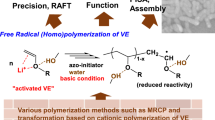
Abstract
Polymeric peroxide is an equimolar alternating copolymer formed by the reaction of a monomer with molecular oxygen (O2). Various polyperoxides have been successfully synthesized using different techniques, such as free radical polymerization, condensation polymerization, and insertion polymerization in the solid state. A wide variety of physical and chemical characteristics are displayed by these polyperoxides, making them attractive candidates for various applications. Due to their high exothermal degrading behavior and autocombustibility, polyperoxides are a viable alternative to fuels derived from petroleum. Additionally, polyperoxides have a wide range of applications, such as free radical initiators, curatives, biocompatible drug carriers, coating materials, dismantlable adhesives, and molding precursors. In this focused review, we report on recent efforts in developing vinyl homo- and copolyperoxides, their physicochemical behaviors, and various applications. Finally, the existing opportunities, possible challenges, and some viewpoints on future directions in vinyl polyperoxide research are highlighted.
Similar content being viewed by others
Introduction
Organic peroxides are a class of materials that contain at least one peroxy linkage (-O-O-) in their chemical structure. The first synthesis of an organic peroxide (benzoyl peroxide) was reported by Brodie in 1858 [1]. In the early 1900s, organic peroxides found their earliest industrial application as an effective bleaching agent. Their use as initiators during the free radical polymerization reactions began to develop significantly during the Second World War. Since then, organic peroxide chemistry has emerged systematically due to peroxide versatility in terms of applications, such as accelerators, bleaching and disinfecting agents, radical initiators, cross-linking agents, and vulcanization agents.
Staudinger reported poly(α-phenylstyrene peroxide) (PAPSP) as the first polyperoxide in 1922 [2]. PAPSP contains multiple -O-O- linkages within a single polymer chain. Thereafter, various other polyperoxides have been prepared and characterized for several applications. The following are the four main methods to prepare polyperoxides: (a) free radical polymerization of a monomer in the presence of high O2 pressure, (b) alternating radical copolymerization of 1,3-diene-based monomer units with O2 at atmospheric pressure, (c) condensation reaction between organic dibasic acids and sodium peroxide (Na2O2), and (d) polymerization of reactive monomers with O2 in the solid-state [3].
Although commonly used polymers degrade endothermically, polymeric peroxides undergo degradation through an exothermic process [4]; thus, polyperoxides are a special class of polymers. Additionally, polyperoxides can exhibit enzymatic degradation and biocompatibility under certain conditions. Because of these special features, polyperoxides have been explored as autocombustible fuel. In addition, this class of polymers could be used as dismantlable adhesives [5], branched polymer precursors [6], curators in coating and molding applications [7], drug carriers [8], polymeric initiators [9], etc.
Various methods of polyperoxide synthesis, characterization techniques, physicochemical properties, and applications have been reviewed elsewhere [3]. This focus review will emphasize the recent achievements in the synthesis, characterization, degradation, biocompatibility, and enzymatic degradation of vinyl polyperoxides and copolyperoxides from our group. Finally, we provide our outlook on the future development possibilities and existing challenges of vinyl polyperoxides for various potential applications.
Vinyl polyperoxides
In vinyl polyperoxides, the main chain contains only one type of monomer alternating with oxygen, as shown in Scheme 1. The preparation of vinyl polyperoxides is usually achieved by conventional free radical polymerization of the vinyl monomer using 2,2’-azobis(isobutyronitrile) (AIBN) as an initiator in the presence or absence of solvents at 45–55 °C. The pathway of oxidative free radical polymerization of vinyl monomers is similar to conventional free radical polymerization and follows three fundamental steps, namely, initiation, propagation, and termination. The rate of oxidative polymerization (Rp) can be calculated using the slope of the O2 consumption versus time plot [10]. In Table 1, the synthesis details and characterization data of several recently developed vinyl polyperoxides are provided.
