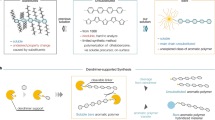
Abstract
Phenylazomethine dendrimers have metal coordination sites whose base strength gradually increases toward the inner positions due to the potential gradient. This feature enables the stepwise assembly of Lewis acidic molecules and metal salts in the dendrimers. In this review, we focus on functionalities for luminous materials, sensors, and subnanosize reactors based on the structural and electronic nature of the phenylazomethine dendrimer. Integration of the photoluminescent units in a dendrimer is achieved using bismuth salts. A detailed description of the performance of a subnanosized reactor with a reducing capsule method, which is useful for synthesizing ultrasmall metal particles, is provided. The atomicity-controlled assembly feature facilitates the solution-phase synthesis of superatoms that mimic the properties of elemental atoms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ottaviani MF, Bossmann S, Turro NJ, Tomalia DA. Characterization of starburst dendrimers by the EPR technique. 1. Copper complexes in water solution. J Am Chem Soc. 1994;116:661–71.
Tomalia DA, Naylor AM, Goddard WA. Starburst dendrimers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Ed. 1990;29:138–75.
Tomalia DA, Frechet JMJ. Discovery of dendrimers and dendritic polymers: a brief historical perspective. J Polym Sci Part A: Polym Chem. 2002;40:2719–28.
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith PA. New class of polymers: starburst-dendritic macromolecules. Polym J. 1985;17:117–32.
de Brabander-van den Berg EMM, Meijer EW. Poly(propylene imine) dendrimers: large-scale synthesis by hetereogeneously catalyzed hydrogenations. Angew Chem Int Ed 1993;32:1308–11.
Bosman AW, Janssen HM, Meijer EW. About dendrimers: structure, physical properties, and applications. Chem Rev. 1999;99:1665–88.
Vögtle F, Gestermann S, Kauffmann C, Ceroni P, Vicinelli V, Balzani V. Coordination of Co2+ ions in the interior of poly(propylene amine) dendrimers containing fluorescent dansyl units in the periphery. J Am Chem Soc. 2000;122:10398–404.
Higuchi M, Shiki S, Ariga K, Yamamoto K. First synthesis of phenylazomethine dendrimer ligands and structural studies. J Am Chem Soc. 2001;123:4414–20.
Higuchi M, Yamamoto K. Novel π-conjugated nano-supramolecules having fine-controlled metal-assembling functions. Bull Chem Soc Jpn. 2004;77:853–74.
Yamamoto K, Imaoka T. Precision synthesis of subnanoparticles using dendrimers as a superatom synthesizer. Acc Chem Res. 2014;47:1127–36.
Kitazawa H, Albrecht K, Yamamoto K. Synthesis of a dendrimer reactor for clusters with a magic number. Chem Lett. 2012;41:828–30.
Kambe T, Haruta N, Imaoka T, Yamamoto K. Solution-phase synthesis of Al13– using a dendrimer template. Nat Commun. 2017;8:2046.
Imaoka T, Kawana Y, Kurokawa T, Yamamoto K. Macromolecular semi-rigid nanocavities for cooperative recognition of specific large molecular shapes. Nat Commun. 2013;4:2581.
Suzuki M, Nakajima R, Tsuruta M, Higuchi M, Einaga Y, Yamamoto K. Synthesis of ferrocene-modified phenylazomethine dendrimers possessing redox switching. Macromolecules. 2006;39:64–9.
Takanashi K, Yamamoto K. Divergent approach for synthesis and terminal modifications of dendritic polyphenylazomethines. Org Lett. 2007;9:5151–4.
Kimoto A, Masachika K, Cho JS, Higuchi M, Yamamoto K. Synthesis and electroluminescence properties of novel main chain poly(p-phenylenevinylene)s possessing pendant phenylazomethine dendrons as metal ligation sites. Chem Mater. 2004;16:5706–12.
Albrecht K, Kasai Y, Kimoto A, Yamamoto K. The synthesis and properties of carbazole−phenylazomethine double layer-type dendrimers. Macromolecules. 2008;41:3793–800.
Satoh N, Cho JS, Higuchi M, Yamamoto K. Novel triarylamine dendrimers as a hole-transport material with a controlled metal-assembling function. J Am Chem Soc. 2003;125:8104–5.
Cho JS, Takanashi K, Higuchi M, Yamamoto K. Phenylazomethine dendrimer complexes as novel hole-transporting materials of organic light-emitting diodes. Synth Met. 2005;150:79–82.
Albrecht K, Matsuoka K, Fujita K, Yamamoto K. Carbazole dendrimers as solution-processable thermally activated delayed-fluorescence materials. Angew Chem Int Ed. 2015;54:5677–82.
