Abstract
The use of cinnamic acid provides an approach to the research and development of biobased plastics for the reduction of global warming caused by the increasing amounts of carbon dioxide in the atmosphere. Cinnamic acids in the metabolic systems of plants and microorganisms have been extensively studied. These cinnamates are reactive to ultraviolet (UV) light, and polymers based on these acids exhibit unique properties. In this focus review, we describe our recent work on the development of materials based on cinnamates. Polyesters are obtained by the polycondensation of hydroxycinnamic acid abundant in plants. The amorphous polycinnamate films exhibit characteristic photodeformability upon UV irradiation owing to E-Z isomerization and [2 + 2] cycloaddition reactions. The [2 + 2] cycloaddition reaction of cinnamate can also be used to obtain truxillic and truxinic acids with excellent symmetry. Using these truxillic/truxinic acids, organic solvents or water-soluble polyimides and high-strength polyamides with high transparency can be derived, and biobased plastics comparable to existing high-performance plastics can be obtained.
Similar content being viewed by others
Introduction
The development of high-performance materials using natural and biologically derived raw materials as starting materials is an effective means for building a sustainable society [1,2,3,4]. Polyesters such as poly(lactic acid), poly(ε-caprolactone), and poly(butylene succinate) have attracted attention as plastics that can degrade in the environment [5,6,7]. Even typical polyesters have durability problems limiting their applications. This is because the polymer structure does not contain structures, such as aromatic rings, heteroatoms, reactive functional groups, and π-conjugated structures, that would confer high durability on the material. Accordingly, the material does not achieve sufficient thermal and mechanical properties. Therefore, the development of new biobased polymers with high performance and high functionality by employing biomasses as the raw material is a pressing issue among researchers [8].
Cinnamic acid is a phenolic plant compound [9]. Coumaric acids such as 4-hydroxycinnamic acid (4HCA), caffeic acid (3,4-dihydroxycinnamic acid, dHCA), and ferulic acid (3-methoxy-4-hydroxycinnamic acid, 3M4HCA) contain phenyl groups, vinyl groups, and hydroxy acids in their molecules [10]. These cinnamic acids are present in the biosynthetic pathway of plant lignin and are also known as photoactive yellow proteins, a component protein of certain photosynthetic bacteria [11,12,13]. Ultraviolet (UV) light induces the E-Z isomerization of the vinyl group of cinnamic acid and controls the protein’s on/off function. This makes cinnamic acids useful functional groups in nature. Furthermore, as the enzymatic synthetic pathway for these cinnamic acids that uses amino acids as starting materials has already been established in the field of biochemistry, they can be mass produced using simple bioproduction methods [10].
4HCA is said to be abundant in nature; it can be made into polyesters by polycondensation because it has hydroxy and carboxyl groups [14,15,16,17,18,19,20]. Polyesters synthesized from 4HCA (P4HCA) have long been found to exhibit liquid crystalline properties owing to their rigid molecular chains. However, due to their rigid molecular structures, it is difficult to achieve a high molecular weight. Thus, because of their brittle nature, no P4HCAs have been used as a general biobased plastic. In addition, these P4HCAs have high crystallinity, and although they are biodegradable, they have very low degradability. By copolymerizing dHCA (which has a branched structure) with P4HCA, it is possible to soften its rigidity and obtain a copolymer P4HCA-co-PdHCA with a high molecular weight and improved flexibility. It has been reported that this copolymerization reduces the crystallinity of polycinnamate and improves its biodegradability [21]. Furthermore, as the cinnamate unit induces a [2 + 2] cycloaddition reaction to UV light, cyclobutanes can be formed from the internal olefins of the main chain, greatly reducing its crystallinity. This property can further accelerate soil degradability. The light-induced cyclobutane formation reaction of cinnamic acid is one of its notable characteristics [22]. As such, researchers have widely investigated its use as a light-responsive material for forming cyclobutane by introducing a cinnamic acid unit into the side chain [23, 24].
Cinnamic acid has one major chemical property; it exhibits an E-Z isomerization of the vinylene moiety and [2 + 2] cycloaddition (photodimerization) between two cinnamic acid molecules under UV light. The photodimerization of these cinnamic acid crystals has long been studied. Photodimerization is a topochemical reaction occurring in the solid phase from cinnamic acid crystals to produce (truxillic/truxinic acid) crystals [23]. Notably, the backbone of the cinnamate derivative crystal is retained before and after the reaction. For example, if the phenylene moiety is the head and the carbonyl moiety is the tail, cinnamic acid has three types of crystal forms: an alpha (α) form with head-tail (H–T) packing and beta (β) or delta (δ) forms with head-head (H–H) packing. Thus, by examining the substituents and crystallization methods of cinnamate derivatives, it is theoretically possible to obtain cinnamate dimers of any structure for use in a wide range of polymer syntheses.
This review presents an investigation of the E-Z isomerization of these cinnamate derivatives and the creation of new biobased materials based on their photodimerization properties. The aim is to inspire researchers to expand the use of cinnamate as a biobased material and design new molecules (Scheme 1).