A substantial number of works regarding polyperoxides from styrene derivatives with different physical properties have been reported. Preparation of polyperoxides from various substituted styrene derivatives, such as 4-flurostyrene, 4-chlorostyrene, 4-bromostyrene, 4-methylstyrene, 4-methoxystyrene, 4-tert butylstyrene and 4-tert-butoxystyrene, was undertaken to understand the kinetics of oxidative polymerizations with both electron-donating and electron-withdrawing groups in the para-position of the phenyl ring [11]. Both electron-donating and electron-withdrawing groups showed enhancement of the oxidative polymerization rate, supported by theoretical calculations using density functional theory (DFT) calculations. To understand the effect of the α-substitution group in styrene on oxidative polymerization, various α-substituted styrenic monomers were polymerized at 100 psi oxygen pressure using AIBN as an initiator at 40-50 °C in toluene [12]. The chain dynamics in solution of polyperoxides prepared from oxygen and monomers such as p-methylstyrene, p-tert-butylstyrene, p-bromostyrene [13], and methacrylonitrile [14] were also studied by measuring the 13C spin-lattice relaxation time (T1) to gain insight into the main chain flexibility of these compounds [15]. Several ortho- and meta-substituted polystyrene polyperoxides were successfully synthesized and characterized [16]. The incorporation of different oligo(ethylene glycol) (OEG) chains in styrenic polyperoxide exhibited aqueous solubility with a relatively low critical solution temperature (LCST) in water, which varied from 43.5 to 67.0 °C depending on the length of the OEG chain [17]. The oxygen pressure-dependent kinetics and distinct fluorescence properties of polyperoxide from 9-vinyl anthracene were thoroughly explored [18]. Generally, polyperoxides are gummy liquids or semisolids because of their low molecular weights and presence of flexible -O-O- linkages in the main chain. Recently, a series of fatty acids containing solid-type styrenic polyperoxides from 4-vinylbenzyl fatty ester (VBFE) were reported with crystalline behavior, depending on the length of the fatty acid chains [19]. The poly(1,3-diisopropenylbenzene peroxide) (PDIPBP) degraded exothermically with heat of degradation (−230.51 kJ/mol), which is slightly higher than that reported earlier for vinyl polyperoxides, possibly because of the cross-linked nature of the PDIPBP [20].
Razuvaev and coworkers reported polyperoxides from methacrylate monomers such as phenyl methacrylate [21], but De and coworkers further characterized the polymer using spectroscopic techniques [22]. Subsequently, several methacrylate-based vinyl monomers were polymerized under high O2 pressure. Poly(2-(2-methoxyethoxy)-ethyl methacrylate) peroxide (PMEO2MAP) was produced by the bulk oxidation of 2-(2-methoxyethoxy)ethyl methacrylate at 10–300 psi O2 pressure and 50 °C using an AIBN initiator. Notably, this water-soluble polyperoxide is a thermoresponsive polymer, and it decomposes exothermically. The cloud point of the LCST-type phase transition of PMEO2MAP was found to be 14.4 °C in water [23]. The homo and copolyperoxides of 2-(acetoacetoxy)ethyl methacrylate (AEMA) were also studied in detail [24, 25]. Therefore, a number of new vinyl polyperoxides were designed and characterized from various styrenic and methacrylate monomers.
Cyclic monomers present a promising avenue for creating a new class of polyperoxides because of their higher rigidity as well as steric effects, which can confer unique physical properties to the resultant polymers. Several cyclic monomers, such as 2,3-dihydrofuran (DHF), 2,3-dihydro-5-methyl-furan (MDHF), benzo[b]furan (BF), and indene (Ind), were used to prepare a series of polyperoxides, after which an examination of the high-pressure kinetics associated with the corresponding oxidative polymerizations was conducted [26]. It was observed that Rp varied depending on the O2 pressure for each of the cyclic monomers, with saturation pressures of 100 psi and 200 psi being observed for MDHF and DHF, respectively, whereas a saturation pressure of 300 psi was noted for BF and Ind.
For the synthesis of polyperoxide, catalysts can be used either with or without an initiator. It has been demonstrated that N-hydroxyphthalimide (NHPI) can effectively catalyze the oxidation of styrene at 35–55 °C at different oxygen pressures in order to produce a quicker rate of oxidative polymerization. The suggested mechanistic root for the oxidative polymerization reaction of styrene in the presence of NHPI was based on the kinetic information and poly(styrene peroxide) (PSP) characterization [27].
Vinyl copolyperoxides
Copolyperoxides with the generic formula -[(-X-O-O-)x-(-Y-O-O-)y]n- (where X and Y represent two different vinyl monomer units) were synthesized by oxidative copolymerization of two different monomers with oxygen. Although this was a terpolymerization reaction, it corresponded to a binary copolymerization based on -XO2•/-YO2• macroradicals in the propagation step. Therefore, the monomer feed composition and monomer composition in copolyperoxide were used to determine the reactivity ratios [28]. The monomer sequence distribution, average chain length of the repeating unit and reactivity ratios of the monomers were investigated thoroughly by Jayanthi and Kishore [29], followed by De and coworkers to determine reactivity ratios for the oxidative copolymerization of various monomer pairs. In addition to the reactivity ratios and monomer sequence distribution, the thermal properties of copolyperoxides were studied in detail to establish their exothermic degradation.
