Abstract
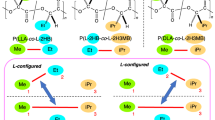
Polylactide (PLA) stereocomplexes (SCs) formed by poly(L,L-lactide) (PLLA) and poly(D,D-lactide) (PDLA) are well known, and their derivatives and copolymers have also been reported. However, little research has been conducted on SCs with a combination of one enantiomer of a copolymer and a homopolymer. In this study, we selected a PLA copolymer with poly(ethylene glycol) (PEG) and a urethane capping PLA bearing almost the same molecular weights as PLA segments and prepared various SC samples. Depending on the combination of L-isomers and D-isomers, the SCs were classified as a Homo-SC, Co-SC, and Multi-SC: the Homo-SC had the same polymer chain ends and copolymers, the Co-SC had different polymer chain ends and copolymers, and the Multi-SC had a mixture of various polymer chain ends and copolymers. Depending on the chain end structures, the melting points of the SCs changed in the case of Homo-SC and Co-SC, but Multi-SC showed two broad melting points at ~200 °C. We propose that these findings are the results of the various combinations of polymer–polymer interactions in the case of Multi-SC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ikada Y, Tsuji H. Biodegradable polyesters for medical and ecological applications. Macromol Rapid Commun. 2000;21:117–32.
Pretula J, Slomkowski S, Penczek S. Polylactides—methods of synthesis and characterization. Adv Drug Del Rev. 2016;107:3–16.
Wu C-S. Renewable resource-based composites of recycled natural fibers and maleated polylactide bioplastic: characterization and biodegradability. Polym Degrad Stab. 2009;94:1076–84.
Huang QY, Hiyama M, Kabe T, Kimura S, Iwata T. Enzymatic self-biodegradation of poly(L-lactic acid) films by embedded heat-treated and immobilized proteinase K. Biomacromolecules. 2020;21:3301–07.
Tsuji H, Tezuka Y. Alkaline and enzymatic degradation of L-lactide copolymers, 1 Amorphous—made films of L-lactide copolymers with D-lactide, glycolide and e-caprolactone. Macromol Biosci. 2005;5:135–48.
Tsuji H, Ishida T. Poly(L-lactide). X. Enhanced surface hydrophilicity and chain-scission mechanisms of poly(L-lactide) film in enzymatic, alkaline, and phosphate-buffered solutions. J Appl Polym Sci. 2003;87:1628–33.
Ikada Y, Jamshidi K, Tsuji H, Hyon S-H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules. 1987;20:904–6.
Tsuji H. Poly(lactide) stereocomlexes: formation, structure, properties, degradation, and applications. Macromol Biosci. 2005;5:569–97.
Tsuji H. Poly(lactic acid) stereocomplexes: a decade of progress. Adv Drug Del Rev. 2016;107:97–135.
Ajiro H, Hsiao Y-J, Thi TH, Fujiwara T, Akashi M. A stereocomplex of poly(lactide)s with chain end modification: simultaneous resistances to melting and thermal decomposition. Chem Commun. 2012;48:8478–80.
Ajiro H, Ito S, Kan K, Akashi M. Catechin-modified polylactide stereocomplex at chain end improved antibiobacterial property. Macromol Biosci. 2016;16:694–704.
Kan K, Akashi M, Ajiro H. Polylactides bearing vanillin at chain end provided dual dynamic interactions: stereocomplex formation and nanostructure control. Macromol Chem Phys. 2016;217:2679–85.
Kumamoto N, Chantheaset N, Ajiro H. Polylactide stereocomplex bearing vinyl groups at chain ends prepared by allyl alcohol, malic acid, and citric acid. Polym Degrad Stab. 2020;180:109311.
Chanthaset N, Ajiro H. Synthetic biodegradable polymers with chain end modification: polylactide, poly(butylene succinate), and poly(hydroxyalkanoate). Chem Lett. 2021;50:767–77.
Fukushima K, Kimura Y. Stereocomlexed polylactides (Neo-PLA) as high-performance bio-based polymers: their formation, properties, and application. Polym Int. 2006;55:626–42.
Isono T, Kondo Y, Otsuka I, Nishiyama Y, Borsali R, Kakuchi T, et al. Synthesis and stereocomplex formation of star-shaped stereoblock polylactides consisting of poly(L-lactide) and poly(D-lactide) arms. Macromolecules. 2013;46:8509–18.
Tsuji H, Tamai K, Kimura T, Kubota A, Tahahashi A, Kuzuya A, et al. Stereocomplex- and homo-crystallization of blends from 2-armed poly(L-lactide) and poly(D-lactide) with identical and opposite chain directional architectures and of 2-armed stereo diblock poly(lactide). Polymer. 2016;96:167–81.