Syntheses of polycinnamates and mechanistic analysis of photodeformation
Light (such as UV or visible light) is one of the most useful and familiar forms of energy owing to its remoteness and controllability (wavelength, intensity, and direction) [25]. In particular, the development of actuators that can change shapes and sizes in response to external stimuli has attracted the attention of several researchers since the first establishment of this concept [26,27,28,29,30,31,32,33]. If a material absorbs light and changes its shape and volume, the material directly converts light energy into mechanical force, i.e., direct energy conversion. [29, 34,35,36,37,38] This characteristic is expected to be useful for sustainability, such as by downsizing the system and reducing energy losses. In addition, as the light used for external stimulation is expected to have excellent remoteness and easy handling (such as by providing light-based manipulation and quick responses), a wide range of applications can be expected in industrial, medical, and other fields [39]. Photoresponsivity is a major research topic in the field of polymers. In this context, cinnamic acid has attracted attention as a photocrosslinking unit for forming crosslinked structures between polymers in response to UV light [40,41,42,43,44,45,46,47]. If a structure with cinnamate in the main chain is used, it is possible to obtain a film that dynamically deforms by UV light [48,49,50].
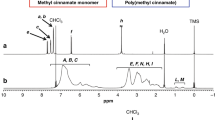
Using 4HCA, 3M4HCA, 3-hydroxycinnamic acid (3HCA), and dHCA as various hydroxycinnamic acids, polyesters have been synthesized by a two-step reaction method of acidolysis polymerization by acetylation and deacetylation of the hydroxyl groups (Scheme 2). These polymer synthesis methods were used when synthesizing the aromatic polyester “Vectra” and are a common approach to obtaining polyesters by polycondensation [51]. It is well known that P4HCA has a rigid macromolecular structure. This makes it difficult for P4HCA to take a high molecular weight form; moreover, it has low solubility owing to the high crystallinity of 4HCA. Similarly, P3M4HCA with a 4-hydroxy group at the aromatic ring also exhibits a low molecular weight and low solubility owing to its high rigidity. However, poly(3-hydroxycinnamic acid) (P3HCA) and hyperbranched poly(3,4-dihydroxycinnamic acid) (PdHCA) are soluble in amide and strong acid solvents such as N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), and trifluoroacetic acid and can therefore be made into films using solution casting methods [50]. UV irradiation of P3HCA and PdHCA films with UV light (280–450 nm) causes a decrease in absorbance as a function of irradiation time owing to the vinylene moiety. By using Fourier transform infrared (FT-IR) spectroscopy, it was confirmed that the UV-irradiated surface peaks at 1640 cm−1 and 980 cm−1 broadened and that the absorption from the vinyl groups was reduced.
Furthermore, P3HCA and PdHCA show different deformations in the convex and concave directions, respectively, despite having the same cinnamate unit in the monomer unit. A structural analysis of P3HCA and PdHCA under UV irradiation can help to elucidate the mechanism(s) of the photoresponsive behaviors of P3HCA and PdHCA. The fluorescence lifetime measurements revealed a difference in the type of excited state for each, suggesting the presence of two different excited states in P3HCA. Further detailed investigations using time-resolved FT-IR measurements revealed that only P3HCA showed a trend toward increased absorption owing to cis isomerization upon UV irradiation (Fig. 1a, b). This indicated for the first time that cis isomerization causes a difference in the photodeformability of P3HCA-based polyesters. From this mechanism, it is concluded that the convex deformation of P3HCA films is caused by “photoexpansion”, which is a decrease in the film surface density resulting from intramolecular cis isomerization (Fig. 1c). The penetration of UV light into the film and the effect of the partial photodeformation on the overall bending of the film are similar to those reported for azobenzene and other liquid crystalline elastomers and are probable mechanisms for light-responsive film deformation [52,53,54].
a 3D image of the step-scanning FT-IR spectrum of poly(3-hydroxycinnamic acid) (P3HCA) during ultraviolet (UV) irradiation, b Time-resolved differential of the absorption for –CH=CH– of P3HCA based on the step-scanning FT-IR spectra, c Structural changes in P3HCA. Reproduced with permission from ref. [50]. Copyright 2021 American Chemical Society
Organic-solvent-soluble polyimides using cinnamate dimer
As mentioned above, cinnamic acid is reactive to UV light, and cinnamate-based polymers are generally expected to function as photoresponsive and biobased plastics. However, it is difficult to avoid [2 + 2] cycloaddition reactions, in addition to E-Z isomerization in cases of polyesters such as P3HCA or PdHCA. Dimerization occurs preferentially for cinnamic acid monomers in the crystalline state [23]. By taking advantage of this property, materials can be developed based on photodimerized cinnamates. Cinnamates occur naturally in a wide variety of forms, and enzymatic reaction systems have been established in the field of bioproduction. In addition to these enzymatic reaction systems, the aromatic diamine 4-aminophenylalanine (4APhe) obtained in microbial production can be subsequently converted to 4-aminocinnamic acid (4ACA) by phenylalanine ammonia-lyase. This can be photodimerized to produce 4,4′-diamino-α-truxillic acid derivatives such as aromatic diamine, a monomer for high-performance plastics [55, 56]. Moreover, cinnamates can be dimerized while maintaining their crystalline structures; by selecting the functional group, it is possible to induce cinnamate dimers with various stereo structures, e.g., truxillic/truxinic acids. Typical hydroxycinnamic acids produce an α-type truxillic acid derived from an α-type crystal structure upon UV irradiation [23]. In contrast, cinnamate derivatives with substituted aromatic rings such as 4ACA are very different from hydroxycinnamic acids in terms of photoreactivity (Table 1). For example, the UV irradiation of 4ACA in solution dispersion hardly leads to a dimerization reaction. Nevertheless, it is possible to obtain α-type truxillic acid by converting the amine to a hydrochloride or acetamide, β-type truxinic acid by dimerizing the nitro-substituted cinnamate, and δ-type truxinic acid by introducing nitro groups and hydroxysuccinimide esters [57]. The α-type truxillate diamine can be used to synthesize polyimides and polyamides for use as biobased high-performance polymers.