Although vinyl acetate (VAc) did not produce stable polyperoxide, it was successfully copolymerized with styrene and α-methylstyrene (AMS) through oxidative polymerization [30]. Investigations into methyl methacrylate (MMA) and VAc copolymerization under high oxygen pressure revealed that a greater percentage of MMA moieties were statistically positioned in the main chain, which was validated by theoretical evaluation [31]. The oxidative copolymerization kinetics of styrene with AMS have been studied at different O2 pressures, and it was found that the rate of reaction is dependent on O2 pressure with unusually higher values of the activation volume (ΔV#) [32]. Furthermore, the copolymerization of styrene and AMS at 50 °C in toluene under 100 psi O2 pressure was studied in the presence of different initiating catalysts, including AIBN, cobalt(II) phthalocyanine pyridine [CoIIPc(Py)] and cobalt(II) tetraphenylporphyrin pyridine [CoIITPP(Py)] complexes [33]. Interestingly, the incorporation of styrene or AMS in the copolyperoxides was independent of the initiating system.
Although Ind does not copolymerize normally with other vinyl monomers by conventional free radical polymerization, it was oxidatively copolymerized with styrene (St), AMS, and α-phenylstyrene (APS), where the resultant polymers were sticky to solid depending on the Ind content in the copolymer [34]. In addition, Ind was copolymerized in the presence of O2 with a series of monomers, such as MMA/methacrylonitrile [35], methyl/ethyl/butyl acrylates [36], p-tert-butylstyrene [37], and VAc/isopropenyl acetate [38]. The spectral characterization, thermal degradation, and reactivity ratios were thoroughly investigated. Recently, composition-dependent crystallization behavior of copolyperoxides was reported for the oxidative copolymerization of MMA and 4-vinylbenzyl stearate (VBS) [39]. To develop degradable polyperoxide metal complexes, copolyperoxides of AEMA have been designed with styrene and MMA [25]. For the above copolyperoxide systems, the activation energy of thermal degradation (Ed), thermal stability, reactivity ratios, etc., were studied in detail. The synthesis details and characterization data of several vinyl copolyperoxides are given in Table 2.
Characterization of different types of polyperoxides
Polyperoxides were characterized by determining the molecular weight, as well as using various spectroscopic techniques, thermal analysis, and analytical methods such as elemental analysis and active oxygen content determination. The 1:1 ratio of vinyl monomer and oxygen in the polyperoxide chain has been confirmed by elemental analysis of numerous polyperoxides. Due to their easily degradable nature throughout their synthetic procedure, polyperoxides often have low molecular weights in the range of 1000–7000 g/mol and yield small molecules such as ketones, alcohols, and aldehydes. These molecules in the polymerization system can act as chain transfer agents to limit the chain length through the reactions between these decomposed products and the macroperoxy radicals produced during propagation [40]. The spectral analysis of polyperoxides by FTIR spectroscopy exhibited a characteristic strong band between 990 and 1050 cm−1 because of the stretching vibration of the -O-O- bond. Various end groups, such as keto, hydroxyl, aldehyde, hydroperoxide, and -C=C-. have been identified by FTIR, NMR spectroscopy, and matrix-assisted laser desorption ionization-time of flight-mass spectrometry (MALDI-TOF-MS) [41].
The chemical structures of vinyl polyperoxides were characterized extensively using NMR spectroscopy [42]. Generally, the main chain -CH- and -CH2- protons from styrenic monomers were shifted downfield in polyperoxides compared to their polystyrenic homopolymers because of two electronegative oxygen atoms that are attached to them directly. Similarly, the main chain -CH2- protons from methacrylate monomers in the polyperoxide showed a downfield shift compared to their polymethacrylate homopolymers. In the 13C NMR spectra, significant downfield shifts of the backbone carbons are also observed compared to their homopolymers without -O-O- links.
