Abstract
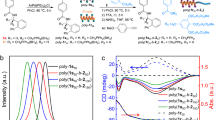
A novel substituted phenylacetylene with a pendant betulin derivative group was designed and synthesized successfully, and the chemical structure of the monomer was confirmed by nuclear magnetic resonance spectroscopy (NMR, 1H, 13C), high-resolution mass spectrometry (HRMS) and Fourier transform infrared spectroscopy (FTIR). The obtained monomer was further polymerized in an achiral [Rh(nbd)Cl]2/triethylamine (TEA) catalytic system, and the resulting polymer exhibited a strong Cotton effect at the main-chain absorption regions in the circular dichroism (CD) spectra. This one-handed helical backbone was induced by the chiral source on the side chain and maintained by the rigid betulin derivative pendant flats. Furthermore, the rigid betulin derivatives also contributed to the porous morphology and perfect thermal stability of the polymer. This study will provide a new method for preparing porous chiral polymers as efficient separation materials to solve practical issues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gowers DM, Halford SE. Protein motion from non-specific to specific DNA by three-dimensional routes aided by supercoiling. EMBO J. 2003;22:1410–8.
van den Broek B, Lomholt MA, Kalisch SM, Metzler R, Wuite GJ. How DNA coiling enhances target localization by proteins. Proc Natl Acad Sci USA 2008;105:15738–42.
Zhang D, Yang Z, Wen X, Xiang Z, He L, Ran S, et al. Helical conformations of semiflexible polymers confined between two concentric cylinders. J Phys Chem B. 2011;115:14333–40.
Aoki T, Kaneko T, Maruyama N, Sumi A, Takahashi M, Sato T, et al. Helix-sense-selective polymerization of phenylacetylene having two hydroxy groups using a chiral catalytic system. J Am Chem Soc. 2003;125:6346–7.
Liu L, Zang Y, Jia H, Aoki T, Kaneko T, Hadano S, et al. Helix-sense-selective polymerization of achiral phenylacetylenes and unique properties of the resulting cis-cisoidal polymers. Polym Rev. 2017;57:89–118.
Shi J, Kimura Y, Takeda S, Xu C, Shoji K, Teraguchi M, et al. Improvement of oxygen permselectivity of a rigid helical polyphenylacetylene: effect of flexible groups, degree of polymerization, composites, thickness, orientation, and network formation. Polymer. 2021;228:123900.
Yashima E, Maeda K, Iida H, Furusho Y, Nagai K. Helical polymers: synthesis, structures, and functions. Chem Rev. 2009;109:6102–211.
Zou H, Wu QL, Zhou L, Hou XH, Liu N, Wu ZQ. Chiral recognition and resolution based on helical polymers. Chinese J Polym Sci. 2021;39:1521–7.
Zang Y, Qu Y, Aoki T, Teraguchi M, Kaneko T, Jia H, et al. Simultaneous improvement of permeability and selectivity in enantioselective permeation through solid chiral membranes from a newly synthesized one-handed helical polyphenylacetylene with aldehyde pendant groups by enantioselective reaction. Polymer. 2019;171:45–49.
Zhao B, Pan K, Deng J, Deng J. Intense circularly polarized luminescence contributed by helical chirality of monosubstituted polyacetylenes. Macromolecules. 2018;51:7104–11.
Yin G, Liu L, Mottate K, Teraguchi M, Kaneko T, Aoki T. On-off reversible switching of the chirality of one-handed helical Poly(phenylacetylene)s by polarity stimuli. Polymer. 2021;237:124347.
Shi Z, Teraguchi M, Aoki T, Kaneko T. Helical conformation stability of Poly 3,5-bis(hydroxymethyl)phenylacetylene s depending on the length of their rigid and linear pi-conjugated side groups. Chem Lett. 2015;44:1413–5.
Shi Z, Kwak G, Jin YJ, Teraguchi M, Aoki T, Kaneko T. Solvent-tuned dual emission of a helical poly[3,5-bis(hydroxymethyl)phenylacetylene] connected with a π-conjugated chromophore. Polym J. 2018;50:533–7.
Shi Z, Wang J, Teraguchi M, Aoki T, Kaneko T. Helix-sense-selective polymerization of 3,5-bis(hydroxymethyl)phenylacetylene rigidly bearing galvinoxyl residues and their chiroptical properties. Polymers. 2019;11:1877.