Truxillate (α-type)-based polyimides have extremely low solubility in organic solvents. In the molecular design of polyimides, attempts are usually made to improve the solvent solubility and formability by adding flexibility to the tetracarboxylic acid dianhydride. For example, by using meso-butane-1,2,3,4-tetracarboxylic dicarboxylic anhydride, 1,2,3,4-cyclopentane tetracarboxylic anhydride, 1,2,4,5-cyclohexane tetracarboxylic anhydride, and mellophanic dianhydride as tetracarboxylic acid dianhydrides, it has been possible to create biobased polyimides soluble in organic solvents (NMP, DMSO, DMF, DMAc) while maintaining high heat resistance [58, 59]. Aromatic diamines can also introduce flexibility to conventional polyimides. However, the most common angles are approximately 180, 120, and 60° for diamines added directly to benzene, approximately 120° for diamines via an ether bond, and approximately 110° for diamines via isopropylidene. The use of biobased aromatic diamines results in a diamine configuration with a twisted structure bonded to cyclobutane, further resulting in aromatic diamines differing from the existing bond angles. The nitrogen angles in the diamine with the four-membered ring of α, β, and δ-type truxillate/truxinate diamines as the center of gravity, as determined via density functional theory calculations, are 156, 70, and 101°, respectively (Fig. 2a) [57]. These unique angles affect the physical properties of the polyimides. Polyimides synthesized with dimethyl 4,4’-diamino-δ-truxinate as the δ-type diamine exhibit good solubility in organic solvents, including DMF, DMAc, and DMSO, as well as in low-boiling solvents, such as dichloromethane and chloroform (Fig. 2b). Proton nuclear magnetic resonance (1H NMR) measurements have shown that peaks originate only from polyimide; this indicates that this solubility is not derived from poly(amic acid), which is a precursor but is rather unique to polyimide. The maximum molecular weight is approximately 60,000 g mol−1, making it possible to form a flexible film. It has excellent recyclability and can be dissolved again in a solvent after forming the film and then can be remolded. The heat resistance is lower than that of the previously studied α-type polyimide, but this is believed to be caused by the differences in the substituents on cyclobutane.
Syntheses of water soluble polyimides and polyimide hydrogels
Water-soluble polymers dissolve in water, making them important in green chemistry. They are expected to contribute to sustainability through environmentally low-impact handling, film production in environments without large facilities such as air intake drafts, compositing with other water-soluble materials (e.g., cellulose nanofibers [60]), and molding using water. It is well known that sodium polyacrylate, a representative example of a water-soluble polymer, is made water soluble by sodium ionization of the side-chain carboxylic acid of poly(acrylic acid) with sodium hydroxide. The water solubilization of biobased polyimides synthesized from 4ACA dimers can be achieved by such poly(acrylic acid)-like approaches owing to the presence of ester groups in the side chains. To obtain water-soluble polyimides, the side-chain carboxylic acid must be alkali metal-substituted (saponified) in an alkaline solution such as potassium hydroxide. In one study, a dicarboxylic acid-type diamine was reacted with tetracarboxylic acid dianhydride in the same manner as other polyimides to obtain a side-chain carboxylic acid-type biobased polyimide by thermal imidization [61]. The resulting polyimide was not soluble in organic solvents, but the presence of COOH groups was confirmed using FT-IR spectroscopy. As the COOH group was present in the polyimide side chain and was not consumed by decarboxylation or other processes during thermal imidization, the polyimide was dissolved via immersion in an aqueous potassium hydroxide solution. Water-soluble polyimides were prepared using 1,2,3,4-cyclobutane tetracarboxylic dianhydride, pyromellitic dianhydride, 4,4′-biphthalic anhydride, and 3,′,4,4′-diphenylsulfone tetracarboxylic dianhydride [62].