Polyperoxides are generally unstable materials due to the presence of thermally labile weak peroxy bonds in the main chain of the polymer. Although the stability of polymers relies on their structural motif, they are usually stored in a refrigerator in dark conditions to improve their shelf life. The thermal stability of polyperoxides was studied extensively by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). The glass transition temperature (Tg) of polyperoxides was lower than that of their respective homopolymers because of the alternating labile peroxy bonds in the polymer main chain. In the DSC thermogram of polyperoxides, degradation usually started exothermically between approximately 80–120 °C, although it depended on the heating rate. The resulting products of the thermal degradation of polyperoxides were analyzed using positive ion electron ionization mass spectrometry (EI-MS), which was suitable as an investigation tool to analyze the principal degradation products of polyperoxides. Size exclusion chromatography (SEC) analysis and FT-IR spectroscopy are other tools used to track degradation [31].
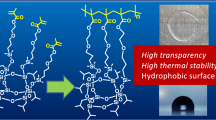
Mostly, polyperoxides are a gummy liquid or semisolid in nature due to their Tg and low molar mass. Moreover, polyperoxides can solidify if they have high Tg values or are crystalline in nature. To form crystalline domains, polyperoxides often need structural symmetry, adequate regularity in the structure, and an optimal degree of flexibility. Recently, different lengths of hydrocarbon chains (fatty acids) were attached at the para position of styrene monomers to introduce side chain crystallinity in the resulting polyperoxides (Fig. 1) [19]. The crystallinity of stearic acid-based polyperoxides was further tuned by introducing MMA units as an amorphous moiety in the copolymer chain. The crystalline melting temperature (Tm) was observed to change in the range of 48–68 °C according to how much MMA was integrated into the polymers [39]. To examine the crystalline behavior in the synthesized polyperoxides, transmission electron microscopy (TEM), polarized optical microscopy (POM), and powder X-ray diffraction (PXRD) examinations were carried out.
Development of crystalline domain in polyperoxides prepared from side-chain fatty acid derived monomers. Reproduced with permission from the American Chemical Society [19]
Degradation of polyperoxides
Degradation of polyperoxides usually occurs highly exothermically by cleavage of the backbone -O-O- bonds in the presence of heat, light, acid, base, and enzyme [43]. Although the thermal degradation of polyperoxides in bulk is typically controlled by the dissociation of ‘O-O’ bonds in the backbone, their photodegradation depends on the stability of the bialkoxy radicals [3]. The impact of heat and enzymes on polyperoxide degradation will be covered in this section.
Although vinyl polyperoxides are quite stable in cold and dark environments, they decompose exothermically at relatively high temperatures or in the presence of light by cleaving the -O-O- bonds in the backbone and producing macroalkoxy radicals, which undergo two categories of reactions: unimolecular decomposition and disproportionation reactions, as shown in Scheme 2. PSP is degraded into two carbonyl molecules, formaldehyde and benzaldehyde, by a unimolecular process via chain unzipping, where the macroalkoxy radicals undergo scission at the bond β- to the radical center. Phenylglycol, α-hydroxyacetophenone, and minor amounts of phenylglyoxal are the primary products in the disproportionation route. In this disproportionation reaction, hydrogen atoms are transferred between the radicals to obtain nonradical products, which have been monitored by gas chromatography‒mass spectrometry (GC‒MS) analysis [3, 40].
Vinyl polyperoxide degradation under thermal conditions. Reproduced with permission from the Taylor & Francis Group [3]
Using Kissinger’s approach, the activation energy (Ed) of thermal degradation of various polyperoxides was calculated [44] from the slope of the ln(ϕ/Tm2) versus 1/Tm plot (Tm: peak temperature in Kelvin, ϕ: heating rate in DSC analysis). The Ed values of various homo- and copolyperoxides were found to be between ~20 and 47 kcal/mol (Table 1 and Table 2), which are comparable to the bond dissociation energy of peroxy bonds in simple organic peroxides. Furthermore, the thermal degradation of PSP, poly(α-methylstyrene) peroxide (PAMSP) and PAPSP was carried out at different temperatures in toluene, and the activation energy of degradation was evaluated [45]. Styrene, MMA, and AMS were oxidatively terpolymerized to generate polyperoxides of different compositions, which were then examined thermally and spectroscopically to ascertain their characteristics [46]. The Ed values for the homopolyperoxides and copolyperoxides were obtained by various methods and found to be in reasonable agreement with each other. To understand the influence of electron-donating and electron-attracting substituents on the thermal degradation of a series of para-substituted PSPs, TGA was performed [47]. Interestingly, a linear relationship between the Hammett constant for the substituents and the maximum rate of degradation temperature (Tmax) was observed (Fig. 2). Their thermal degradation kinetics in toluene at 65–95 °C were also studied to obtain Ed values of 18–22 kcal/mol [48].
