer 4. Even though alkoxide nucleophiles can cleave aryl–SO2 bonds [41,42,43,44], depolymerization successfully occurred with the aryl–SO2 main chains remaining intact, as in our previous study [35]. The obtained dianion 6 was almost insoluble in the present solution such that the formed precipitate was separated from other products by filtration before the treatment with HCl to form BPA (3). At that time, NaOMe caused the demethylation of 2 and generated 5 so that exact amounts of NaOH (approximately 2 equiv.) should be used for this depolymerization.
We applied the present methanolysis depolymerization to other super engineering plastics, such as polyphenylsulfone (PPSU), polyetherethersulfone (PEES), and PEEK (Fig. 8 and Supplementary Table S5-S7). We used a baby bottle made up of PPSU (Mn 17932 and Mw 43133 analyzed by high-temperature GPC using polystyrene) [35] as a consumer resin. Small pieces of the baby bottle (7) were subjected to depolymerization with methanol and NaOH at 80 °C to give BPS-Me2 (2) and 4,4’-dihydroxybiphenyl (8). PEES (9) (Mn 21458 and Mw 45338 determined with high-temperature GPC using polystyrene) [35] and PEEK (11) (average Mw ~ 20,800 by LS, average Mn ~ 10,500 by MO as in the catalog specification) main chains included less-reactive hydroquinone units [34, 35]. In addition, PEEK is normally insoluble in organic solvents. Despite these disadvantages, resins 9 and 11 underwent the reaction at 120 °C to form the corresponding products 2 or 4,4’-dimethoxybenzophenone (12) with hydroquinone (10), albeit with lower yields than those from PSU. This trend was similar to the previous case using CsOH·H2O at 150 °C [35].
Finally, we examined other alcohols for this depolymerization (Fig. 9 and Supplementary Table S8). Ethanol was applied to the depolymerization of PSU (1) pellets instead of methanol at 100 °C to form the corresponding depolymerization products bis(4-ethoxyphenyl)sulfone (13) and BPA (3) in 54 and 83% yields, respectively. Isopropyl alcohol was suitable for depolymerization at 100 °C to provide bis(4-isopropoxyphenyl)sulfone (14) and 3 in 49 and 51% yields, respectively. These results indicated that the depolymerization reactivity was attributable to the structure of the main chains and the nucleophilicity of the alcohols.
Conclusion
We have demonstrated that alcoholysis can be used for low-temperature depolymerization of super engineering plastics such as PSU, PPSU, PEES, and PEEK with a combination of alcohols and sodium hydroxide in DMI solvent. When using methanol, this depolymerization proceeded quickly to give low molecular weight products, followed by formation of the final products. Sodium hydroxide was an effective activator by promoting depolymerization and providing insoluble sodium salts that were separable by simple filtration. The depolymerization method proceeded without substrate dissolution, although the reactivity was reduced. Further efforts are underway to exploit catalytic alcoholysis of various super engineering plastics and related robust polymer materials.
Experimental method
Depolymerization of PSU (1) pellets by methanol with sodium hydroxide (Table 1, Entry 12)
To a mixture of PSU pellets (87.6 mg, 0.198 mmol relative to the molecular weight of the monomer), sodium hydroxide (17.8 mg, 0.44 mmol), and methanol (100 μL, 79.1 mg, 2.47 mmol) were added 1,3-dimethyl-2-imidazolidinone (DMI, 0.40 mL) in a 3 mL vial under argon atmosphere. The resultant mixture was stirred at 80 °C for 18 h. The reaction mixture was cooled to room temperature. HCl aq. (2 M, 0.5 mL) was added to quench the reaction. This mixture was analyzed by 1H NMR to determine the yields of the products 1,1’-sulfonylbis[4-methoxybenzene] (2) (92%), 4,4’-(propane-2,2-diyl)diphenol (3) (94%), 4 (6%), and 5 (1%) by using CDCl3 and mesitylene as internal standards. Product 2 was obtained in 83% yield (45.9 mg, 0.165 mmol) by preparative TLC (hexane/ethyl acetate 10:3 to 10:6).
References
Hong M, Chen EY-X. Chemically recyclable polymers: a circular economy approach to sustainability. Green Chem. 2017;19:3692–706.
Kosloski-Oh SC, Wood ZA, Manjarrez Y, de los Rios JP, Fieser ME. Catalytic methods for chemical recycling or upcycling of commercial polymers. Mater Horiz. 2021;8:1084–129.
Kim JG. Chemical recycling of poly(bisphenol A carbonate). Polym Chem. 2020;1:4830–49.
Payne J, Jones MD. The chemical recycling of polyesters for a circular plastics economy: challenges and emerging opportunities. ChemSusChem. 2021;14:4041–70.