Fox TG, Garrett BS, Goode WE, Gratch S, Kincaid JF, Spell A, et al. Crystalline polymers of methyl methacrylate. J Am Chem Soc. 1958;80:1768–9.
Jiang Z, Boyer MT, Sen A. Chiral and steric recognition in optically active, isotactic, alternating a-olefin-carbon monoxide copolymers. Effect on physical properties and chemical reactivity. J Am Chem Soc. 1995;117:7037–8.
Marín R, Alla A, Muñoz-Guerra S. Stereocomplex formation from enantiomeric polyamides derived from Tartaric acid. Macromol Rapid Commun. 2006;27:1955–61.
Tsuji H, Okumura A. Stereocomplex formation between enantiomeric substituted poly(lactide)s: blends of poly[(S)−2-hydroxybutyrate] and poly[(R)−2-hydroxybutyrate]. Macromolecules. 2009;42:7263–6.
Nakano K, Hashimoto S, Nakamura M, Kamada T, Nozaki K. Stereocomoplex of poly(propylene carbonate): synthesis of stereogradient poly(propylnene carbonate) by regio- and enantioselective copolymerization of propylene oxide with carbon dioxide. Angew Chem Int Ed. 2011;50:4868–71.
Slager J, Gladnikoff M, Domb AJ. Stereocomplexes, based on biodegradable polymers and bioactive macromolecules. Macromol Symp. 2001;175:105–15.
Slager J, Domb AJ. Hetero-stereocomplexes of D-poly(lactic acid) and the LHRH analogue leuprolide. Application in controlled release. Eur J Pharm Biopharm. 2004;58:461–9.
Ishii D, Kimishima M, Otake K, Iwata T. Enhanced crystallization of poly(lactide) stereocomplex by xylan propionate. Polym Int. 2016;65:339–45.
Tsuji H, Matsuoka H. Stereoselective interaction between isotactic and optically active poly(lactic acid) and phenyl-substituted poly(lactic acid). Macromol Rapid Commun. 2008;29:1372–7.
Tsuji H, Yamamoto S, Okumura A, Sugiura Y. Heterostereocomplexation between biodegradable and optically active polyesters as a versatile preparation method for biodegradable materials. Biomacromolecules. 2010;11:252–8.
Tsuji H, Sobue T. Stereocomplexation of quaternary or ternary monomer units and dual stereocomplexation in enantiomeric binary and quaternary polymer blends of poly(2-hydroxybutanoic acid)s, poly(2-hydroxybutanoic acid-co-lactic acid)s, and poly(lactic acid)s. RSC Adv. 2015;5:83331–42.
Tsuji H, Masaki N, Arakawa Y, Iguchi K, Sobue T. Ternary stereocomplex and hetero-stereocomplex crystallizability of substituted and unsubstituted poly(lactic acid)s. Cryst Growth Des. 2018;18:521–30.
Shirahama H, Ichimaru A, Tsutsumi C, Nakayama Y, Yasuda H. Characteristics of the biodegradability and physical properties of stereocomplexes between poly(Llactide) and poly(D-lactide) copolymers. J Polym Sci A Polym Chem. 2005;43:438–54.
Wanamaker CL, Bluemle MJ, Pitet LM, O’Leary LE, Tolman WB, Hillmyer MA. Consequences of polylactide stereochemistry on the properties of polylactide-polymenthide-polylactide thermoplastic elastomers. Biomacromolecules. 2009;10:2904–11.
Kobayashi K, Kanmuri S, Kimura Y, Masutani K. Synthesis and properties of stereo mixtures of enantiomeric block copolymers of polylactide and aliphatic polycarbonate. Polym Int. 2015;64:641–6.
Fan X, Wang Z, Yuan D, Sun Y, Li Z, He C. Novel linear-dendritic-like amphiphilic copolymers: synthesis and self-assembly characteristics. Polym Chem. 2014;5:4069–75.
Li Z, Yuan D, Fan X, Tan BH, He C. Poly(ethylene glycol) conjugated poly(lactide)-based polyelectrolytes: synthesis and formation of stable selfassemblies induced by stereocomplexation. Langmuir. 2015;31:2321–33.
Wolf FK, Hofmann AM, Frey H. Poly(isoglycerol methacrylate)-b-poly(D- or L-lactide) copolymers: a novel hydrophilicmethacrylate as building block for supramolecular aggregates. Macromolecules. 2010;43:3314–24.
Uehara H, Karaki Y, Wada S, Yamanobe T. Stereocomplex crystallization of poly(lactic acid)s in block-copolymer phase separation. ACS Appl Mater Interfaces. 2010;2:2707–10.
Grancharov G, Coulembier O, Surin M, Lazzaroni R, Dubois P. Stereocomplexed materials based on poly(3-hexylthiophene)-b-poly(lactide) block copolymers: synthesis by organic catalysis, thermal properties, and microscopic morphology. Macromolecules. 2010;43:8957–64.