Wang J, Shi Z, Zang Y, Jia H, Teraguchi M, Kaneko T, et al. Macromolecular design for oxygen/nitrogen permselective membranes-top-performing polymers in 2020. Polymers. 2021;13:3012.
O’Connell MM, Bentley MD, Campbell CS, Cole BJ. Betulin and lupeol in bark from four white-barked birches. Phytochemistry. 1988;27:2175–6.
Ohara S. Utilization of wood extractives I. Extractives from the bark of Betula platyphylla sakatcher ver. Japonica. Mokuzai Gakkaishi. 1986;132:266–73.
Hodon J, Borkova L, Pokorny J, Kazakova A, Urban M. Design and synthesis of pentacyclic triterpene conjugates and their use in medicinal research. Eur J Med Chem. 2019;182:111653–77.
Curia S, Dautle S, Satterfield B, Yorke K, Cranley CE, Dobson BE. Betulin-based thermoplastics and thermosets through sustainable and industrially viable approaches: new insights for the valorization of an underutilized resource. ACS Sustain. Chem Eng. 2019;7:16371–81.
Chen Y, Song Q, Zhao J, Gong X, Schlaad H, Zhang G. Betulin-constituted multiblock amphiphiles for broad-spectrum protein resistance. ACS Appl Mater Interfaces. 2018;10:6593–6600.
Okada M, Suzuki K, Mawatari Y, Tabata M. Biopolyester prepared using unsaturated betulin (Betulinol) extracted from outer birch bark and dicarboxylic acid dichlorides and its thermal-induced crosslinking. Eur Polym J. 2019;113:12–17.
Auclair N, Kaboorani A, Riedl B, Landry V. Acrylated betulin as a comonomer for bio-based coatings. Part I: Characterization, photo-polymerization behavior and thermal stability. Ind Crop Prod. 2015;76:530–7.
Auclair N, Kaboorani A, Riedl B, Landry V. Acrylated betulin as a comonomer for bio-based coatings. Part II: Mechanical and optical properties. Ind Crop Prod. 2016;82:118–26.
Claude B, Viron‐Lamy C, Haupt K, Morin P. Synthesis of a molecularly imprinted polymer for the solid-phase extraction of betulin and betulinic acid from plane bark. Phytochem Anal. 2010;21:180–5.
Ma Z, Jia YG, Zhu XX. Glycopolymers bearing galactose and betulin: synthesis, encapsulation, and lectin recognition. Biomacromolecules. 2017;18:3812–8.
Takata T, Ishiwari F, Sato T, Seto R, Koyama Y. Synthesis, structure, and properties of polyacetylenes possessing chiral spirobifluorene moieties in the side chain. Polym J. 2008;40:846–53.
Aoki T, Kokai M, Shinohara KI, Oikawa E. Chiral helical conformation of the polyphenylacetylene having optically-active bulky substituent. Chem Lett. 1993;22:2009–12.
San Jose BA, Yan J, Akagi K. Dynamic switching of the circularly polarized luminescence of disubstituted polyacetylene by selective transmission through a thermotropic chiral nematic liquid crystal. Angew Chem Int Ed. 2014;53:10641–4.
Kaneko T, Umeda Y, Yamamoto T, Teraguchi M, Aoki T. Assignment of helical sense for poly(phenylacetylene) bearing achiral galvinoxyl chromophore synthesized by helix-sense-selective polymerization. Macromolecules. 2005;38:9420–6.
Kaneko T, Umeda Y, Jia H, Hadano S, Teraguchi M, Aoki T. Helix-sense tunability induced by achiral diene ligands in the chiral catalytic system for the helix-sense-selective polymerization of achiral and bulky phenylacetylene monomers. Macromolecule. 2007;40:7098–102.
Liu L, Zhang G, Aoki T, Wang Y, Kaneko T, Teraguchi M, et al. Synthesis of one-handed helical block copoly(substituted acetylene)s consisting of dynamic cis-transoidal and static cis-cisoidal block: chiral teleinduction in helix-sense-selective polymerization using a chiral living polymer as an initiator. ACS Macro Lett. 2016;5:1381–5.
Yashima E, Matsushima T, Okamoto Y. Chirality assignment of amines and amino alcohols based on circular dichroism induced by helix formation of a stereoregular poly((4-carboxyphenyl)acetylene) through acid-base complexation. J Am Chem Soc. 1997;119:6345–59.
Yashima E, Maeda K. Chirality-responsive helical polymers. Macromolecules. 2008;41:3–12.