As it was confirmed that 4ACA-based polyimides can be water-solubilized, structural analysis was performed with 1H NMR using D2O. From the 1H NMR spectra, broad peaks specific to the polymer derived from the dimer of 4ACA and tetracarboxylic anhydride were confirmed. Furthermore, all water-soluble polyimides were able to be made into films by casting on glass substrates, and transparent films were obtained. These results imply that no significant degradation of the polyimide main chain occurs during treatment when a potassium hydroxide solution is used and that the obtained polyimides maintain a sufficiently high molecular weight. Water-soluble polyimides have been similarly synthesized using LiOH, NaOH, and CsOH to obtain water-soluble polyimides with different side-chain cations. The heat resistance (Td10) of water-soluble polyimides is slightly lower at approximately 350 °C relative to the ca. 400 °C of 4ACA-based polyimides without water solubility. This is believed to be due to the weakening of the interaction between the polyimide side chains by the introduction of alkali metal-derived cations. The introduction of cations into polyimide side chains also affects the mechanical properties. Polyimides with cations introduced into side chains show nearly half the fracture strength of those without cations but up to 12% higher elongation. The elongation ratio increases with an increasing ionic radius of the side-chain cations (Li, Na, K, and Cs), indicating that there is a correlation between the ionic radius and mechanical properties. The effect of side-chain cations on the physical properties in turn affects the optical properties.
Water-soluble biobased polyimides can be used as precursor polymers for a variety of functional materials, such as hydrogels with pH-responsive carboxylates in side chains (Fig. 3a) [62]. In one study, gelation was carried out using the amino acid 4-aminophenylalanine or 4ACA dimer potassium salt (4ATA-K) and the condensing agent 4-(4,6-dimethoxy-1,3,5-triazine-2-yl)-4-methylmorpholinium chloride. The cross-linker moieties of the resulting polyimide hydrogels and COOK groups derived from the polyimide backbone underwent reversible proton transfer when the pH was changed. When the polyimide hydrogel was immersed in an aqueous solution with a low pH (~1.45), the volume of the polyimide hydrogel gradually decreased (Fig. 3b). The volume of the polyimide hydrogel was reduced to approximately 20% of that in the original neutral solution (the volume of the hydrogel was calculated by measurement of the XYZ-axis of each hydrogel under various pH conditions). In contrast, when the pH of the solvent containing the volume-shrunk polyimide hydrogel was gradually increased from 1.45 to 12.50 using a potassium hydroxide solution, the polyimide hydrogel swelled and recovered to approximately 80% of its original hydrogel volume. This property indicates that the polyimide hydrogel has a stimulus-responsive property with a volume change in response to pH. The results of the gel compression test also confirmed a change in the density of the gel. In polyimide hydrogels, the COOK moiety of the polyimide backbone and COOK side chain derived from 4ATA-K/4APhe introduced as a cross-linker are converted to COOH under acidic conditions. The COOH interaction is enhanced, making the molecules inside the gel become hydrophobic and causing volume contraction. Furthermore, as the pH of the external environment increases, the COOH sites are converted to COOK, and the hydrophilicity increases, causing partial swelling of the hydrogel and reswelling.
a Synthesis of water-soluble polyimides and polyimide hydrogel. b Photographs of polyimide hydrogel in different pH environments. Reproduced with permission from ref. [62]. Copyright 2021 Springer Nature
Syntheses of functional and high-performance polyamides
Polyamides can also be synthesized using the dicarboxylic acid and diamine obtained by the photodimerization of 4ACA [56]. As general polyamides do not have a side-chain structure, no attempt has been made to control performance via side-chain conversion. However, when using biobased polyamides, the transparency can be improved by introducing fluoroalkyl groups to the side chain to increase the transparency of the molded film and reduce the yellowish color of the resin. Fluorine atoms have the greatest electronegativity, making them the most likely of all elements to attract electrons in the intramolecular vicinity. In addition, fluorine has a higher C–F bond energy than hydrogen or other halogen atoms, making it more stable in response to external energies. Thus, fluorine atoms with optical and electrical functions are often introduced into the side chains of highly heat-resistant resins such as polyimides because they exhibit various properties such as high heat resistance, weather resistance, a dielectric breakdown voltage, and flame retardance. 4ACA reacts with trifluoroacetic anhydride to form 4-trifluoroacetamido cinnamic acid. This acid can be dispersed in hexane and irradiated with UV light to obtain 4ACA-based dicarboxylic acid with trifluoromethyl groups. By polycondensation of this trifluoromethylated dicarboxylic acid with the diamine derived from 4ACA, a fluorinated biobased polyamide with trifluoromethyl groups on the side chain can be synthesized (Scheme 3) [63]. The thermophysical properties of the fluorinated polyamides are similar to those of nonfluorinated polyamides (Td10, 350 °C; Tg, 274 °C, approx.). The transparency of the film also shows a high transmittance of approximately 90% at 450 nm, and the yellowness index of the film is 3.0, i.e., less yellow than that of a nonfluorinated polyamide film at 4.8.
