Abstract
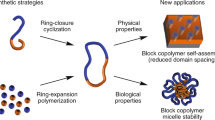
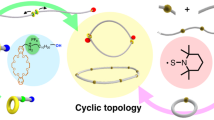
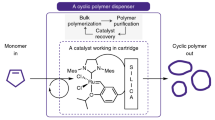
The relationship between the primary structures of polymers and their properties has long been recognized as an important research subject for polymer chemists. Advanced chemical procedures allowing precise control of the structure also enable us to examine the topological effects on polymer properties. Cyclic polymers possess unique characteristics due to the absence of polymer termini, showing different bulk and solution properties from their corresponding linear counterparts, i.e., smaller hydrodynamic volume and radius of gyration (Rg) [1], lower intrinsic viscosity [2], high critical solution temperature [2], accelerated rate of crystallization [3], and high refractive indices [4]. To make progress in this research field, innovative synthetic procedures are essential. The synthetic strategy for cyclic polymers has two typical pathways: the ring closure of functional linear polymers and ring expansion polymerization using cyclic monomers, an initiator, or a catalyst. This review describes the recent synthetic evolution of cyclic polymers, focusing on our new strategy: ring closing without highly dilute conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




















Similar content being viewed by others
References
Lonsdale DE, Bell CA, Monteiro MJ. Strategy for rapid and high-purity monocyclic polymers by CuAAC “click” reactions. Macromolecules. 2010;43:3331–9.
Qiu XP, Tanaka F, Winnik FM. Temperature-induced phase transition of well-defined cyclic poly(N-isopropylacrylamide)s in aqueous solution. Macromolecules. 2007;40:7069–71.
Shin EJ, Jeong W, Brown HA, Koo BJ, Hedrick JL, Waymouth RM. Crystallization of cyclic polymers: synthesis and crystallization behavior of high molecular weight cyclic poly(epsilon-caprolactone)s. Macromolecules. 2011;44:2773–9.
Bannister DJ, Semlyen JA. Studies of cyclic and linear poly(dimethyl siloxanes): 6. Effect of heat. Polymer. 1981;22:377–81.
Romio M, Trachsel L, Morgese G, Ramakrishna SN, Spencer ND, Benetti EM. Topological polymer chemistry enters materials science: expanding the applicability of cyclic polymers. ACS Macro Lett. 2020;9:1024–33.
Geiser D, Hocker H. Synthesis and investigation of macrocyclic polystyrene. Macromolecules. 1980;13:653–6.
Hild G, Kohler A, Rempp P. Synthesis of ring-shaped macromolecules. Eur Polym J. 1980;16:525–7.
Schappacher M, Deffieux A. Synthesis of macrocyclic poly(2-chloroethyl vinyl ether)s. Makromol Chem Rapid Commun. 1991;12:447–53.
Schappacher M, Deffieux A. Alpha-acetal-omega-bis(hydroxymethyl) heterodifunctional polystyrene: synthesis, characterization, and investigation of intramolecular end-to-end ring closure. Macromolecules. 2001;34:5827–32.
Laurent BA, Grayson SM. An efficient route to well-defined macrocyclic polymers via “click” cyclization. J Am Chem Soc. 2006;128:4238–9.
Xu J, Ye J, Liu SY. Synthesis of well-defined cyclic poly(N-isopropylacrylamide) via click chemistry and its unique thermal phase transition behavior. Macromolecules. 2007;40:9103–10.
Chen FG, Liu GM, Zhang GZ. Synthesis of cyclic polyelectrolyte via direct copper(I)-catalyzed click cyclization. J Polym Sci A: Polym Chem. 2012;50:831–5.
Ren JM, Satoh K, Goh TG, Blencowe A, Nagai K, Ishitake K, et al. Stereospecific cyclic poly(methyl methacrylate) and its topology-guided hierarchically controlled supramolecular assemblies. Angew Chem Int Ed. 2014;53:459–64.