Matsuoka K, Albrecht K, Yamamoto K, Fujita K. Mono-substituted carbazole dendrimers as solution processable host materials for phosphorescent organic light-emitting diodes. J Photopolym Sci Technol. 2016;29:323–6.
Matsuoka K, Albrecht K, Yamamoto K, Fujita K. Mulifunctional dendritic emitter: aggregation-induced emission enhanced, thermally activated delayed fluorescent material for solution-processed multilayered organic light-emitting diodes. Sci Rep. 2017;7:41780.
Albrecht K, Matsuoka K, Yokoyama D, Sakai Y, Nakayama A, Fujita K, Yamamoto K. Thermally activated delayed fluorescence OLEDs with fully solution processed organic layers exhibiting nearly 10% external quantum efficiency. Chem Commun. 2017;53:2439–42.
Matsuoka K, Albrecht K, Nakayama A, Yamamoto K, Fujita K. Highly efficient thermally activated delayed fluorescence organic light-emitting diodes with fully solution-processed organic multilayered architecture: impact of terminal substitution on carbazole–benzophenone dendrimer and interfacial engineering. ACS Appl Mater Interfaces. 2018;10:33343–52.
Albrecht K, Matsuoka K, Fujita K, Yamamoto K. A dendrimer emitter doped in a dendrimer host: efficient thermally activated delayed fluorescence OLEDs with fully-solution processed organic-layers. Mater Chem Front. 2018;2:1097–103.
Amruth C, Luszczynska B, Szymanski MZ, Ulanski J, Albrecht K, Yamamoto K. Inkjet printing of thermally activated delayed fluorescence (TADF) dendrimer for OLEDs applications. Org Electron. 2019;74:218–27.
Kambe T, Watanabe A, Imaoka T, Yamamoto K. Bismuth complexes in phenylazomethine dendrimers: controllable luminescence and emission in the solid state. Angew Chem Int Ed. 2016;55:13151–4.
Kambe T, Imaoka S, Imaoka T, Yamamoto K. Build-up enhancement of photoluminescence from phenylazomethine bismuth dendrimer using Bi(OTf)3. J Nanopart Res. 2018;20:118.
Kambe T, Imaoka T, Yamamoto K. Insight into the effect of dendrimer structure on photoluminescence from assembled bismuth complexes. J Inorg Organomet Polym Mater. 2018;28:463–6.
Kawakami J, Mizuguchi T, Ito S. Poly(amine ester) dendrimer with naphthyl units as a fluorescent chemosensor for Al(III), Cu(II), and Zn(II). Anal Sci. 2006;22:1383–4.
Nakajima S, Albrecht K, Kushida S, Nishibori E, Kitao T, Uemura T, Yamamoto K, Bunz UHF, Yamamoto Y. A fluorescent microporous crystalline dendrimer discriminates vapour molecules. Chem Commun. 2018;54:2534–7.
Satija J, Sai VVR, Mukherji S. Dendrimers in biosensors: concept and applications. J Mater Chem. 2011;21:14367–86.
Valério C, Fillaut JL, Ruiz J, Guittard J, Blais JC, Astruc D. The dendritic effect in molecular recognition: ferrocene dendrimers and their use as supramolecular redox sensors for the recognition of small inorganic anions. J Am Chem Soc. 1997;119:2588–9.
Daniel MC, Ruiz J, Astruc D. Supramolecular H-bonded assemblies of redox-active metallodendrimers and positive and unusual dendritic effects on the recognition of H2PO4-. J Am Chem Soc. 2003;125:1150–1.
Daniel MC, Ba F, Ruiz J, Astruc D. Assemblies of redox-active metallodendrimers using hydrogen bonding for the electrochemical recognition of the H2PO4- and adenosine-triphosphate (ATP2-) anions. Inorg Chem. 2004;43:8649–57.
Yamamoto K, Higuchi M, Shiki S, Tsuruta M, Chiba H. Stepwise radial complexation of imine groups in phenylazomethine dendrimers. Nature. 2002;415:50911.
Satoh N, Nakashima T, Kamikura K, Yamamoto K. Quantum size effect in TiO2 nanoparticles prepared by finely controlled metal assembly on dendrimer templates. Nat Nanotechnol. 2008;3:106–11.
Yamamoto K, Imaoka T, Chun WJ, Enoki O, Katoh H, Takenaga M, Sonoi A. Size-specific catalytic activity of platinum clusters enhances oxygen reduction reactions. Nat Chem. 2009;1:397–402.
Takahashi M, Imaoka T, Hongo Y, Yamamoto K. A highly active and poison-tolerant Pt12 sub-nanocluster catalyst for the reductive amination of aldehydes with amines. Dalton Trans. 2013;42:15919–21.
Takahashi M, Imaoka T, Hongo Y, Yamamoto K. Formation of a Pt12 cluster by single-atom control that leads to enhanced reactivity: hydrogenation of unreactive olefins. Angew Chem Int Ed. 2013;52:7419–21.
