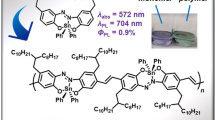
Abstract
An alternating conjugated polymer with thiophene-substituted aza-boron dipyrromethene (BODIPY) was synthesized in which the carbon atom at the meso-position in the ligand moiety was replaced by nitrogen. Initially, it was found that the synthesized polymer had a significant near-infrared (NIR) light-absorbing ability. In the absorption spectrum, a large absorption band (molar extinct coefficient εmax = 48,000 M−1 cm−1) with a peak at 864 nm was detected in the deep NIR region, even above 1300 nm. From cyclic voltammetry (CV) data, the energy levels of the frontier orbitals were determined. Accordingly, the polymer had a deep lowest unoccupied molecular orbital (LUMO) level (−4.01 eV), and this value was similar to that of the monomer. This result indicates that extension of π-conjugation throughout the polymer main-chain influenced only the highest occupied molecular orbital (HOMO) level while preserving the LUMO.
Similar content being viewed by others
Introduction
Construction of conjugated systems based on main-chain-type polymers containing heteroatoms is a valid strategy not only for obtaining desirable electronic properties according to preprogrammed designs but also for achieving superior functions distinct from each element. From this viewpoint, light-absorption ability and narrow-band gap electronic structures have been often enhanced by introducing boron into the conjugated system throughout the polymer main-chains [1, 2]. For instance, by employing boron dipyrromethenes (BODIPYs), efficient light-harvesting antennae [3,4,5] and multi-luminescent materials [6,7,8,9] can be readily fabricated with conjugated polymers. Therefore, the development of main-chain-type conjugated polymers containing organoboron units is an area of particular interest with high relevance, especially for producing advanced organic optoelectronic materials [1, 2, 10, 11].
We have recently proposed a new concept for material design based on “element-blocks,” which are defined as a minimal functional unit composed of heteroatoms [12, 13]. Simultaneously, by combination and assembly of element-blocks, “element-block polymers” can be obtained, and the realization of new characteristics exceeding the monomer properties is expected. We have focused on boron-containing “element-block polymers” as a platform for constructing functional luminescent materials because of their versatile optical properties [14,15,16,17,18,19,20]. Near-infrared (NIR) light-absorbing polymers are useful for a wide variety of applications, such as photovoltaics and biological systems, and by employing BODIPY derivatives with narrow-band gaps as a monomer, the desired functions were obtained [21,22,23,24,25,26,27,28,29]. BODIPYs are also recognized as a promising candidate for realizing highly efficient NIR-absorbing polymers because of their intrinsic large molar extinct coefficient ε values and sharp absorption bands observed via spectroscopy [30]. Aza-BODIPYs with a nitrogen atom instead of carbon at the meso-position in the dipyrromethene ligand are a promising candidate for NIR dyes because of their intrinsic narrow band gaps [31,32,33,34]. According to investigations on electronic structures, it was suggested that π-conjugation can be expanded more effectively by introducing co-monomers at the bottom aryl groups [35]. Indeed, it was shown that the conjugated polymers containing aza-BODIPYs in their main-chains provided deeply redshifted absorption and emission bands compared to their monomers [36, 37]. Recently, thiophene-substituted aza-BODIPYs were reported, and their superior NIR absorption and emission properties were revealed [38,39,40,41,42,43]. To achieve deep NIR absorption by aza-BODIPYs, we have also designed and synthesized aza-BODIPYs substituted with furan instead of thiophene for improving molecular planarity [44]. From optical measurements, it was demonstrated that the absorption and emission bands were shifted to the longer wavelength region. In these studies, it was also proposed that the extension of π-conjugation should be responsible for narrowing band gaps. Based on these data, we presumed that further extension can be expected by employing conjugated polymers with aza-BODIPYs. Hence, we designed a conjugated polymer with thiophene-substituted aza-BODIPY.
Herein, the synthesis and photophysical properties of an alternating conjugated polymer comprising thiophene-substituted aza-BODIPY in the main-chain are reported for the first time. An aza-BODIPY monomer with four thiophenes in the dipyrromethene ligand and two bromines at the bottom thiophenes was prepared and polymerized via the Pd-catalyzed Suzuki–Miyaura coupling reaction. The polymer showed a considerably redshifted absorption band compared to that of the monomer in the NIR region. Evaluation of the energy levels of the polymer revealed that the narrower band gap of the polymer compared to that of the monomer unit originated from increasing only the HOMO level by polymerization.