4ACA-based polyamides are characterized by their high heat resistance performances, with glass transition temperatures (Tg) all above 150 °C and Td10 mostly above 350 °C. In addition to the introduction of fluorine, high transparency can be achieved by selecting the appropriate dicarboxylic acids. The reason for this high transparency can be attributed to the cyclobutane incorporated in the main chain and ester moiety in the side chain. Polyamide has been traditionally considered a representative high-strength material similar to aramid and nylon; correspondingly, biobased polyamide also has the potential to be similar to a high-strength material. To obtain high-strength biobased polyamides, various aliphatic dicarboxylic acids were copolymerized with biobased polyamides in predetermined proportions, and the changes in their thermal and mechanical properties were systematically evaluated (Fig. 4a) [64]. Consequently, biobased copolyamide showed a slight decrease in heat resistance but significant increases in strength and elongation, with a tensile strength of up to 400 MPa and an elongation at break up to 36% in the fiber state. Plastics with such high transparency and strength are expected to have a variety of applications in transparent materials. To confirm the mechanism by which these biobased polyamides and their copolymers exhibit high strength, FT-IR spectroscopy during tensile testing confirmed that when the elongation of the polyamide copolymer exceeds approximately 50%, the cyclobutane derived from truxillate is tautomerized, resulting in a more rigid structure similar to that of cyclobutadiene (Fig. 4b, c).
a Structure of biobased copolyimide using 4-aminocinnamic acid (4ACA)-dimers and sebacic acid. b Differential Fourier transform-IR spectra of copolyamide at 10, 30, and 65% elongation. c Tautomerization model of a copolyamide fiber while an external force is being applied. d Photograph of self-standing nanomembrane. Reproduced with permission from ref. [64]. Copyright 2022 American Chemical Society
Owing to the high transparency and high strength of these biobased polyamides, the molding of thin nanofilms based on them has been considered for application in device substrates. Nanofilms are used as coating materials in fields such as architecture, electronics, and biomedicine to protect materials from external forces. Recently, they have attracted attention in device-related lightweight and flexible device applications, and there are high expectations for the development of such nanofabricated biobased materials. However, it is very difficult to physically remove the nanofilm from the support substrate while maintaining its mechanical properties; the mechanical properties of the resin are critical to achieving this effect. A biobased polyamide has higher thermal stability and better mechanical properties than conventional transparent resins, making it ideal for fabricating nanofilms. In light of the ability to synthesize biobased polyamides with high toughness, nanofilms have been fabricated and isolated using spin casting on glass substrates. The polyamide thin film was prepared by casting using a spin-coater, and the thickness was measured by a refractometer. The results showed that a polyamide nanofilm of 200 nm was formed. Normally, a film of this thinness is impossible to achieve, but the biobased polyamide copolymer can be isolated as a nanofilm. In addition, despite being a nanolevel thin film, it is a freestanding thin film. This clearly contributes to the high Young’s modulus and toughness of this biobased polyamide. These results indicate that nanofilms fabricated from biobased polyamides are an innovative technology opening up new approaches for thin, flexible materials for electronic displays, organic memories, and other devices, as well as for contributing to miniaturized machines (such as nanoscale machines) in in vivo operated mini-robots.
Conclusions
Various biobased polymers can be obtained using cinnamic acid, which is abundant in nature, and 4ACA, a special amino acid that utilizes genetically engineered microorganisms for metabolism. These polymers have specific properties that are not found in conventional petroleum-based polymers or biobased polymers. We believe that they are unique because they are biobased. Hydroxy cinnamate-based polyesters are degradable, and 4ACA-based polyimides and polyamides can be chemically recycled by the reversible photocleavage of main chain cyclobutane under shortwavelength UV light. Because of these properties, cinnamate-based polymers also have degradability characteristics that are not found in conventional high heat-resistant and high-strength plastics. Generally, there are concerns regarding low yields and low purity during bioproduction; however, the production system of cinnamic acid has been widely studied in the field of bioproduction. If the production system of cinnamic acid can be optimized in the future, the associated costs can be reduced to a level comparable to those of petroleum-based monomers. In recent years, research on and examples of practical applications of biobased plastics have been rapidly increasing, with similar levels of interest in industry, academia, and government. As described above, the use of natural products and bioproducts is becoming the norm for achieving sustainability. Thus, there are high expectations for the future development of functions unique to biobased plastics.
