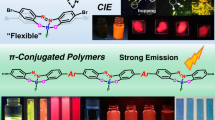
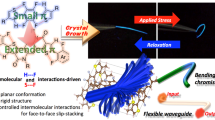
Abstract
Flexible molecules are unfavorable for designing luminescent dyes because their excitation states rapidly decay through molecular motions. We recently found that some flexible boron complexes, which potentially show a larger degree of structural relaxation in the excited state, and their polymers exhibit unique optical properties with high environmental sensitivity, such as aggregation-induced emission and luminochromism triggered by external stimuli, upon the addition of structural restrictions. Moreover, these optical properties were drastically changed by modulating the connecting points in the polymers. In this review, recent progress in the development of luminescent polymer films with stimuli responsiveness is illustrated. In particular, the influence of the alteration of connecting points on luminescent behaviors is explained. Polymerization is a versatile strategy not only for transforming a class of nonemissive molecules into luminescent dyes but also for precisely regulating the optical properties of film materials; the resulting materials are promising for application as scaffolds for advanced chemical sensors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout















Similar content being viewed by others
References
Chujo Y, Tanaka K. New polymeric materials based on element-blocks. Bull Chem Soc Jpn. 2015;88:633–43.
Gon M, Sato K, Tanaka K, Chujo Y. Controllable intramolecular interaction of 3D arranged π-conjugated luminophores based on a POSS scaffold, leading to highly thermostable and emissive materials. RSC Adv. 2016;6:78652–60.
Tanaka K, Chujo Y. Recent progress of optical functional nanomaterials based on organoboron complexes with β-diketonate, ketoiminate and diiminate. NPG Asia Mater. 2015;7:e223.
Buyuktemiz M, Duman S, Dede Y. Luminescence of BODIPY and dipyrrin: an MCSCF comparison of excited states. J Phys Chem A. 2013;117:1665–9.
Tanaka K, Chujo Y. Advanced luminescent materials based on organoboron polymers. Macromol Rapid Commun. 2012;33:1235–55.
Tanaka K, Yamane H, Yoshii R, Chujo Y. Efficient light absorbers based on thiophene-fused boron dipyrromethene (BODIPY) dyes. Bioorg Med Chem. 2013;21:2715–9.
Yamane H, Ohtani S, Tanaka K, Chujo Y. Synthesis of furan-substituted aza-BODIPYs having strong near-infrared emission. Tetrahedron Lett. 2017;58:2989–92.
Yoshii R, Yamane H, Tanaka K, Chujo Y. Synthetic strategy for low-band gap oligomers and homopolymers using characteristics of thiophene-fused boron dipyrromethene. Macromolecules. 2014;47:3755–60.
Yeo H, Tanaka K, Chujo Y. Effective light-harvesting antennae based on BODIPY-tethered cardo polyfluorenes via rapid energy transferring and low concentration quenching. Macromolecules. 2013;46:2599–605.
Yeo H, Tanaka K, Chujo Y. Tunable optical property between pure red luminescence and dual-emission depended on the length of light-harvesting antennae in the dyads containing the cardo structure of BODIPY and oligofluorene. Macromolecules. 2016;49:8899–904.
Yamane H, Tanaka K, Chujo Y. Pure-color and dual-color emission from BODIPY homopolymers containing the cardo boron structure. Polym Chem. 2018;9:3917–21.
Gon M, Tanaka K, Chujo Y. Creative synthesis of organic–inorganic molecular hybrid materials. Bull Chem Soc Jpn. 2017;90:463–74.
Kajiwara Y, Nagai A, Tanaka K, Chujo Y. Efficient simultaneous emission from RGB-emitting organoboron dyes incorporated into organic-inorganic hybrids and preparation of white light-emitting. Mater J Mater Chem C. 2013;1:4437–44.
Yoshii R, Nagai A, Tanaka K, Chujo Y. Highly NIR emissive boron di(iso)indomethene (BODIN)-based polymer: drastic change from deep-red to NIR emission via quantitative polymer reaction. J Polym Sci Part A. 2013;51:1726–33.
Yamane H, Tanaka K, Chujo Y. Synthesis of a near-infrared light-absorbing polymer based on thiophene-substituted aza-BODIPY. Polym J 2018;50:271–5.
Tanaka K, Yanagida T, Yamane H, Hirose A, Yoshii R, Chujo Y. Liquid scintillators with near infrared emission based on organoboron conjugated polymers. Bioorg Med Chem Lett 2015;25:5331–4.
Yoshii R, Yamane H, Nagai A, Tanaka K, Taka H, Kita H, et al. π-Conjugated polymers composed of BODIPY or aza-BODIPY derivatives exhibiting high electron mobility and low threshold voltage in electron-only devices. Macromolecules. 2014;47:2316–23.