Stamenovic MM, Espeel P, Baba E, Yamamoto T, Tezuka Y, Du Prez FE. Straightforward synthesis of functionalized cyclic polymers in high yield via RAFT and thiolactone-disulfide chemistry. Polym Chem. 2013;4:184–93.
McGraw ML, Chen EYX. Lewis pair polymerization: perspective on a ten-year journey. Macromolecules. 2020;53:6102–22.
Haque FM, Grayson SM. The synthesis, properties and potential applications of cyclic polymers. Nat Chem. 2020;12:433–44.
Arduengo AJ, Harlow RL, Kline M. A stable crystalline carbene. J Am Chem Soc. 1991;113:361–3.
Matsuoka S, Ota Y, Washio A, Katada A, Ichioka K, Takagi T, et al. Organocatalytic tail-to-tail dimerization of olefin: umpolung of methyl methacrylate mediated by N-heterocyclic carbene. Org Lett. 2011;13:3722–5.
Kato T, Ota Y, Matsuoka S, Takagi K, Suzuki M. Experimental mechanistic studies of the tail-to-tail dimerization of methyl methacrylate catalyzed by N-heterocyclic carbene. J Org Chem. 2013;78:8739–47.
Biju AT, Padmanaban M, Wurz NE, Glorius F. N-heterocyclic carbene catalyzed umpolung of michael acceptors for intermolecular reactions. Angew Chem Int Ed 2011;50:8412–5.
Zhang Y, Schmitt M, Falivene L, Caporaso L, Cavallo L, Chen EY-X. Organocatalytic conjugate-addition polymerization of llinear and cyclic acrylic monomers by N-heterocyclic carbenes: mechanisms of chain initiation, propagation, and termination. J Am Chem Soc. 2013;135:17925–41.
Hosoi Y, Takasu A, Matsuoka S, Hayashi M. N-heterocyclic carbene initiated anionic polymerization of (E,E)-methyl sorbate and subsequent ring-closing to cyclic poly(alkyl sorbate). J Am Chem Soc. 2017;139:15005–12.
Maruoka K, Itoh T, Sakurai M, Nonoshita K, Yamamoto H. Amphiphilic reactions by means of exceptionally bulky organoaluminum reagents. Rational approach for obtaining unusual equatorial, anti-Cram, and 1,4 selectivity in carbonyl alkylation. J Am Chem Soc. 1998;110:3588–97.
Hirabayashi T, Yamamoto H, Kojima T, Takasu A, Inai Y. Synthesis of head-to-tail and head-to-head poly(propylene-alt-methyl methacrylate)s via anionic polymerization of methyl 2,4-alkadienoates. Macromolecules. 2000;33:4304–6.
Takasu A, Ishii M, Inai Y, Hirabayashi T. Highly threo diastereoselective anionic polymerization of (E,E)-methyl sorbate catalyzed by a bulky organoaluminum Lewis acid. Macromolecules. 2001;34:6548–50.
Takasu A, Ishii M, Inai Y, Hirabayashi T, Inomata K. Threo-disyndiotactic polymerization of (E,E)-alkyl sorbates assisted by bulky organoaluminum Lewis acid via “alternating turning over polymerization (ATOP)” mechanism. Macromolecules. 2003;36:7055–64.
Oga Y, Hosoi Y, Takasu A. Synthesis of cyclic poly(methyl methacrylate) via N-heterocyclic carbene (NHC) initiated-anionic polymerization and subsequent ring-closing without need of highly dilute conditions. Polymer. 2020;186:122019.
Naruse K, Takasu A, Higuchi M. Direct observation of a cyclic vinyl polymer prepared by anionic polymerization using N-heterocyclic carbene and subsequent ring-closure without highly diluted conditions. Macromol Chem Phys. 2020;221:202000004.
Muramatsu Y, Oga Y, Takasu A, Higuchi M. Direct observation of cyclic poly(N-substituted maleimide)s with broad size distributions synthesized by anionic polymerization using an N-heterocyclic carbene and successive ring closure without high dilutions. Polym J. 2020;52:1253–61.