Results and discussion
The synthetic route for the thiophene-substituted aza-BODIPY monomer (Br2-TTAB) is outlined in Scheme 1. Hetero-aldol condensation followed by conjugate addition proceeded quantitatively, and the brominated precursors 1 and 2 were obtained in good yields, while elimination of bromine atoms occurred during the reaction to afford 3. Additionally, it was difficult to isolate 3 from the mono- and non-substituted ligands. Thus, purification was performed after complexation with boron difluoride. Although the reaction yield was very low due to the generation of byproducts and the intrinsically low coupling yield, the dibromide monomer Br2-TTAB was successfully obtained. The product showed good stability toward air and moisture, and significant degradation was hardly observed under visible light irradiation. Moreover, owing to the good solubility of the monomer in common organic solvents such as chloroform, tetrahydrofuran (THF), and toluene, characterization and then polymerization were feasible.
The polymerization of the monomer (Br2-TTAB) with the fluorene co-monomer was achieved via the Pd-catalyzed Suzuki–Miyaura coupling reaction (Scheme 1). The polymer properties were effectively determined using size-exclusion chromatography calibrated with polystyrene standards in THF (Mn = 1500, Mw/Mn = 3.4). According to the molecular weights of the repeated units (aza-BODIPY: 518, fluorene: 499), it was observed that the products should be oligomers containing several repeating units. Although it was proposed that the effective conjugation length should be much longer than that of the synthesized polymer, we assumed that the lengths obtained were long enough for evaluating the influence of extending π-conjugation throughout the polymer main-chains on electronic properties. The polymeric products showed good stability and moderate solubility in organic solvents, allowing 1H and 11B NMR spectra to be obtained (Charts S1–S4). From the chemical shifts of the signal peaks in both NMR spectra, we concluded that the products after polymerization have the desired structure.
The optical properties of the synthesized polymers were investigated (Table 1). Tetrathienyl aza-BODIPY (TTAB, Fig. 1a) and the monomer (Br2-TTAB) were also analyzed for comparison. TTAB was prepared according to previous reports [38, 39]. Figure 1b shows the ultraviolet (UV)–visible (Vis) absorption spectra in THF (1.0 × 10−5 mol L-1). Both TTAB and Br2-TTAB showed strong and sharp absorption bands at approximately 750 nm derived from the S0 → S1 (π → π*) transition. The absorption band of the polymer p(TTAB-FL) was observed in a longer wavelength region than those of TTAB and Br2-TTAB. A strong S0 → S1 (π → π*) transition was observed in the NIR region with a peak at 864 nm. The peak wavelength was shifted by over 100 nm compared to those of the small molecules. Furthermore, significant absorption was detected even above 1300 nm. This result clearly indicates that conjugation should be efficiently extended throughout the fluorene copolymers. We also investigated the emissive properties of the polymer, and the emission intensity was under a detectable level, suggesting that the polymer has weak emission properties, possibly because of the heavy-atom effect as well as structural distortion of the polymer main-chains [44].
To investigate the electronic states, CV measurements were performed (Table 1, Fig. S1). All three compounds showed two reduction peaks. These results indicate that these BODIPY compounds can easily accept electrons. The LUMO energy levels were estimated from the onsets of the first reduction waves by an empirical formula [45]. The HOMO energy levels were calculated with the LUMO energy levels and optical energy band gaps (Egopt) of the corresponding compounds (Table 1). Accordingly, it was found that the LUMO energy level of the polymer was very similar to those of the monomer and TTAB. In contrast, the Egopt of the polymer was significantly smaller. These data indicate that the HOMO energy level was elevated by the extension of conjugation after polymerization and the resulting narrower band gap of the polymer. Finally, the absorption band could be detected in the longer wavelength region.
To gather detailed information on the electronic structures of the compounds, computer calculations were performed using density functional theory (DFT). FL-TTAB-FL, which is the alternative model of p(TTAB-FL) and TTAB, was evaluated by calculation (Fig. 2). It was clearly shown that all the HOMOs and LUMOs delocalized throughout the TTAB moieties. It should be noted that the HOMO of FL-TTAB-FL spread even to the fluorene units, whereas the LUMO seemed to be delocalized within the TTAB moieties. It was proposed that the electron-donating character of the fluorene units could be responsible for only the HOMO being extended. These results correspond to the CV data. It was suggested that the HOMO energy level of the polymer model became higher than that of TTAB by extending π-conjugation throughout the polymer main-chains. On the other hand, the LUMO energy level was slightly influenced by the co-monomer units.
Conclusion
A deep NIR-absorbing polymer with thiophene-substituted aza-BODIPY was synthesized via a conventional Pd-catalyzed cross-coupling reaction based on the strategy for expanding conjugated systems by employing polymer structures. The synthetic polymer showed a strong absorption band even in the region above 1300 nm. Furthermore, the LUMO energy level of the polymer was revealed from CV measurements, which showed that the low-lying LUMO of the thiophene-substituted aza-BODIPY was maintained after polymerization. If the polymer is used as an electron-carrier material in organic optoelectronic devices, modulation of each energy level should be essential for optimizing device efficiencies. In this study, it was demonstrated that only the HOMO level can be altered by polymerization. Thus, our findings and materials could be particularly applicable for fabricating efficient polymer-based electric devices.
