Abstract
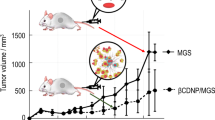
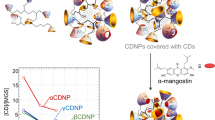
We previously developed cyclodextrin-based nanoparticles by utilizing a conventional polymerization method with α-, β-, γ cyclodextrin and epichlorohydrin, which successfully encapsulated a hydrophobic xanthone compound named α-mangostin (MGS). The drug release profile of MGS showed two release modes, quick release in the early stage and slow release in the late stage, which can be characterized by the lifetimes of complexes τ1 and τ2, respectively. Although these nanoparticles satisfied the demand of the Enhancement Permeation and Retention effect, only β–cyclodextrin-based nanoparticles containing MGS exhibited anticancer efficacy in vivo. Thus, the aims of the present study are to create libraries of β–cyclodextrin-based nanoparticles (CDNPs), characterize the CDNPs and clarify the relationships between the physical parameters, release behavior, and anticancer efficacy. When surfactants were present during the polymerization process, the radius of the obtained CDNPs could exceed 10 nm. τ2 was linearly dependent on the nanoparticle density and showed a relationship with anticancer efficacy. We assumed that MGS released from CDNPs would accumulate in the tumor region if the optimal range of τ2 was approximately 90–140 h. These results suggest that τ2 can be a critical quality attribute for designing our CDNPs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jung H-A, Su B-N, Keller JW, Mehta GR, Kinghorn DA. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J Agric Food Chem. 2006;54:2077–82.
Nakatani K, Nakahata N, Arakawa T, Yasuda H, Ohizumi Y. Inhibiton of cyclooxygenase and prostaglandin E2 synthesis by γ-MGS, a xanthone dervative in mangosteen, in C6 rat glioma cells. Biochem Pharm. 2002;63:73–79.
Janhom P, Dharmasaroja P. Neuroprotective Effects Of Alpha-mangostin on MPP(+)-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. J Toxicol. 2015;2015:919058–69.
Gutierrez-Orozco F, Chitchumroonchokchai C, Lesinski BG, Suksamrarn S, Failla LM. alpha-Mangostin: anti-inflammatory activity and metabolism by human cells. J Agric Food Chem. 2013;61:3891–900.
Mohan S, Suvitha S, Abdelwahab IS, Thangavel N. An anti-inflammatory molecular mechanism of action of alpha-mangostin, the major xanthone from the pericarp of Garcinia mangostana: an in silico, in vitro and in vivo approach. Food Funct. 2018;9:3860–71.
Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, Pérez-Rojas MJ. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem Toxicol. 2008;46:3227–39.
Akao Y, Nakagawa Y, Nozawa Y. Anti-cancer effects of xanthones from pericarps of Mangosteen. Int J Mol Sci. 2008;9:355–70.
Fourmentin S, Crini G, Lichtfouse E. Cyclodextrin applications in medicine, food, environment and liquid crystals. 1st ed. Cham: Springer; 2018.
Wathoni N, Rusdin A, Motoyama K, Joni MI, Lesmana R, Muchtaridi M. Nanoparticle drug delivery systems for alpha-mangostin. Nanotechnol Sci Appl. 2020;13:23–36.
Verma RK, Yu W, Shrivastava A, Shankar S, Srivastava KR. alpha-Mangostin-encapsulated PLGA nanoparticles inhibit pancreatic carcinogenesis by targeting cancer stem cells in human, and transgenic (Kras(G12D), and Kras(G12D)/tp53R270H) mice. Sci Rep. 2016;6:32743–56.
Yostawonkul J, Surassmo S, Namdee K, Khongkow M, Boonthum C, Pagseesing S, et al. Nanocarrier-mediated delivery of alpha-mangostin for non-surgical castration of male animals. Sci Rep. 2017;7:16234–44.
Hotarat W, Phunpee S, Rungnim C, Wolschann P, Kungwan N, Ruktanonchai U, et al. Encapsulation of alpha-mangostin and hydrophilic beta-cyclodextrins revealed by all-atom molecular dynamics simulations. J Mol Liq. 2019;288:110965–73.
Phunpee S, Suktham K, Surassmo S, Jarussophon S, Rungnim C, Soottitantawat A, et al. Controllable encapsulation of alpha-mangostin with quaternized beta-cyclodextrin grafted chitosan using high shear mixing. Int J Pharm. 2018;538:21–29.
Rungnim C, Phunpee S, Kunaseth M, Namuangruk S, Rungsardthong K, Rungrotmongkol T, et al. Co-solvation effect on the binding mode of the alpha-mangostin/beta-cyclodextrin inclusion complex. Beilstein J Org Chem. 2015;11:2306–17.