Gon M, Kato K, Tanaka K, Chujo Y. Elastic and mechanofluorochromic hybrid films with POSS-capped polyurethane and polyfluorene. Mater Chem Front. 2019;3:1174–1180.
Kato K, Gon M, Tanaka K, Chujo Y. Stretchable conductive hybrid films consisting of cubic silsesquioxane-capped polyurethane and poly(3-hexylthiophene). Polymers. 2019;11:1195.
Nakamura R, Narikiyo H, Gon M, Tanaka K, Chujo Y. An optical sensor for discriminating the chemical compositions and sizes of plastic particles in water based on water-soluble networks consisting of polyhedral oligomeric silsesquioxane presenting dual-color luminescence. Mater Chem Front. 2019;3:2690–5.
Gon M, Tanaka K, Chujo Y. Concept of excitation-driven boron complexes and their applications for functional luminescent materials. Bull Chem Soc Jpn. 2019;92:7–18.
Gon M, Wakabayashi J, Tanaka K, Chujo Y. Unique substitution effect at 5,5’-positions of fused azobenzene–boron complexes with a N=N π‐conjugated system. Chem Asian J. 2019;14:1837–43.
Ohtani S, Gon M, Tanaka K, Chujo Y. Construction of the luminescent donor–acceptor conjugated systems based on boron-fused azomethine acceptor. Macromolecules. 2019;52:3387–93.
Gon M, Tanaka K, Chujo Y. A highly efficient near-infrared-emissive copolymer with a N=N double-bond π-conjugated system based on a fused azobenzene-boron complex. Angew Chem Int Ed. 2018;57:6546–51.
Matsumoto T, Takamine H, Tanaka K, Chujo Y. Design of bond-cleavage-induced intramolecular charge transfer emission with dibenzoboroles and their application to ratiometric sensors for discriminating chain lengths of alkanes. Mater Chem Front. 2017;1:2368–75.
Ohtani S, Gon M, Tanaka K, Chujo Y. Flexible fused azomethine–boron complex: thermally-induced switching of crystalline-state luminescent property and thermosalient behaviors based on phase transition between polymorphs. Chem Eur J. 2017;23:11827–33.
Hirose A, Tanaka K, Tamashima K, Chujo Y. Synthesis for dual-emissive organometallic complexes containing heterogeneous metal elements. Tetrahedron Lett. 2014;55:6477–81.
Zhang G, Palmer GM, Dewhirst MW, Fraser CL. A dual-emissive-materials design concept enables tumour hypoxia imaging. Nat Mater. 2009;8:747–51.
Wang F, DeRosa CA, Daly ML, Song D, Sabat M, Fraser CL. Multi-stimuli responsive luminescent azepane-substituted β-diketones and difluoroboron complexes. Mater Chem Front. 2017;1:1866–74.
Morris WA, Butler T, Kolpaczynska M, Fraser CL. Stimuli responsive furan and thiophene substituted difluoroboron β-diketonate materials. Mater Chem Front. 2017;1:158–66.
Zhang G, Lu J, Sabat M, Fraser CL. Polymorphism and reversible mechanochromic luminescence for solid-state difluoroboron avobenzone. J Am Chem Soc. 2010;132:2160–2.
Wang L, Wang K, Zou B, Ye K, Zhang H, Wang Y. Luminescent chromism of boron diketonate crystals: distinct responses to different stresses. Adv Mater. 2015;27:2918–22.
Galer P, Korošec RC, Vidmar M, Šket B. Crystal structures and emission properties of the BF2 complex 1-phenyl-3-(3,5-dimethoxyphenyl)-propane-1,3-dione: multiple chromisms, aggregation- or crystallization-induced emission, and the self-assembly effect. J Am Chem Soc. 2014;136:7383–94.
Tanaka K, Tamashima K, Nagai A, Okawa T, Chujo Y. Facile modulation of optical properties of diketonate-containing polymers by regulating complexation ratios with boron. Macromolecules. 2013;46:2969–75.
Yoshii R, Tanaka K, Chujo Y. Conjugated polymers based on tautomeric units: regulation of main-chain conjugation and expression of aggregation induced emission property via boron-complexation. Macromolecules. 2014;47:2268–78.
Luo J, Xie Z, Lam JW, Cheng L, Chen H, Qiu C, et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem Commun. 2001:1740–1.
Tong H, Hong Y, Dong Y, Häußler M, Lam JWY, Li Z, et al. Fluorescent “light-up” bioprobes based on tetraphenylethylene derivatives with aggregation-induced emission characteristics. Chem Commun. 2006:3705‒7.
Hu YB, Lam JWY, Tang BZ. Recent progress in AIE-active polymers. Chin J Polym Sci. 2019;37:289–301.