References
Tanaka K, Chujo Y. Advanced luminescent materials based on organoboron polymers. Macromol Rapid Commun. 2012;33:1235–55.
Tanaka K, Chujo. Recent progress of optical functional nanomaterials based on organoboron complexes with β-diketonate, ketoiminate and diiminate. NPG Asia Mater. 2015;7:e223.
Yeo H, Tanaka K, Chujo Y. Effective light-harvesting antennae based on bodipy-tethered cardo polyfluorenes via rapid energy transferring and low concentration quenching. Macromolecules. 2013;46:2599–605.
Yamane H, Ito S, Tanaka K, Chujo Y. Preservation of main-chain conjugation through BODIPY-containing alternating polymers from electronic interactions with side-chain substituents by cardo boron structures. Polym Chem. 2016;7:2799–807.
Yoshii R, Yamane H, Tanaka K, Chujo Y. Synthetic strategy for low-band gap oligomers and homopolymers using characteristics of thiophene-fused boron dipyrromethene. Macromolecules. 2014;47:3755–60.
Kajiwara Y, Nagai A, Tanaka K, Chujo Y. Efficient simultaneous emission from RGB-emitting organoboron dyes incorporated into organic-inorganic hybrids and preparation of white light-emitting materials. J Mater Chem C. 2013;1:4437–44.
Yeo H, Tanaka K, Chujo Y. Tunable optical property between pure red luminescence and dual-emission depended on the length of light-harvesting antennae in the dyads containing the cardo structure of BODIPY and oligofluorene. Macromolecules. 2016;49:8899–904.
Yeo H, Tanaka K, Chujo Y. Energy transfer through heterogeneous dyes-substituted fluorene-containing alternating copolymers and their dual-emission properties. J Polym Sci A: Polym Chem. 2015;53:2026–35.
Yeo H, Tanaka K, Chujo Y. Synthesis of dual-emissive polymers based on ineffective energy transfer through cardo fluorene-containing conjugated polymers. Polym (Guildf). 2015;60:228–33.
Jäkle F. Lewis acidic organoboron polymers. Coord Chem Rev. 2006;250:1107–21.
Cheng F, Jäkle F. Boron-containing polymers as versatile building blocks for functional nanostructured materials. Polym Chem. 2011;2:2122–32.
Chujo Y, Tanaka K. New polymeric materials based on element-blocks. Bull Chem Soc Jpn. 2015;88:633–43.
Gon M, Tanaka K & Chujo Y. Recent progress in the development of advanced element-block materials. Polym. J. https://doi.org/10.1038/pj.2017.56.
Matsumoto T, Ito S, Tanaka K & Chujo Y. Synthesis, properties and structure of borafluorene-based conjugated polymers including kinetically and thermodynamically stabilized tetra-coordinated boron atoms. Polym. J. https://doi.org/10.1038/pj.2017.56.
Tanaka K, Yanagida T, Hirose A, Yamane H, Yoshii R, Chujo Y. Synthesis and color tuning of boron diiminate conjugated polymers with aggregation-induced scintillation properties. RSC Adv. 2015;5:96653–9.
Hirose A, Tanaka K, Yoshii R, Chujo Y. Film-type chemosensors based on boron diminate polymers having oxidation-induced emission properties. Polym Chem. 2015;6:5590–5.
Yoshii R, Hirose A, Tanaka K, Chujo Y. Functionalization of boron diiminates with unique optical properties: Multicolor tuning of crystallization-induced emission and introduction into the main-chain of conjugated polymers. J Am Chem Soc. 2014;136:18131–9.
Yoshii R, Nagai A, Tanaka K, Chujo Y. Boron ketoiminate-based polymers: fine-tuning of the emission color and expression of strong emission both in the solution and film state. Macromol Rapid Commun. 2014;35:1315–9.
Yoshii R, Tanaka K, Chujo Y. Conjugated polymers based on tautomeric units: Regulation of main-chain conjugation and expression of aggregation induced emission property via boron-complexation. Macromolecules. 2014;47:2268–78.
Tanaka K, Tamashima K, Nagai A, Okawa T, Chujo Y. Facile modulation of optical properties of diketonate-containing polymers by regulating complexation ratios with boron. Macromolecules. 2013;46:2969–75.
Kubo Y, Watanabe K, Nishiyabu R, Hata R, Murakami A, Shoda T, Ota H. Near-infrared absorbing boron-dibenzopyrromethenes that serve as light-harvesting sensitizers for polymeric solar cells. Org Lett. 2011;13:4574–7.