Chen H, Wan K, Zhang Y, Wang Y. Waste to wealth: chemical recycling and chemical upcycling of waste plastics for a great future. ChemSusChem. 2021;14:4123–36.
Xu G, Wang Q. Chemically recyclable polymer materials: polymerization and depolymerization cycles. Green Chem. 2022;24:2321–46.
Chu M, Liu Y, Lou X, Zhang Q, Chen J. Rational design of chemical catalysis for plastic recycling. ACS Catal. 2022;12:4659–79.
Kurokawa H, Ohshima M, Sugiyama K, Miura H. Methanolysis of polyethylene terephthalate (PET) in the presence of aluminium tiisopropoxide catalyst to form dimethyl terephthalate and ethylene glycol. Poly Degrad Stab. 2003;79:529–33.
Pardal F, Tersac G. Comparative reactivity of glycols in PET glycolysis. Poly Degrad Stab. 2006;91:2567–78.
Fukushima K, Coulembie O, Lecuyer JM, Almegren HA, Alabdulrahman AM, Alsewailem FD, et al. Organocatalytic Depolymerization of Poly(ethylene terephthalate). J Polym Sci A Polym Chem. 2011;49:1273–81.
Pham DD, Cho J. Low-energy catalytic methanolysis of poly(ethyleneterephthalate). Green Chem. 2021;23:511–25.
Tanaka S, Sato J, Nakajima Y. Capturing ethylene glycol with dimethyl carbonate towards depolymerisation of polyethylene terephthalate at ambient temperature. Green Chem. 2021;23:9412–16.
Abe R, Komine N, Nomura K, Hirano M. La(III)-Catalysed degradation of polyesters to monomers via transesterifications. Chem Commun. 2022;58:8141–44.
Hu L-C, Oku A, Yamada E. Alkali-catalyzed methanolysis of polycarbonate. A study on recycling of bisphenol A and dimethyl carbonate. Polymer. 1998;39:3841–45.
Liu M, Guo J, Gu Y, Gao J, Liu F, Yu S. Pushing the limits in alcoholysis of waste polycarbonate with dbu-based ionic liquids under metal- and solvent-free conditions. ACS Sustain Chem Eng. 2018;6:13114–21.
Liu M, Guo J, Gu Y, Gao J, Liu F. Versatile imidazole-anion-derived ionic liquids with unparalleled activity for alcoholysis of polyester wastes under mild and green conditions. ACS Sustain Chem Eng. 2018;6:15127–34.
Jehanno C, Demarteau J, Mantione D, Arno MC, Ruipérez F, Hedrick JL, et al. Synthesis of functionalized cyclic carbonates through commodity polymer upcycling. ACS Macro Lett. 2020;9:443–7.
Kamimura A, Yamamoto S. A novel depolymerization of nylons in ionic liquids. Polym Adv Technol. 2008;19:1391–95.
Yamamoto S, Kamimura A. Chem Lett. 2009;38:1016–7.
Kamimura K, Shiramatsu Y, Kawamoto T. Green Energy Environ. 2019;4:166–70.
Alberti C, Figueira R, Hofmann M, Koschke S, Enthaler S. Chemical recycling of end-of-life polyamide 6 via ring closing depolymerization. ChemistrySelect. 2019;4:12638–42.
Wursthorn L, Beckett K, Rothbaum JO, Cywar RM, Lincoln C, Kratish Y, et al. Selective lanthanide-organic catalyzed depolymerization of nylon-6 to ɛ-caprolactam. Angew Chem Int Ed. 2023;62:e202212543.
Kumar A, Luk J Catalytic Hydrogenation of Urea Derivatives and Polyureas. Eur J Org Chem. 2021;4546–50.
Iwasaki T, Tsuge K, Naito N, Nozaki K. Chemoselectivity change in catalytic hydrogenolysis enabling urea-reduction to formamide/amine over more reactive carbonyl compounds. Nat Commun. 2023;14:3279.
Nguyen ST, McLoughlin EA, Cox JH, Fors BP, Knowles RR. Depolymerization of hydroxylated polymers via light-driven C−C bond cleavage. J Am Chem Soc. 2021;143:12268–77.
Nguyen ST, Fries LR, Cox JH, Ma Y, Fors BP, Knowles RR. Chemical recycling of thiol epoxy thermosets via light-driven C–C bond cleavage. J Am Chem Soc. 2023;145:11151–60.
Ahrens A, Bonde A, Sun H, Wittig NK, Hammershøj HCD, Batista GMF, et al. Catalytic disconnection of C–O bonds in epoxy resins and composites. Nature. 2023;617:730–7.
Yu ZL, Miao GX, Chen YR. Synthesis of thiol- and carboxyl-terminated poly(p-phenylene sulfide) oligomers. Macromol Chem Phys. 1996;197:4061–468.