References
Miller SA. Sustainable polymers: opportunities for the next decade. ACS Macro Lett. 2013;2:550–4. https://doi.org/10.1021/mz400207g
Isikgor FH, Becer CR. Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem. 2015;6:4497–559. https://doi.org/10.1039/c5py00263j
Froidevaux V, Negrell C, Caillol S, Pascault JP, Boutevin B. Biobased amines: from synthesis to polymers; present and future. Chem Rev. 2016;116:14181–224. https://doi.org/10.1021/acs.chemrev.6b00486
Kawaguchi H, Takada K, Elkasaby T, Pangestu R, Toyoshima M, Kahar P, et al. Recent advances in lignocellulosic biomass white biotechnology for bioplastics. Bioresour Technol. 2022;344:126165 https://doi.org/10.1016/j.biortech.2021.126165
Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–46. https://doi.org/10.1016/S0142-9612(00)00101-0
Wang G-X, Huang D, Ji J-H, Völker C, Wurm FR. Seawater-degradable polymers—fighting the marine plastic pollution. Adv Sci. 2021;8:2001121 https://doi.org/10.1002/advs.202001121
Rosenboom J-G, Langer R, Traverso G. Bioplastics for a circular economy. Nat Rev Mater. 2022;7:117–37. https://doi.org/10.1038/s41578-021-00407-8
Rose M, Palkovits R. Isosorbide as a renewable platform chemical for versatile applications—Quo Vadis? ChemSusChem. 2012;5:167–76. https://doi.org/10.1002/cssc.201100580
Fonseca AC, Lima MS, Sousa AF, Silvestre AJ, Coelho JFJ, Serra AC. Cinnamic acid derivatives as promising building blocks for advanced polymers: synthesis, properties and applications. Polym Chem. 2019;10:1696–723. https://doi.org/10.1039/C9PY00121B
Shuab R, Lone R, Koul KK. Cinnamate and cinnamate derivatives in plants. Acta Physiol Plant. 2016;38:64 https://doi.org/10.1007/s11738-016-2076-z
Kort R, Vonk H, Xu X, Hoff WD, Crielaard W, Hellingwerf KJ. Evidence for trans-cis isomerization of the p-coumaric acid chromophore as the photochemical basis of the photocycle of photoactive yellow protein. FEBS Lett. 1996;382:73–78. https://doi.org/10.1016/0014-5793(96)00149-4
Tenboer J, Basu S, Zatsepin N, Pande K, Milathianaki D, Frank M, et al. Time-resolved serial crystallography captures high-resolution intermediates of photoactive yellow protein. Science. 2014;346:1242–6. https://doi.org/10.1126/science.1259357
Anderson S, Srajer V, Pahl R, Rajagopal S, Schotte F, Anfinrud P, et al. Chromophore conformation and the evolution of tertiary structural changes in photoactive yellow protein. Structure. 2004;12:1039–45. https://doi.org/10.1016/j.str.2004.04.008
Jin X, Carfagna C, Nicolais L, Lanzetta R. Synthesis, characterization, and in vitro degradation of a novel thermotropic ternary copolyester based on p-hydroxybenzoic acid, glycolic acid, and p-hydroxycinnamic acid. Macromolecules. 1995;28:4785–94. https://doi.org/10.1021/ma00118a001
Stumpe J, Ziegler A, Berghahn M, Kricheldorf HR. Photoreactive cholesteric polyesters derived from 4-carboxycinnamic acid and novel chiral spacers. Macromolecules. 1995;28:5306–11. https://doi.org/10.1021/ma00119a021
Sapich B, Stumpe J, Krawinkel T, Kricheldorf HR. New Polymer Syntheses. 95. Photosetting cholesteric polyesters derived from 4-hydroxycinnamic acid and isosorbide. Macromolecules. 1998;31:1016–23. https://doi.org/10.1021/ma971027e
Reina A, Gerken A, Zemann U, Kricheldorf HR. New polymer syntheses, 101. Liquid-crystalline hyperbranched and potentially biodegradable polyesters based on phloretic acid and gallic acid. Macromol Chem Phys. 1999;200:1784–91. https://doi.org/10.1002/(SICI)1521-3935(19990701)200:73.0.CO;2-B.
Kimura K, Inoue H, Kohama S-I, Yamashita Y.,Sakaguchi Y. Self-organizing polycondensation of (E)-4-acetoxycinnamic acid for preparation of poly(p-oxycinnamoyl) microspheres. Macromolecules. 2003;36:7721–9. https://doi.org/10.1021/ma030197q
Kaneko T, Matsusaki M, Hang TT, Akashi M. Thermotropic liquid-crystalline polymer derived from natural cinnamoyl biomonomers. Macromol Rapid Commun. 2004;25:673–7. https://doi.org/10.1002/marc.200300143
Thi TH, Matsusaki M, Shi D, Kaneko T, Akashi M. Synthesis and properties of coumaric acid derivative homo-polymers. J Biomater Sci Polym Ed. 2008;19:75–85. https://doi.org/10.1163/156856208783227668
Kaneko T, Thi TH, Shi DJ, Akashi M. Environmentally degradable, high-performance thermoplastics from phenolic phytomonomers. Nat Mater. 2006;5:966–70. https://doi.org/10.1038/nmat1778
Schmidt GMJ, 385. Topochemistry. Part III. The crystal chemistry of some trans-cinnamic acids. J Chem Soc (Resumed). 1964:2014–21. https://doi.org/10.1039/JR9640002014.
Pattabiraman M, Kaanumalle LS, Natarajan A, Ramamurthy V. Regioselective photodimerization of cinnamic acids in water: templation with cucurbiturils. Langmuir. 2006;22:7605–9. https://doi.org/10.1021/la061215a
Mallette JR, Casale JF. Rapid determination of the isomeric truxillines in illicit cocaine via capillary gas chromatography/flame ionization detection and their use and implication in the determination of cocaine origin and trafficking routes. J Chromatogr A. 2014;1364:234–40. https://doi.org/10.1016/j.chroma.2014.08.072
Beach ES, Cui Z, Anastas PT, Green chemistry: a design framework for sustainability. Energy Environ Sci. 2009;2. https://doi.org/10.1039/b904997p.