Hammett plot of Tmax values in various para-substituted PSPs. Reproduced with permission from Elsevier [47]
Enzymatic degradation and biocompatibility of vinylpolyperoxides
The active site of the horseradish peroxidase (HRP) enzyme exhibits a heterolytic breakdown of the -O-O- bond in hydrogen peroxide (H2O2) [49]. Therefore, enzymatic degradation of vinyl polyperoxides such as PAMSP, PSP, and poly(methyl methacrylate peroxide) (PMMAP) was investigated at room temperature in the presence of HRP enzyme (Fig. 3) [50]. With increasing HRP concentration and reaction time, the enzymatic degradation of polyperoxides was enhanced (Fig. 3), and decomposition products were monitored by 1H NMR spectroscopy. Benzaldehyde and formaldehyde were the resulting main products of PSP degradation via cleavage of -O-O- bonds in the polymer main chain. Additionally, minute amounts of phenylglycol and α-hydroxyacetophenone were produced via the disproportion pathway. In the presence of HRP, PMMAP and PAMSP produced the unimolecular decomposition products acetophenone and methyl pyruvate, respectively, and formaldehyde. Similar first-order kinetics were also observed for HRP enzyme-triggered degradation of PAEMAP, with the main degradation products being formaldehyde and 2-(2-oxopropanoyloxy)ethyl 3-oxobutanoate [24].
Horseradish peroxidase (HRP) enzyme-mediated degradation of polyperoxides at 26 °C; A PSP/HRP = 10:1 (■), 25:1 (●), 30:1 (▲) and 40:1 (▼) (by weight). B PMMAP/HRP = 10:1 (by weight) (◆) and PAMSP/HRP = 10:1 (by weight) (►). Reproduced with permission from the Taylor & Francis Group [50]
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye reduction assay on a tumor (HeLa) cell line was used to test the biocompatibility of PAMSP, PSP, and PMMAP [50]. PMMAP and PSP exhibited a nontoxic nature up to 60 μg/mL, and PAMSP was found to be nontoxic up to 120 μg/mL. Similarly, the biocompatible behavior study for PAEMAP exhibited an appreciable nontoxicity profile on HeLa cells for polymer concentrations up to 240 μg/mL [24].
Applications of polyperoxides
Due to the presence of -O-O- bonds in their main chain, polyperoxides can serve as free radical initiators in a manner similar to simple organic peroxide initiators. The use of polymeric initiators offers several advantages over traditional small-molecule initiators, and one of the key benefits is the ability to prepare block copolymers through the incorporation of a specific initiator segment into the main polymer chain, which could be converted into macroradicals under suitable conditions [51]. Additionally, the degradation of polyperoxides can lead to changes in their chemical structure, molecular weight, and mechanical and physical properties. This property has been explored in the development of dismantlable adhesives, where degradable polymers offer a promising option [5, 52]. Additionally, polyperoxides have demonstrated versatility in various applications, especially in drug delivery, due to their unique properties, including stimuli-induced degradation and the ability to form nanostructures in an aqueous medium. Their stimuli-induced degradation allows for controlled drug release, making them suitable for sustained and targeted drug delivery [8]. Apart from these, polyperoxides have been investigated for their potential use as fuels due to their highly exothermic decomposition [53], coating and molding applications, plastic waste management, etc. In our research group, we explored vinyl polyperoxides as initiators during free radical polymerization reactions. Free radical polymerization of MMA has been studied using poly(9-vinyl anthracene peroxide) (PVAnP) as a macroinitiator at 90 °C in toluene [18]. The synthesized poly(methyl methacrylate) (PMMA) contained a fluorescent anthracene moiety at the chain end, as confirmed by 1H NMR, UV‒visible and fluorescence spectroscopy (Fig. 4). The polyperoxide from 4-vinylbenzyl methoxypoly(oxyethylene) ether (PEGSt) was also successfully used to prepare a water soluble polymer from N,N-dimethylacrylamide (DMA) in toluene [17].