Qiu Z, Liu X, Lam JWY, Tang BZ. The marriage of aggregation-induced emission with polymer science. Macromol Rapid Commun. 2019;40:1800568.
Qin A, Lam JWY, Tang BZ. Luminogenic polymers with aggregation-induced emission characteristics. Prog Polym Sci. 2012;37:182–209.
Hu R, Kang Y, Tang BZ. Recent advances in AIE polymers. Polym J. 2016;48:359–70.
Tang BZ. Luminogenic polymers. Macromol Chem Phys. 2009;210:900–2.
Mei J, Leung NL, Kwok RT, Lam JW, Tang BZ. Aggregation-induced emission: Together We Shine, United We Soar! Chem Rev. 2015;115:11718–940.
Yoshii R, Nagai A, Tanaka K, Chujo Y. Highly emissive boron ketoiminate derivatives as new class of aggregation-induced emission fluorophores. Chem Eur J. 2013;19:4506–12.
Tanaka K, Nishino K, Ito S, Yamane H, Suenaga K, Hashimoto K, et al. Development of solid-state emissive o-carborane and theoretical investigation of the mechanism of the aggregation-induced emission behaviors of organoboron “element-blocks”. Faraday Discuss. 2017;196:31–42.
Yoshii R, Nagai A, Tanaka K, Chujo Y. Boron ketoiminate-based polymers: fine-tuning of the emission color and expression of strong emission both in the solution and film state. Macromol Rapid Commun. 2014;35:1315–9.
Zhao C-H, Wakamiya A, Yamaguchi S. Highly emissive poly(aryleneethynylene)s containing 2,5-diboryl-1,4-phenylene as a building unit. Macromolecules. 2007;40:3898–3900.
Zhao C-H, Sakuda E, Wakamiya A, Yamaguchi S. Highly emissive diborylphemlene-containing bis(phenylethynyl)benzenes: Structure-photophysical property correlations and fluoride ion sensing. Chem Eur J. 2009;15:10603–12.
Yin X, Guo F, Lalancette RA, Jäkle F. Luminescent main-chain organoborane polymers: highly robust, electron-deficient poly(oligothiophene borane)s via stille coupling polymerization. Macromolecules. 2016;49:537–46.
Meng B, Ren Y, Liu J, Jäkle F, Wang L. p-π conjugated polymers based on stable triarylborane with n-type behavior in optoelectronic devices. Angew Chem Int Ed. 2018;57:2183–7.
Yamane H, Tanaka K, Chujo Y. Simple and valid strategy for the enhancement of the solid-emissive property based on boron dipyrromethene. Tetrahedron Lett. 2015;56:6786–90.
Yamane H, Ito S, Tanaka K, Chujo Y. Preservation of main-chain conjugation through BODIPY-containing alternating polymers from electronic interactions with side-chain substituents by cardo boron structures. Polym Chem. 2016;7:2799–807.
Yeo H, Tanaka K, Chujo Y. Synthesis and energy transfer through heterogeneous dyes-substituted fluorene-containing alternating copolymers and their dual-emission properties. J Polym Sci A. 2015;53:2026–35.
Yeo H, Tanaka K, Chujo Y. Synthesis of dual-emissive polymers based on ineffective energy transfer through cardo fluorene-containing conjugated polymers. Polymer. 2015;60:228–33.
Nishino K, Yamamoto H, Tanaka K, Chujo Y. Development of solid-state emissive materials based on multi-functional o-carborane-pyrene dyads. Org Lett. 2016;18:4064–7.
Naito H, Nishino K, Morisaki Y, Tanaka K, Chujo Y. Luminescence color tuning of stable luminescent solid materials from blue to NIR based on bis-o-carborane-substituted oligoacenes. Chem Asian J. 2017;12:2134–8.
Naito H, Nishino K, Morisaki Y, Tanaka K, Chujo Y. Highly-efficient solid-state emissions of the anthracene–o-carborane dyads with various substituents and their thermochromic luminescent properties. J Mater Chem C. 2017;4:10047–54.
Mori H, Nishino K, Wada K, Morisaki Y, Tanaka K, Chujo Y. Modulation of luminescent chromic behaviors and environment-responsive intensity changes by substituents in bis-o-carborane-substituted conjugated molecules. Mater Chem Front. 2018;2:573–9.
Suenaga K, Yoshii R, Tanaka K, Chujo Y. Sponge-type emissive chemosensors for the protein detection based on boron ketoiminate-modifying hydrogels with aggregation-induced blueshift emission property. Macromol Chem Phys. 2016;217:414–7.
Yoshii R, Suenaga K, Tanaka K, Chujo Y. Mechanofluorochromic materials based on aggregation-induced emission-active boron ketoiminates: regulation of the direction of the emission color changes. Chem Eur J. 2015;21:7231–7.