Kaneko T, Yamaoka K, Osada Y, Gong JP. Thermoresponsive shrinkage triggered by mesophase transition in liquid crystalline physical hydrogels. Macromolecules. 2004;37:5385–8. https://doi.org/10.1021/ma049096y
Li MH, Keller P, Li B, Wang X, Brunet M. Light-driven side-on nematic elastomer actuators. Adv Mater. 2003;15:569–72. https://doi.org/10.1002/adma.200304552
Kim J, Kim JW, Kim HC, Zhai L, Ko H-U, Muthoka RM. Review of soft actuator materials. Int J Precis Eng Manuf. 2019;20:2221–41. https://doi.org/10.1007/s12541-019-00255-1
Lv JA, Liu Y, Wei J, Chen E, Qin L, Yu Y. Photocontrol of fluid slugs in liquid crystal polymer microactuators. Nature. 2016;537:179–84. https://doi.org/10.1038/nature19344
Ren H, Qiu X-P, Shi Y, Yang P, Winnik FM. Light, temperature, and pH control of aqueous azopyridine-terminated poly(N-isopropylacrylamide) solutions. Polym Chem. 2019;10:5080–6. https://doi.org/10.1039/C9PY01086F
Cui K, Gong JP. Aggregated structures and their functionalities in hydrogels. Aggregate. 2021;n/a(n/a):e33 https://doi.org/10.1002/agt2.33
Mohd Jani J, Leary M, Subic A, Gibson MA. A review of shape memory alloy research, applications and opportunities. Mater Des (1980-2015). 2014;56:1078–113. https://doi.org/10.1016/j.matdes.2013.11.084
Hager MD, Bode S, Weber C, Schubert US. Shape memory polymers: past, present and future developments. Prog Polym Sci. 2015;49-50:3–33. https://doi.org/10.1016/j.progpolymsci.2015.04.002
Yu H, Ikeda T. Photocontrollable liquid-crystalline actuators. Adv Mater. 2011;23:2149–80. https://doi.org/10.1002/adma.201100131
Iwaso K, Takashima Y, Harada A. Fast response dry-type artificial molecular muscles with [c2]daisy chains. Nat Chem. 2016;8:625–32. https://doi.org/10.1038/nchem.2513
Barrett CJ, Mamiya J-i, Yager KG, Ikeda T. Photo-mechanical effects in azobenzene-containing soft materials. Soft Matter. 2007;3:1249–61. https://doi.org/10.1039/B705619B
Yamada M, Kondo M, Mamiya J, Yu Y, Kinoshita M, Barrett CJ, et al. Photomobile polymer materials: towards light-driven plastic motors. Angew Chem Int Ed Engl. 2008;47:4986–8. https://doi.org/10.1002/anie.200800760
Ube T, Nakayama R, Ikeda T. Photoinduced motions of thermoplastic polyurethanes containing azobenzene moieties in main chains. Macromolecules. 2022;55:413–20. https://doi.org/10.1021/acs.macromol.1c01827
Kondo M. Photomechanical materials driven by photoisomerization or photodimerization. Polym J 2020;52:1027–34. https://doi.org/10.1038/s41428-020-0367-0
Guo A, Liu G, Tao J. Star polymers and nanospheres from cross-linkable diblock copolymers. Macromolecules. 1996;29:2487–93. https://doi.org/10.1021/ma951354r
Ding J, Liu G. Hairy, semi-shaved, and fully shaved hollow nanospheres from polyisoprene-block-poly(2-cinnamoylethyl methacrylate). Chem Mater. 1998;10:537–42. https://doi.org/10.1021/cm970546t
Subramanian K, Krishnasamy V, Nanjundan S, Rami Reddy AV. Photosensitive polymer: synthesis, characterization and properties of a polymer having pendant photocrosslinkable group. Eur Polym J. 2000;36:2343–50. https://doi.org/10.1016/S0014-3057(00)00008-2
Liu F, Liu G. Poly(solketal methacrylate)-block-poly(2-cinnamoyloxyethyl methacrylate)-block-poly(allyl methacrylate): synthesis and micelle formation. Macromolecules. 2001;34:1302–7. https://doi.org/10.1021/ma0016230
Jiang X, Luo S, Armes SP, Shi W, Liu S. UV irradiation-induced shell cross-linked micelles with pH-responsive cores using ABC triblock copolymers. Macromolecules. 2006;39:5987–94. https://doi.org/10.1021/ma061386m
Chen J, Vaino AR, Smith RL, Collins SC. Photomediated crosslinking of cinnamated PDMS for in situ direct photopatterning. J Polym Sci Part A Polym Chem. 2008;46:3482–7. https://doi.org/10.1002/pola.22653
Hu X, Chen X, Cheng H, Jing X. Cinnamate-functionalized poly(ester-carbonate): synthesis and its UV irradiation-induced photo-crosslinking. J Polym Sci Part A Polym Chem. 2009;47:161–9. https://doi.org/10.1002/pola.23134
Lendlein A, Jiang H, Jünger O, Langer R. Light-induced shape-memory polymers. Nature. 2005;434:879–82. https://doi.org/10.1038/nature03496