A 1H NMR spectrum of PMMA obtained from polymerization of MMA in the presence of PVAnP macroinitiator, B absorption spectra of VAn (0.1 mmol), PVAnP (0.1 mg/mL) and PMMA (2 mg/mL) in tetrahydrofuran (THF), and C fluorescence spectra of VAn (0.1 mmol), PVAnP (0.1 mg/mL) and PMMA (2 mg/mL) excited at 368 nm in THF. Reproduced with permission from Elsevier [18]
Conclusion and future perspective
In summary, we reviewed recent developments in the research area of vinyl polyperoxides, a unique class of polymers that undergo highly exothermic degradation. The article covers various aspects of vinyl polyperoxides, including their synthesis, physico-chemical behavior, and various applications. Vinyl polyperoxides undergo easy degradation reactions during their synthesis and thus have a low molar mass with a gum-like, semisolid nature. The highly exothermic degradation and gum-like nature of polyperoxides make them difficult to handle. Thoughtful incorporation of long-chain fatty acid pendants into vinyl polyperoxides resulted in solid crystalline polyperoxides with improved thermal stability, as fatty acid units underwent side-chain crystallization through the all-trans conformation of long alkyl chains.
We have demonstrated vinyl polyperoxides as thermal initiators during the conventional free radical polymerizations of vinyl monomers in solution. The PVAnP-initiated polymerization of MMA in toluene installed a fluorescent anthracene moiety at the PMMA chain ends [18]. However, initiator efficiency was not measured. Additionally, the detailed polymerization mechanism and further scope of vinyl monomers, such as PVAnP-initiated polymerization, should be studied to develop water-soluble polymers with fluorescent moieties as chain ends for further biological study. Although water soluble vinyl polyperoxide from PEGSt was used to polymerize DMA in organic solvent to prepare water soluble polymers, water as a polymerization medium should be explored as a green solvent.
As the focus on sustainable and environmentally friendly materials grows, there is potential in this area, as molecular oxygen is one of the components in vinyl polyperoxides. Such materials could find applications in biodegradable adhesives [54], although no work has been reported thus far with vinyl polyperoxides. At the same time, the stimuli-induced degradation of vinyl polyperoxides can be leveraged to create smart materials that respond to external stimuli, such as temperature. A limited number of water-soluble vinyl polyperoxides with thermoresponsive properties in an aqueous medium below their degradation temperature have been reported. Thus, there is tremendous opportunity to develop thermoresponsive vinyl polyperoxides, which could be crystalline solids with enhanced thermal stability.
Although small molecule organic peroxides find extensive applications in diverse areas, polyperoxides are yet to find technological relevance. This is mostly because of their explosive nature and low yield during synthesis. Although it is always important to study the effect of O2 in vinyl polymerizations, more research is needed to better understand and control polyperoxide synthesis and enhance their stability. We believe that these fundamental issues will be addressed in the near future, and polyperoxides will find technological importance.
References
Brodie BC. Ueber die bildung der hyperoxyde organischer säureradicale. Justus Liebigs Ann Chem. 1858;108:79–83. https://doi.org/10.1002/jlac.18581080117
Staudinger H. Erfahrungen über einige explosionen. Angew Chem. 1922;35:657–9. https://doi.org/10.1002/ange.19220359302
Samanta P, Mete S, Pal S, De P. Polymeric peroxides: synthesis, characterization, degradation and applications. J Macromol Sci Part A. 2022;59:711–30.
Kishore K, Mukundan T. Poly(styrene peroxide): an auto-combustible polymer fuel. Nature. 1986;324:130–1.
Sato E, Tamura H, Matsumoto A. Cohesive force change induced by polyperoxide degradation for application to dismantlable adhesion. ACS Appl Mater Interfaces. 2010;2:2594–601.
Kurochkin SA, Silant′ev MA, Perepelitsyna EO, Grachev VP. Molecular oxygen as a regulator of primary chain length of branched polymers formed in 3D radical polymerization. Oxidative polymerization of styrene. Polymer. 2013;54:31–42.
Subramanian K, Kishore K. Application of polystyrene peroxide as a curative in coating and molding compositions. Eur Polym J. 1997;33:1365–7.
Fujioka T, Taketani S, Nagasaki T, Matsumoto A. Self-assembly and cellular uptake of degradable and water-soluble polyperoxides. Bioconjugate Chem. 2009;20:1879–87.
Murthy KS, Kishore K, Mohan VK. Vinyl monomer based polyperoxides as potential initiators for radical polymerization: an exploratory investigation with poly(α-methylstyrene peroxide). Macromolecules. 1994;27:7109–14.
Nanda AK, Kishore K. Autocatalytic oxidative polymerization of indene by cobalt porphyrin complex and kinetic investigation of the polymerization of styrene. Macromolecules. 2001;34:1600–5.
Pal S, Ghorai PK, De P. Oxidative polymerization of para-substituted styrene derivatives: synthesis, characterization and kinetics study. Polymer. 2012;53:3687–94.
Pal S, Ghorai PK, De P. Kinetic and thermochemical study of the oxidative polymerizations of α-substituted styrenes. Polym Bull. 2012;69:149–61.