Yamaguchi M, Ito S, Hirose A, Tanaka K, Chujo Y. Modulation of sensitivity to mechanical stimulus in mechanofluorochromic properties by altering substituent positions in solid-state emissive diiodo boron diiminates. J Mater Chem C. 2016;3:5314–9.
Suenaga K, Tanaka K, Chujo Y. Heat-resistant mechanoluminescent chromism of the hybrid molecule based on boron ketoiminate-modified octa-substituted polyhedral oligomeric silsesquioxane. Chem Eur J. 2017;23:1409–14.
Suenaga K, Tanaka K, Chujo Y. Design and luminescent chromism of fused boron complexes having constant emission efficiencies in solution and in the amorphous and crystalline states. Eur J Org Chem. 2017;2017:5191–6.
Suenaga K, Uemura K, Tanaka K, Chujo Y. Stimuli-responsive luminochromic polymers consisting of multi-states emissive fused boron ketoiminate. Polym Chem. https://doi.org/10.1039/C9PY01733J.
Yoshii R, Hirose A, Tanaka K, Chujo Y. Boron diiminate with aggregation-induced emission and crystallization-induced emission enhancement characteristics. Chem Eur J. 2014;20:8320–4.
Tanaka K, Yanagida T, Hirose A, Yamane H, Yoshii R, Chujo Y. Synthesis and color tuning of boron diiminate conjugated polymers with aggregation-induced scintillation properties. RSC Adv. 2015;5:96653–9.
Yoshii R, Hirose A, Tanaka K, Chujo Y. Functionalization of boron diiminates with unique optical properties: multicolor tuning of crystallization-induced emission and introduction into the main chain of conjugated polymers. J Am Chem Soc. 2014;136:18131–9.
Hirose A, Tanaka K, Yoshii R, Chujo Y. Film-type chemosensors based on boron diminate polymers having oxidation-induced emission properties. Polym Chem. 2015;6:5590–5.
Ito S, Tanaka K, Chujo Y. Characterization and photophysical properties of a luminescent aluminum hydride complex supported by a β-diketiminate ligand. Inorganics. 2019;7:100.
Ito S, Hirose A, Yamaguchi M, Tanaka K, Chujo Y. Size-discrimination for volatile organic compounds utilizing gallium diiminate by luminescent chromism of crystallization-induced emission via encapsulation-triggered crystal-crystal transition. J Mater Chem C. 2016;3:5564–71.
Ito S, Hirose A, Yamaguchi M, Tanaka K, Chujo Y. Synthesis of aggregation-induced emission-active conjugated polymers composed of group 13 diiminate complexes with tunable energy levels via alteration of central element. Polymers. 2017;9:68–78.
Yamaguchi M, Ito S, Hirose A, Tanaka K, Chujo Y. Luminescent color tuning with polymer films composed of boron diiminate conjugated copolymers by changing connection points to comonomers. Polym Chem. 2018;9:1942–6.
Yamaguchi M, Ito S, Hirose A, Tanaka K, Chujo Y. Control of aggregation-induced emission versus fluorescence aggregation-caused quenching by bond existence at a single site in boron pyridinoiminate complexes. Mater Chem Front. 2017;1:1573–9.
Yamaguchi M, Tanaka K, Chujo Y. Design of conjugated molecules presenting short-wavelength luminescence by utilizing heavier atoms of the same element group. Chem Asian J. 2018;13:1342–7.
Yamaguchi M, Tanaka K, Chujo Y. Control of solution and solid-state emission with conjugated polymers based on the boron pyridinoiminate structure by ring fusion. Polymer. 2018;142:127–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tanaka, K., Chujo, Y. Modulation of the solid-state luminescent properties of conjugated polymers by changing the connecting points of flexible boron element blocks. Polym J 52, 555–566 (2020). https://doi.org/10.1038/s41428-020-0316-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-0316-y
This article is cited by
-
New strategy for lowering the energy levels of one frontier molecular orbital in conjugated molecules and polymers based on Aza-substitution at the isolated HOMO or LUMO
Polymer Journal (2024)
-
π-Conjugated polymers based on flexible heteroatom-containing complexes for precise control of optical functions
Polymer Journal (2023)
-
Frustrated element-blocks: A new platform for constructing unique stimuli-responsive luminescent materials
Polymer Journal (2023)
-
PPV-type π-conjugated polymers based on hypervalent tin(IV)-fused azobenzene complexes showing near-infrared absorption and emission
Polymer Journal (2021)
-
Evaluation-oriented exploration of photo energy conversion systems: from fundamental optoelectronics and material screening to the combination with data science
Polymer Journal (2020)