Yasaki K, Suzuki T, Yazawa K, Kaneko D, Kaneko T. Effects of double photoreactions on polycoumarate photomechanics. J Polym Sci Part A Polym Chem. 2011;49:1112–8. https://doi.org/10.1002/pola.24525
Wang SQ, Kaneko D, Okajima M, Yasaki K, Tateyama S, Kaneko T. Hyperbranched polycoumarates with photofunctional multiple shape memory. Angew Chem Int Ed Engl. 2013;52:11143–8. https://doi.org/10.1002/anie.201305647
Takada K, Yasaki K, Rawat S, Okeyoshi K, Kumar A, Murata H, et al. Photoexpansion of biobased polyesters: mechanism analysis by time-resolved measurements of an amorphous polycinnamate hard film. ACS Appl Mater Interfaces. 2021;13:14569–76. https://doi.org/10.1021/acsami.0c22922
Volokhina AV. High-strength high-modulus fibres from liquid-crystalline aromatic polyesters. Fibre Chem. 1990;22:187–97. https://doi.org/10.1007/BF00548343
Ikeda T, Nakano M, Yu Y, Tsutsumi O, Kanazawa A. Anisotropic bending and unbending behavior of azobenzene liquid-crystalline gels by light exposure. Adv Mater. 2003;15:201–5. https://doi.org/10.1002/adma.200390045
Camacho-Lopez M, Finkelmann H, Palffy-Muhoray P, Shelley M. Fast liquid-crystal elastomer swims into the dark. Nat Mater. 2004;3:307–10. https://doi.org/10.1038/nmat1118
Mamiya J-i. Photomechanical energy conversion based on cross-linked liquid-crystalline polymers. Polym J. 2013;45:239–46. https://doi.org/10.1038/pj.2012.140
Suvannasara P, Tateyama S, Miyasato A, Matsumura K, Shimoda T, Ito T, et al. Biobased polyimides from 4-aminocinnamic acid photodimer. Macromolecules. 2014;47:1586–93. https://doi.org/10.1021/ma402499m
Tateyama S, Masuo S, Suvannasara P, Oka Y, Miyazato A, Yasaki K, et al. Ultrastrong, transparent polytruxillamides derived from microbial photodimers. Macromolecules. 2016;49:3336–42. https://doi.org/10.1021/acs.macromol.6b00220
Noda T, Iwasaki T, Takada K, Kaneko T. Soluble biobased polyimides from diaminotruxinic acid with unique bending angles. Macromolecules. 2021;54:10271–8. https://doi.org/10.1021/acs.macromol.1c01273
Dwivedi S, Kaneko T, Molecular design of soluble biopolyimide with high rigidity. Polymers. 2018;10. https://doi.org/10.3390/polym10040368.
Takada K, Karikome S, Dwivedi S, Kaneko T. Fully bio-based aromatic polyimide using 4-aminocinnamic acid and mellophanic dianhydride as bio-derived monomers. ECS Trans. 2018;88:99–105. https://doi.org/10.1149/08801.0099ecst
Fujisawa S. Material design of nanocellulose/polymer composites via Pickering emulsion templating. Polym J. 2021;53:103–9. https://doi.org/10.1038/s41428-020-00408-4
Dwivedi S, Nag A, Sakamoto S, Funahashi Y, Harimoto T, Takada K, et al. High-temperature resistant water-soluble polymers derived from exotic amino acids. RSC Adv. 2020;10:38069–74. https://doi.org/10.1039/d0ra06620f
Takada K, Noda T, Kobayashi T, Harimoto T, Singh M, Kaneko T. Synthesis of pH-responsive polyimide hydrogel from bioderived amino acid. Polym J. 2021;53:1223–30. https://doi.org/10.1038/s41428-021-00509-8
Takada K, Mae Y, Kaneko T. Fluorinated and bio-based polyamides with high transparencies and low yellowness index. Polymers. 2018;10. https://doi.org/10.3390/polym10121311.
Funahashi Y, Yoshinaka Y, Takada K, Kaneko T. Self-standing nanomembranes of super-tough plastics. Langmuir. 2022;38:5128–34. https://doi.org/10.1021/acs.langmuir.1c02193
Acknowledgements
The author is deeply indebted to Professor Tatsuo Kaneko (Jiangnan University) for his continuous encouragement and constructive discussions. This work has been financially supported by the Japan Society for the Promotion of Science (JSPS) of Grant-in-Aid for Early-Career Scientists (21K14681) and partially supported by Scientific Research (C) (23K04836), Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takada, K. Synthesis of biobased functional materials using photoactive cinnamate derivatives. Polym J 55, 1023–1033 (2023). https://doi.org/10.1038/s41428-023-00804-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00804-6