De P, Sathyanarayana DN. Para-substituted poly(styrene peroxide)s: synthesis, characterization, thermal reactivities and chain dynamics studies in solution. Macromol Chem Phys. 2002;203:420–6.
De P, Sathyanarayana DN, Sadasivamurthy P. Sridhar S. Synthesis, structural characterization, thermal studies and chain dynamics of poly(methacrylonitrile peroxide) by NMR spectroscopy. Polymer. 2001;42:8587–93.
De P. Comparative study of the chain dynamics of polymers containing peroxy linkages in the backbone. Polym Prepr. 2005;46:852–3.
Pal S, Dhawan A, De P. Ortho- and meta-substituted polystyrene polyperoxides: synthesis, characterization and thermal decomposition studies. Polym Int. 2014;63:746–51.
Mete S, Choudhury N, De P. Degradable alternating polyperoxides from poly(ethylene glycol) substituted styrenic monomers with water solubility and thermoresponsiveness. J Polym Sci Part A Polym Chem. 2018;56:2030–8.
Pal S, De P. Poly(9-vinyl anthracene peroxide): synthesis, characterization, degradation and application as macroinitiator for the polymerization of methyl methacrylate. Polymer. 2013;54:2652–7.
Mete S, Mukherjee P, Maiti B, Pal S, Ghorai PK, De P. Degradable crystalline polyperoxides from fatty acid containing styrenic monomers. Macromolecules. 2018;51:8912–21.
De P, Sathyanarayana DN. Synthesis of poly(1,3-diisopropenylbenzene peroxide). Indian J Chem. 2001;40A:1009–11.
Razuvaev GA, Boguslavskaya LS, Barabashina RA. Synthesis and properties of polymeric peroxides of acrylic acid phenyl esters. Zh Organicheskoi Khimii. 1972;8:1601–8.
De P, Sathyanarayana DN, Sadasivamurthy P. Sridhar S. Synthesis, spectral characterization and thermochemical studies on poly(phenyl methacrylate peroxide). J Appl Polym Sci. 2003;88:2364–8.
Pal S, De P. Water soluble polyperoxides from 2-(2-methoxyethoxy)ethyl methacrylate: influence of molecular oxygen on thermoresponsive properties and thermal degradation. Chem Commun. 2012;48:4229–31.
Pal S, Das A, Maiti S, De P. Synthesis and characterization of a biodegradable polymer prepared via radical copolymerization of 2-(acetoacetoxy)ethyl methacrylate and molecular oxygen. Polym Chem. 2012;3:182–9.
Pal S, Banoth B, Rahithya G, Dhawan A, De P. Copolyperoxides of 2-(acetoacetoxy)ethyl methacrylate with methyl methacrylate and styrene; synthesis, characterization, thermal analysis, and reactivity ratios. Polymer. 2012;53:2583–90.
Mete S, Mukherjee P, Goswami KG, Ghorai PK, De P. Polyperoxides from cyclic monomers: synthesis, characterization and high pressure kinetics study. ACS Appl Polym Mater. 2020;2:4109–17.
Khan EH, Pal S, De P. N-Hydroxyphthalimide-mediated oxidation of styrene by molecular oxygen. Macromol Chem Phys. 2013;214:2181–8.
Mayo FR, Miller AA, Russell GA. The oxidation of unsaturated compounds. IX. The effects of structure on the rates and products of oxidation of unsaturated compounds. J Am Chem Soc. 1958;80:2500–7.
Jayanthi S, Kishore K. Oxidative copolymerization: microstructure analysis of the terpolymer of styrene, methyl methacrylate, and oxygen. Macromolecules. 1993;26:1985–9.
De P, Sathyanarayana DN. Polymerization of vinyl acetate with styrene and α-methylstyrene under high oxygen pressure. Indian J Chem. 2001;40A:1282–7.
De P, Sathyanarayana DN. Reactivity ratios for the terpolymerization of methyl methacrylate, vinyl acetate and molecular oxygen. J Polym Sci Part A Polym Chem. 2002;40:564–72.
De P, Sathyanarayana DN. High-pressure kinetics of oxidative copolymerization of styrene with α-methylstyrene. Macromol Chem Phys. 2002;203:2218–24.
Pal S, Vaish A, De P. The effect of different catalysts on the monomer reactivity ratios in oxidative copolymerization of styrene and α-methylstyrene. Polym Int. 2015;64:541–6.