Muramatsu Y, Takasu A, Higuchi M, Hayashi M. Direct observation of the Formation of a cyclic poly(alkyl sorbate) via chain-growth polymerization by an N-heterocyclic carbene initiator and ring-closing without extreme dilution. J Poymer Sci. 2020;52:2936–2942.
McGraw ML, Clarke RW, Chen EY-X. Synchronous control of chain length/sequence/topology for precision synthesis of cyclic block copolymers from monomer mixtures,. J Am Chem Soc. 2021;143:3318–22.
McGraw ML, Clarke RW, Chen EY-X. Compounded sequence control in polymerization of one-pot mixtures of highly reactive acrylates by differentiating Lewis pairs. J Am Chem Soc. 2020;142:5969–73.
Bielawski CW, Benitez D, Grubbs RH. An “endless” route to cyclic polymers. Science. 2002;297:2041–4.
Boydston AJ, Xia Y, Kornfield JA, Gorodetskaya IA, Grubbs RH. Cyclic ruthenium-alkylidene catalysts for ring-expansion metathesis polymerization. J Am Chem Soc. 2008;130:12775–82.
Niu W, Gonsales SA, Kubo T, Bentz KC, Pal D, Savin DA, et al. Polypropylene: now available without chain ends. Chem. 2019;5:237–44.
Kammiyada H, Ouchi M, Sawamoto M. A study on physical properties of cyclic poly(vinyl ether)s synthesized via ring-expansion cationic polymerization. Macromolecules. 2017;50:841–8.
Kubota H, Yoshida S, Ouchi M. Ring-expansion cationic cyclopolymerization for construction of cyclic cyclopolymers. Polym Chem. 2020;11:3964–71.
Jeong W, Hedrick JL, Waymouth RM. Organic spirocyclic initiators for the ring-expansion polymerization of beta-lactones. J Am Chem Soc. 2007;129:8414–5.
Kamber NE, Jeong W, Gonzalez S, Hedrick JL, Waymouth RM. N-heterocyclic carbenes for the organocatalytic ring-Opening polymerization of epsilon-caprolactone. Macromolecules. 2009;42:1634–9.
Culkin DA, Jeong W, Csihony S, Gomez ED, Balsara NP, Hedrick JL, et al. Zwitterionic polymerization of lactide to cyclic poly(lactide) by using N-heterocyclic carbene organocatalysts. Angew Chem Int Ed. 2007;46:2627–30.
Zhang Y, Liu R, Jin H, Song W, Augustine R, Kim I. Straightforward access to linear and cyclic polypeptides. Commun Chem. 2018;40:1–7.
Guo L, Zhang DH. Cyclic poly(alpha-peptoid)s and their block copolymers from N-heterocyclic carbene-mediated ring-opening polymerizations of N-substituted N-carboxylanhydrides. J Am Chem Soc. 2009;131:18072–4.
Hong M, Chen EY-X. Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone. Nat Chem. 2016;8:42–49.
Zhu J, Watson EM, Tang J, Chen EY-X. A synthetic polymer system with repeatable chemical recyclability. Science. 2018;27:398–403.
Zhu J, Chen EY-X. Living coordination polymerization of a six-five bicyclic lactone to produce completely recyclable polyester. Angew Chem Int Ed. 2020;57:12558–62.
Wang Y, Xu T. Topology-controlled ring-opening polymerization of o-carboxyanhydride. Macromoleculs. 2020;53:8829–36.
Tsurumi N, Takashima R, Aoki D, Kuwata S, Otsuka H. A strategy toward cyclic topologies based on the dynamic behavior of a bis(hindered amino)disulfide linker. Angew Chem Int Ed. 2020;59:4269–73.
Takashima R, Aoki D, Otsuka H. Rational entry to cyclic polymers via thermally induced radical ring-expansion polymerization of macrocycles with one bis(hindered amino)disulfide linkage. Macromolecules. 2020;53:4670–7.