Wang SJ, Bian SG, Yan H, Xiao M, Meng YZ. Novel synthesis of macrocyclic disulfides from poly(phenylene sulfide) by depolymerization reaction. J Appl Poly Sci 2008;110:4049–54.
Lian Z, Bhawal BN, Morandi B. Palladium-catalyzed carbon-sulfur or carbon-phosphorus bond metathesis by reversible arylation. Science. 2017;356:1059–63.
Minami Y, Matsuyama N, Matsuo Y, Tamura M, Sato K, Nakajima Y. Catalytic reductive cleavage of poly(phenylene sulfide) using a hydrosilane. Synthesis. 2021;53:3351–54.
Delcaillau T, Woenckhaus-Alvarez A, Morandi B. Nickel-catalyzed cyanation of aryl thioethers. Org Lett. 2021;23:7018–22.
Yoshida H, Fukunaka, T Method for producing raw material monomer of polyether sulfone. JP 2013249324, 2013.
Minami Y, Matsuyama N, Takeichi Y, Watanabe R, Mathew S, Nakajima Y. Depolymerization of robust polyetheretherketone to regenerate monomer units using sulfur reagents. Commun Chem. 2023;6:14.
Minami Y, Inagaki Y, Tsuyuki T, Sato K, Nakajima Y. Hydroxylation-depolymerization of oxyphenylene-based super engineering plastics to regenerate arenols. JACS Au. 2023;3:2323–32.
Hawthorne JO, Mihelic EL, Morgan MS. 2,2’-Dihydroxydiphenyl sulfone and its monoethers. J Org Chem. 1968;33:446–8.
Perperopoulou FD, Tsoungas PG, Thireou TN, Rinotas VE, Douni EK, Eliopoulos EE, et al. 2,20-Dihydroxybenzophenones and their carbonyl N-analogues as inhibitor scaffolds for MDR-involved human glutathione transferase isoenzyme A1-1. Bioorg Med Chem. 2014;22:3957–70.
Yang Y, Lu Y, Xiang H, Xu Y, Li Y. Study on methanolytic depolymerization of PET with supercritical methanol for chemical recycling. Polym Degrad Stab. 2002;75:185–91.
Genta M, Iwaya T, Sasaki M, Goto M, Hirose T. Depolymerization Mechanism of Poly(ethylene terephthalate) in Supercritical Methanol. Ind Eng Chem Res. 2005;44:3894–900.
Genta M, Goto M, Sasaki M. Heterogeneous continuous kinetics modeling of PET depolymerization in supercritical methanol. J Supercrit Fluids. 2010;52:266–75.
Bennett OF, Saluti G, Quinn FX. Methoxide displacement of the sulfone linkage in thioxanthen-9-one 10,10-dioxides. A facile synthesis of benzophenone-2’-methoxy-2-sulfinic acids. Synth Commun. 1977;7:33–41.
Song J, Jang S, Lee JW, Jung D, Lee S, Min KH. Click chemistry for improvement in selectivity of quinazoline-based kinase inhibitors for mutant epidermal growth factor receptors. Bioorg Med Chem Lett. 2019;29:477–80.
Yanagi T, Tanaka T, Yorimitsu H. Asymmetric systematic synthesis, structures, and (chir)optical properties of a series of dihetero[8]helicenes. Chem Sci. 2021;12:2784–93.
Bai J, Wang T, Dai B, Liu Q, Yu P, Jia T. Radical anion promoted chemoselective cleavage of Csp2−S bond enables formal cross-coupling of aryl methyl sulfones with alcohols. Org Lett. 2021;23:5761–65.
Acknowledgements
This work was supported financially by JST, ERATO (JPMJER2103). The authors would like to thank Prof. Kyoko Nozaki as the supervisor of this ERATO project, Prof. Kohei Takahashi, Prof. Takanori Iwasaki, Prof. Shuhei Kusumoto, Prof. Xiongjie Jin, and her lab members for valuable discussions. Y.M. also acknowledges JST, PRESTO (JPMJPR21N9) and TOBE MAKI Scholarship Foundation. We would like to thank Ms. Sae Imamura for her kind assistance in the high-temperature GPC analysis.
Author information
Authors and Affiliations
Contributions
Y.M. conceived the idea and designed the whole experiment with the assistance of R.H. and Y.I. Y.M. and R.H. performed the experiments and participated in data analyses. Y.M. and R.H. contributed to writing the manuscript and discussions. K. S and M.Y. supported this project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Minami, Y., Honobe, R., Inagaki, Y. et al. Alcoholysis of oxyphenylene-based super engineering plastics mediated by readily available bases. Polym J 56, 369–377 (2024). https://doi.org/10.1038/s41428-023-00870-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00870-w