Sliwa W, Girek T. Cyclodextrins properties and applications. Weinheim, Germany: Wiley Verlag GmbH & Co. KGaA; 2017.
Crini G, Fourmentin S, Lichtfouse E. The history of cyclodextrins. Cham: Springer; 2020.
Doan VTH, Lee JH, Takahashi R, Nguyen TMP, Nguyen TVA, Pham TTH, et al. Cyclodextrin-based nanoparticles encapsulating α-mangostin and their drug release behavior: potential carriers of α-mangostin for cancer therapy. Polym J. 2020;52:457–66.
Doan VTH, Doan TNA, Fujii S, Sakurai K. Enhanced binding constant of cyclodextrin to alpha-mangostin in hyperbranched polymers. Chem Lett. 2020;49:1144–46.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Rel. 2000;65:271–84.
Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–9.
Maeda H, Tsukigawa K, Fang J. A retrospective 30 years after discovery of the enhanced permeability and retention effect of solid tumors: next-generation chemotherapeutics and photodynamic therapy-problems, solutions, and prospects. Microcirculation. 2016;23:173–82.
Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade KR. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl. 2019;98:1252–76.
Nakamura Y, Mochida A, Choyke LP, Kobayashi H. Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug Chem. 2016;27:2225–38.
Dewey HB, Mark TZ, Eric JM. Physicochemical properties, formulation, and drug delivery. In: Drug delivery: principles and applications. 2nd ed. Hoboken, New Jersey, United States: John Wiley & Sons, Inc.; 2016.
Tietgen H, Walden M. Physicochemical properties. In: Drug discovery and evaluation: safety and pharmacokinetic assays. Berlin, Heidelberg: Springer; 2013. p. 1125–38.
Young RJ. Physical properties in drug design. Tactics in contemporary drug design. Berlin, Heidelberg: Springer; 2014. p. 1–68.
Valkó K. Measurements of physical properties for drug design in industry. In: Handbook of Analytical Separation. Amsterdam, Netherlands: Elsevier; 2000.
U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for Industry Q8(R2) Pharmaceutical-Development. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q8r2-pharmaceutical-development 2009.
Osmani RA, Kulkarni P, Manjunatha S, Gowda V, Hani U, Vaghela R, et al. Cyclodextrin nanosponges in drug delivery and nanotherapeutics. Environmental nanotechnology. Springer; 2018. p. 279–342.
Rossi B, Caponi S, Castiglione F, Corezzi S, Fontana A, Giarola M, et al. Networking properties of cyclodextrin-based cross-linked polymers probed by inelastic light-scattering experiments. J Phys Chem B. 2012;116:5323–27.
Venuti V, Rossi B, Mele A, Melone L, Punta C, Majolino D, et al. Tuning structural parameters for the optimization of drug delivery performance of cyclodextrin-based nanosponges. Expert Opin Drug Deliv. 2017;14:331–40.
Castiglione F, Crupi V, Majolino D, Mele A, Rossi B, Trotta F, et al. Effect of cross-linking properties on the vibrational dynamics of cyclodextrins-based polymers: an experimental-numerical study. J Phys Chem B. 2012;116:7952–58.
Sherje AP, Dravyakar RB, Kadam D, Jadhav M. Cyclodextrin-based nanosponges: a critical review. Carbohydr Polym. 2017;173:37–49.
Doan VTH, Takano S, Doan TNA, Nguyen TMP, Nguyen TVA, Pham TTH, et al. Anticancer efficacy of cyclodextrin-based hyperbranched polymer nanoparticles containing alpha-mangostin. Polym J. 2021;53:481–92.
Wiedenhof N, Lammers JNJJ, Eck CLP. Properties of cyclodextrins. Part III*. Cyclodextrin-epichlorhydrin resins: preparation and analysis. Starch. 1969;21:119–23.
Nakama Y. Surfactants. In: Cosmetic science and technology: theoretical principles and applications. Amsterdam, Netherlands: Elsevier; 2017. p. 231–44.
Acknowledgements
This study is financially supported by the JST CREST program, JSPS KAKENHI: Grant-in-Aid for Scientific Research A (20H00668), Grant-in-Aid for Challenging Exploratory Research (20K20449), and Grant-in-Aid for Scientific Research (19K15394).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Doan, V.T.H., Katsuki, J., Takano, S. et al. Determining the critical quality attribute for the delivery of α–mangostin by β–cyclodextrin-based nanoparticles in cancer treatment. Polym J 55, 1367–1378 (2023). https://doi.org/10.1038/s41428-023-00813-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00813-5