Fujiwara T, Mukose T, Yamaoka T, Yamane H, Sakurai S, Kimura Y. Novel thermo-responsive formation of a hydrogel by stereo-complexation between PLLA-PEG-PLLA and PDLA-PEG-PDLA block copolymers. Macromol Biosci. 2001;1:204–8.
Yang L, Ghzaoui AE, Li S. In vitro degradation behavior of poly(lactide)-poly(ethylene glycol) block copolymer micelles in aqueous solution. Int J Pharm. 2010;400:96–103.
Ajiro H, Kuroda A, Kan K, Akashi M. Stereocomplex film using triblock copolymers of polylactide and poly(ethylene glycol) retain paxlitacel on substrates by an aqueous inkjet system. Langmuir. 2015;31:10583–9.
Kricheldorf HR, Rost S, Wutz C, Domb A. Stereocomplex of A-B-A triblock copolymers based on poly(L-lactide) and poly(D-lactide) A blocks. Macromolecules. 2005;38:7018–25.
Liu R, He B, Li D, Lai Y, Tang JZ, Gu Z. Stabilization of pH-sensitive mPEG-PH-PLA nanoparticles by stereocomplexation between enantiomeric polylactides. Macromol Rapid Commun. 2012;33:1061–6.
Geschwind J, Rathi S, Tonhauser C, Schomer M, Hsu SL, Coughlin EB, et al. Stereocomplex formation in polylactide multiarm stars and comb copolymers with linear and hyperbranched mutifunctional PEG. Macromol Chem Phys. 2013;214:1434–44.
Zhou D, Shao J, Sun J, Bian X, Xiang S, Li G, et al. Effect of the different architectures and molecular weights on stereocomplex in enantiomeric polylactides-b-MPEG block copolymers. Polymer. 2017;123:49–54.
Xie Q, Han L, Zhou J, Shan G, Bao Y, Pan P. Homocrystalline mesophase formation and mutistage structural transitions in stereocomplexable racemic blends of block copolymers. Polymer. 2020;189:122180.
Liu Y, Shao J, Sun J, Bian X, Feng L, Xiang S, et al. Improved mechanical thermal properties of PLLA by solvent blending with PDLA-b-PEG-b-PDLA. Polym Degrad Stab. 2014;101:10–7.
Tsuji H, Kikkawa K, Ozawa R, Arakawa Y. Simultaneous stereocomplex cocrystallization from coexisting two types of stereocomplexationable poly(lactide) systems. CrystEngCommn. 2019;21:3158–69.
Zhang J, Sato H, Tsuji H, Noda I, Ozaki Y. Infrared spectroscopic study of CH3…O=C interaction during poly(L-lactide)/poly(D-lactide) stereocomplex formation. Macromolecules. 2005;38:1822–8.
Sarasua JR, Prud’homme RE, Wisniewski M, Borgne AL, Spassky N. Crystallization and melting behavior of polylactides. Macromolecules. 1998;31:3895–905.
Tsuji H, Horii F, Nakagawa M, Ikada Y, Odani H, Kitamaru R. Stereocomplex formation between enantiomeric poly(lactic acid)s. 7. Phase structure of the stereocomplex crystallized from dilute acetonitrile solution as studied by high-resolution solid-state carbon-13 NMR spectopy. Macromolecules. 1992;25:4114–8.
Xu R, Xie J, Lei C. Influence of melt-draw ratio on the crystalline behaviour of a polylactic acid cast film with a chi structure. RSC Adv. 2017;7:39914.
Lorenzo MLD, Androsch R. Influence of α’-/α-crystal polymorphism on properties of poly(L-lactic acid). Polym Int. 2019;68:320–34.
Hsieh YT, Nozaki S, Kido M, Kamitani K, Kojio K, Takahara A. Crystal polymorphoism of polylactide and its composites by X-ray diffraction study. Polym J. 2020;52:755–63.
Acknowledgements
This work was supported by JSPS KAKENHI: Grant-in-Aid for Scientific Research in Innovative Areas (JP20H05223), Grant-in-Aid for Scientific Research (B) (JP20H02799), and Fostering Joint International Research (B) (JP19KK0277). This study was also partly supported by AMED under Grant Number JP20lm0203014.
Author information
Authors and Affiliations
Contributions
Jaeyeong Choi and Hiroharu Ajiro equally contributed to this work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Choi, J., Ajiro, H. Preparation and analyses of stereocomplexes of a polylactide homopolymer and copolymer with poly(ethylene glycol) and urethane capping. Polym J 54, 151–160 (2022). https://doi.org/10.1038/s41428-021-00564-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-021-00564-1