De P, Sathyanarayana DN. Synthesis and characterization of copolyperoxides of indene with styrene, α-methylstyrene and α-phenylstyrene. J Polym Sci Part B Polym Phys. 2002;40:2004–17.
De P, Sathyanarayana DN, Sadasivamurthy P, Sridhar S. Reactivity ratios for the oxidative copolymerizations of indene with methyl methacrylate and methacrylonitrile. Eur Polym J. 2002;38:847–55.
De P, Sathyanarayana DN. Determination of the reactivity ratios for the oxidative copolymerizations of indene with methyl, ethyl and butyl acrylates. Macromol Chem Phys. 2002;203:573–9.
De P, Sathyanarayana DN. Oxidative copolymerization of indene with p-tert-butylstyrene: synthesis, characterization, thermal analysis and reactivity ratios. J Polym Sci Part A Polym Chem. 2002;40:9–18.
De P, Sathyanarayana DN. Free-radical oxidative copolymerization of indene with vinyl acetate and isopropenyl acetate: synthesis and characterization. J Appl Polym Sci. 2002;86:639–46.
Mete S, Goswami KG, De P. Composition dependent crystallization behaviour of copolyperoxides from methyl methacrylate and 4-vinylbenzyl stearate. J Polym Sci. 2020;58:766–78.
Subramanian K. Formation, degradation, and applications of polyperoxides. J Macromol Sci Part C Polym Rev. 2003;43:323–83.
Nanda AK, Ganesh K, Kishore K, Surianarayan M. End-group analysis of vinyl polyperoxides by MALDI-TOF-MS, FT-IR technique and thermochemical calculations. Polymer. 2000;41:9063–72.
Cais RE, Bovey FA. Carbon-13 nuclear magnetic resonance study of the microstructure and molecular dynamics of poly(styrene peroxide). Macromolecules. 1977;10:169–78.
Sato E, Matsumoto A. Facile synthesis of functional polyperoxides by radical alternating copolymerization of 1,3-dienes with oxygen. Chem Rec. 2009;9:247–57.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
De P, Chattopadhyay S, Giridhar M, Sathyanarayana DN. Kinetics of thermal degradation of vinyl polyperoxides in solution. Polym Degrad Stab. 2002;76:161–70.
Sivalingam G, De P, Karthik R, Giridhar M. Thermal degradation kinetics of vinyl polyperoxide copolymers. Polym Degrad Stab. 2004;84:173–9.
De P, Chattopadhyay S, Giridhar M, Sathyanarayana DN. Thermal degradation studies of para-substituted poly(styrene peroxide)s. Polym. Degrad Stab. 2002;76:511–4.
De P, Chattopadhyay S, Giridhar M, Sathyanarayana DN. Thermal degradation kinetics of para-substituted poly(styrene peroxide)s in solution. J Appl Polym Sci. 2002;86:957–61.
Jose NR-L, David JL, Josefa H-R, Alexander NPH, Francisco G-C, Roger NFT. Mechanism of reaction of hydrogen peroxide with horseradish peroxidase: identification of intermediates in the catalytic cycle. J Am Chem Soc. 2001;123:11838–47.
Pal S, Das A, Maiti S, De P. Biodegradation and in vitro biocompatibility of polyperoxides: alternating copolymers of vinyl monomers and molecular oxygen. J Biomat Sci Polym Ed. 2012;23:2105–17.
Silant’ev MA, Perepelitsina EO, Grachev VP, Kurochkin SA. Irregular polystyrene peroxides – a promising macroinitiators synthesized by radical polymerization under oxygen inflow. Eur Polym J. 2017;89:67–77.
Sato E, Hagihara T, Matsumoto A. Facile synthesis of main-chain degradable block copolymers for performance enhanced dismantlable adhesion. ACS Appl Mater Interfaces. 2012;4:2057–64.
Mukundan T, Annakutty KS, Kishore K. A novel solid fuel system based on an auto-pyrolysable polymer. Fuel. 1993;72:688–9.
Sato E, Omori C, Yuri M, Koda Y, Horibe H. Thermal latent reductants for controlled degradation of polyperoxides and their application to high performance dismantlable adhesives. ACS Appl Polym Mater. 2019;1:2140–8.
Acknowledgements
The authors are grateful to Ms. Pampa Chawdhury for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Samanta, P., Mete, S., Pal, S. et al. Synthesis, characterization, degradation and applications of vinyl polyperoxides. Polym J 56, 283–296 (2024). https://doi.org/10.1038/s41428-023-00860-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00860-y