Takahashi M, Imaoka T, Yamamoto K. Reactivities of platinum subnanocluster catalysts for the oxidation reaction of alcohols. RSC Adv. 2015;5:100693–6.
Inomata Y, Albrecht K, Yamamoto K. Size-dependent oxidation state and CO oxidation activity of tin oxide clusters. ACS Catal. 2018;8:451–6.
Huda M, Minamisawa K, Tsukamoto T, Tanabe M, Yamamoto K. Aerobic toluene oxidation catalyzed by subnano metal particles. Angew Chem Int Ed. 2019;58:1002–6.
Yamamoto K, Imaoka T, Tanabe M, Kambe T. New Horizon Of Nanoparticle And Cluster Catalysis With Dendrimers. Chem Rev. 2020;120:1397–437.
Kambe T, Imaoka S, Hasegawa R, Tsukamoto T, Imaoka T, Natsui K, Einaga Y, Yamamoto K. Electrochemical measurement of bismuth clusters in dendrimer through transformation from atomicity controlled complexes. J Inorg Organomet Polym Mater. 2020;30:169–73.
Imaoka T, Kitazawa H, Chun WJ, Omura S, Albrecht K, Yamamoto K. Magic number Pt13 and misshapen Pt12 clusters: which one is the better catalyst? J Am Chem Soc. 2013;135:13089–95.
Yamamoto K, Imaoka T. Dendrimer complexes based on fine-controlled metal assembling. Bull Chem Soc Jpn. 2006;79:511–26.
Wakizaka M, Imaoka T, Yamamoto K. Composition-defined nanosized assemblies that contain heterometallic early 4d/5d-transition-metals. Dalton Trans. 2019;48:14261–8.
Tsukamoto T, Kambe T, Nakao A, Imaoka T, Yamamoto K. Atom-hybridization for synthesis of polymetallic clusters. Nat Commun. 2018;9:3873.
Nakamula I, Yamanoi Y, Imaoka T, Yamamoto K, Nishihara H. A uniform bimetallic rhodium/iron nanoparticle catalyst for the hydrogenation of olefins and nitroarenes. Angew Chem Int Ed. 2011;50:5830–3.
Takahashi M, Koizumi H, Chun WJ, Kori M, Imaoka T, Yamamoto K. Finely controlled multimetallic nanocluster catalysts for solvent-free aerobic oxidation of hydrocarbons. Sci Adv. 2017;3:e1700101–9.
Moriai T, Tsukamoto T, Tanabe M, Kambe T, Yamamoto K. Selective hydroperoxygenation of olefins realized by a coinage multimetallic 1‐nanometer catalyst. Angew Chem Int Ed. 2020;59:23051–5.
Kambe T, Imaoka T, Yamamoto K. Reducing capsule based on electron programming: versatile synthesizer for size‐controlled ultra‐small metal clusters. Chem Eur J. 2016;22:16406–9.
Khannna SN, Jena P. Atomic clusters: building blocks for a class of solids. Phys Rev B. 1995;51:13705–16.
Luo Z, Grover CJ, Reber AC, Khanna SN, Castleman AW. Probing the magic numbers of aluminum–magnesium cluster anions and their reactivity toward oxygen. J Am Chem Soc. 2013;135:4307–13.
Bergeron DE, Castleman AW, Morisato T, Khanna SN. Formation of Al13I-: evidence for the superhalogen character of Al13. Science. 2004;304:84–7.
Luo Z, Castleman AW. Special and general superatoms. Acc Chem Res. 2014;47:2931–40.
Luo Z, Berkdemir C, Smith JC, Castleman AW. Cluster reaction of [Ag8]−/[Cu8]− with chlorine: evidence for the harpoon mechanism? Chem Phys Lett. 2013;582:24–30.
Zhang X, Wang Y, Wang H, Lim A, Gantefoer G, Bowen KH, Reveles JU, Khanna SN. On the existence of designer magnetic superatoms. J Am Chem Soc. 2013;135:4856–61.
Albrecht K, Hirabayashi Y, Otake M, Mendori S, Tobari Y, Azuma Y, Majima Y, Yamamoto K. Polymerization of a divalent/tetravalent metal-storing atom-mimicking dendrimer. Sci Adv. 2016;2:e1601414.
Acknowledgements
We acknowledge Takane Imaoka (Tokyo Institute of Technology) and Takamasa Tsukamoto (Tokyo Institute of Technology) for their assistance with this review. The work was financially supported in part by JST ERATO Grant Number JPMJER1503 and JSPS KAKENHI Grant Nos. JP 15H05757, JP 20K05538 and JP 21H05023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kambe, T., Yamamoto, K. Functionalization of phenylazomethine dendrimers. Polym J 54, 97–105 (2022). https://doi.org/10.1038/s41428-021-00524-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-021-00524-9