Yamamoto T, Tezuka Y. Topological polymer chemistry: a cyclic approach toward novel polymer properties and functions. Polym Chem. 2011;2:1930–41.
Chichak KS, Cantrill SJ, Pease AR, Chiu S-H, Cave GWV, Atwood JL, et al. Molecular borromean rings. Science. 2004;304:1308–12.
Zhang WB, Sun F, Tirrell DA, Arnold FH. Controlling macromolecular topology with genetically encoded spytag-spycatcher chemistry. J Am Chem Soc. 2013;135:13988–97.
Clark PG, Guidry EN, Chan WY, Steinmetz WE, Grubbs RH. Synthesis of a molecular charm bracelet via click cyclization and olefin metathesis clipping. J Am Chem Soc. 2010;132:3405–12.
Ishikawa K, Yamamoto T, Asakawa M, Tezuka Y. Effective synthesis of polymer catenanes by cooperative electrostatic/hydrogen-bonding self-assembly and covalent fixation. Macromolecules. 2010;43:168–76.
Tezuka Y, editor. Topological polymer chemistry: progress of cyclic polymers in syntheses, properties and functions. Singapore: World Scientific; 2013.
Oike H, Imaizumi H, Mouri T, Yoshioka Y, Uchibori A, Tezuka Y. Designing unusual polymer topologies by electrostatic self-assembly and covalent fixation. J Am Chem Soc. 2000;122:9592–9.
Oike H, Kobayashi S, Mouri T, Tezuka Y. Kyklo-telechelics: Tailored synthesis of cyclic poly(tetrahydrofuran)s having two functional groups at opposite positions. Macromolecules. 2001;34:2742–4.
Sugai N, Heguri H, Ohta K, Meng Q, Yamamoto T, Tezuka Y. Effective click construction of bridged- and spiro-multicyclic polymer topologies with tailored cyclic prepolymers (kyklo-Telechelics). J Am Chem Soc. 2010;132:14790–802.
Tezuka Y, Fujiyama K. Construction of polymeric delta-graph: a doubly fused tricyclic topology. J Am Chem Soc. 2005;127:6266–70.
Sugai N, Heguri H, Yamamoto T, Tezuka Y. A programmed polymer folding: click and clip construction of doubly fused tricyclic and triply fused tetracyclic polymer topologies. J Am Chem Soc. 2011;133:19694–7.
Chen CT, Gantzel P, Siegel JS, Baldridge KK, English RB, Ho DM. Synthesis and structure of the nanodimensional multicyclophane “Kuratowski cyclophane”, an achiral molecule with nonplanar K-3,K-3 topology. Angew Chem Int Ed. 1995;34:2657–60.
Craik DJ. Chemistry - Seamless proteins tie up their loose ends. Science. 2006;311:1563–4. 1125248
Alon A, Grossman I, Gat Y, Kodali VK, DiMaio F, Mehlman T, et al. Fass D. The dynamic disulphide relay of quiescin sulphydryl oxidase. Nature. 2012;488:414–8.
de Araujo AD, Mobli M, King GF, Alewood PF. Cyclization of peptides by using selenolanthionine bridges. Angew Chem Int Ed. 2012;51:10298–302.
Suzuki T, Yamamoto T, Tezuka Y. Constructing a macromolecular K-3,K-3 graph through electrostatic self-assembly and covalent fixation with a dendritic polymer precursor. J Am Chem Soc. 2014;136:10148–55.
Acknowledgements
A.T. is grateful for financial support from the Ministry of Education, Science and Culture of Japan (Grant-in-Aid for Development Scientific Research 18K19112 and 20H02786). Y.M. also acknowledges Dr. Mikihiro Hayashi and Prof. Masahiro Higuchi for their fruitful discussions and technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muramatsu, Y., Takasu, A. Synthetic innovations for cyclic polymers. Polym J 54, 121–132 (2022). https://doi.org/10.1038/s41428-021-00560-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-021-00560-5