Maeda K, Hirose D, Okoshi N, Shimomura K, Wada Y, Ikai T, et al. Direct detection of hardly detectable hidden chirality of hydrocarbons and deuterated isotopomers by a helical polyacetylene through chiral amplification and memory. J Am Chem Soc. 2018;140:3270–6.
Zhang Y, Deng J, Pan K. Chiral helical polymer nanomaterials with tunable morphology: prepared with chiral solvent to induce helix-sense-selective precipitation polymerization. Macromolecules. 2018;51:8878–86.
Zhang L, Ma Z, Wang R, Zhu M. Synthesis and characterization of methacrylate-functionalized betulin derivatives as antibacterial comonomer for dental restorative resins. ACS Biomater Sci Eng. 2021;7:3132–40.
Melissaris AP, Litt MH. Economical and convenient synthesis of p-ethynylbenzoic acid and p-ethynylbenzoyl chloride. J Org Chem. 1992;57:6998–9.
Zang Y, Aoki T, Teraguchi M, Kaneko T, Ma L, Jia H. Synthesis and oxygen permeation of novel polymers of phenylacetylenes having two hydroxyl groups via different lengths of spacers. Polymer. 2015;56:199–206.
Spivak AY, Keiser J, Vargas M, Gubaidullin RR, Nedopekina DA, Shakurova ER, et al. Synthesis and activity of new triphenylphosphonium derivatives of betulin and betulinic acid against Schistosoma mansoni in vitro and in vivo. Bioorg Med Chem. 2014;22:6297–304.
Tsepaeva OV, Nemtarev A, Abdullin TI, Grigor’eva LR, Kuznetsova EV, Akhmadishina RA, et al. Design, synthesis, and cancer cell growth inhibitory activity of triphenylphosphonium derivatives of the triterpenoid betulin. J Nat Prod. 2017;80:2232–9.
Saeed I, Khan FZ, Shiotsuki M, Masuda T. Synthesis and properties of carbamate-and amine-containing poly (phenylacetylenes). J Polym Sci Pol Chem. 2009;47:1853–63.
Lai LM, Lam JW, Tang BZ. Synthesis and chiroptical properties ofl-valine-containing poly(phenylacetylene)s with (a)chiral pendant terminal groups. J Polym Sci Pol Chem. 2006;44:2117–29.
Wei H, Wang F, Qian X, Li S, Hu Z, Sun H, et al. Superhydrophobic fluorine-rich conjugated microporous polymers monolithic nanofoam with excellent heat insulation property. Chem Eng J. 2018;351:856–66.
Qian X, Zhu ZQ, Sun HX, Ren F, Mu P, Liang W, et al. Superhydrophobic fluorine-rich conjugated microporous polymers monolithic nanofoam with excellent heat insulation property. ACS Appl Mater Inter. 2016;8:21063–9.
Chen C, Zhao B, Deng J. Optically active porous microspheres consisting of helical substituted polyacetylene prepared by precipitation polymerization without porogen and the application in enantioselective crystallization. Acs Macro Lett. 2015;4:348–52.
Miao T, Cheng X, Ma H, He Z, Zhang Z, Zhou N, et al. Transfer, amplification, storage, and complete self‐recovery of supramolecular chirality in an achiral polymer system. Angew Chem. 2021;133:18714–9.
Shi G, Dai X, Zhou Y, Zhang J, Shen J, Wan X. Synthesis and enantioseparation of proline-derived helical polyacetylenes as chiral stationary phases for HPLC. Polym Chem. 2020;11:3179–87.
Zhang ZG, Deng JP, Li JW, Yang WT. Influence of solvent on the secondary structure of helical poly(N-propargyl-(1R)-camphor-10-sulfamide). Polym J. 2008;40:436–41.
Kaneko T, Liang XJ, Kawami A, Sato M, Namikoshi T, Teraguchi M, et al. Transformation from preformed racemic helical poly(phenylacetylene)s to the enantioenriched helical polymers by chiral solvation, followed by removal of the chiral solvents. Polym J. 2012;44:327–33.
Acknowledgements
This work was partially supported by research projects of basic scientific research operating expenses of provincial universities of Heilongjiang Province of China (135209218).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Z., Wen, J., Zhao, Y. et al. Novel synthesis of porous one-handed helical poly(substituted phenylacetylene) bearing betulin derivatives pendant groups. Polym J 55, 203–211 (2023). https://doi.org/10.1038/s41428-022-00752-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-022-00752-7