Gao L, Senevirathna W, Sauvé G. Azadipyrromethene-based conjugated oligomers with near-IR absorption and high electron affinity. Org Lett. 2011;13:5354–7.
Gao L, Tang S, Zhu L, Sauvé G. Synthesis and characterization of azadipyrromethene-alt-p-phenylene ethynylene conjugated polymers and their chelates. Macromolecules. 2012;45:7404–12.
Wu L, Burgess K. A new synthesis of symmetric boraindacene (BODIPY) dyes. Chem. Commun. 2008;4933–5.
Wu D, O’Shea DF. Synthesis and properties of BF2-3,3′-dimethyldiarylazadipyrromethene near-infrared fluorophores. Org Lett. 2013;15:3392–5.
Ni Y, Wu J. Far-red and near infrared BODIPY dyes: synthesis and applications for fluorescent pH probes and bio-imaging. Org Biomol Chem. 2014;12:3774–91.
Poirel A, Nicola AD, Retailleau P, Ziessel R. Oxidative coupling of 1,7,8-unsubstituted BODIPYs: Synthesis and electrochemical and spectroscopic properties. J Org Chem. 2012;77:7512–25.
Saino S, Saikawa M, Nakamura T, Yamamura M, Nabeshima T. Remarkable red-shift in absorption and emission of linear BODIPY oligomers containing thiophene linkers. Tetrahedron Lett. 2016;57:1629–34.
Shen B-x, Qian Y, Qi Z-q, Lu C-g, Sun Q, Xia X, Cui Y-p. Near-infrared BODIPY-based two-photon ClO− probe based on thiosemicarbazide desulfurization reaction: naked-eye detection and mitochondrial imaging. J Mater Chem B. 2017;5:5854–61.
Yoshii R, Nagai A, Tanaka K, Chujo Y. Highly NIR emissive boron di(iso)indomethene (BODIN)-based polymer: Drastic change from deep-red to NIR emission via quantitative polymer reaction. J Polym Sci Part A: Polym Chem. 2013;51:1726–33.
Gorman A, Killoran J, O’Shea C, Kenna T, Gallagher WM, O’Shea DF. In vitro demonstration of the heavy-atom effect for photodynamic therapy. J Am Chem Soc. 2004;126:10619–31.
Loudet A, Bandichhor R, Wu L, Burgess K. Functionalized BF(2) chelated azadipyrromethene dyes. Tetrahedron. 2008;64:3642–54.
Ge Y, O’Shea DF. Azadipyrromethenes: from traditional dye chemistry to leading edge applications. Chem Soc Rev. 2016;45:3846–64.
Wang J, Wu Y, Sheng W, Yu C, Wei Y, Hao E, Jiao L. Synthesis, structure, and properties of β-vinyl ketone/ester functionalized AzaBODIPYs from formylazaBODIPYs. ACS Omega. 2017;2:2568–76.
Yoshii R, Nagai A, Chujo Y. Highly near-infrared photoluminescence from aza-borondipyrromethene-based conjugated polymers. J Polym Sci A: Polym Chem. 2010;48:5348–56.
Yoshii R, Yamane H, Nagai A, Tanaka K, Taka H, Kita H, Chujo Y. π-Conjugated polymers composed of BODIPY or aza-BODIPY derivatives exhibiting high electron mobility and low threshold voltage in electron-only devices. Macromolecules. 2014;47:2316–23.
Tanaka K, Yanagida T, Yamane H, Hirose A, Yoshii R, Chujo Y. Liquid scintillators with near infrared emission based on organoboron conjugated polymers. Bioorg Med Chem Lett. 2015;25:5331–4.
Gresser R, Hartmann H, Wrackmeyer M, Leo K, Riede M. Synthesis of thiophene-substituted aza-BODIPYs and their optical and electrochemical properties. Tetrahedron. 2011;67:7148–55.
Zhang X, Yu H, Xiao Y. Replacing phenyl ring with thiophene: An approach to longer wavelength aza-dipyrromethene boron difluoride (aza-BODIPY) dyes. J Org Chem. 2012;77:669–73.
Liao J, Wang Y, Xu Y, Zhao H, Xiao X, Yang X. Synthesis, optical and electrochemical properties of novel meso-triphenylamine-BODIPY dyes with aromatic moieties at 3,5-positions. Tetrahedron. 2015;71:5078–84.
Feng Z, Jiao L, Feng Y, Yu C, Chen N, Wei Y, Mu X, Hao E. Regioselective and stepwise syntheses of functionalized BODIPY dyes through palladium-catalyzed cross-coupling reactions and direct C–H arylations. J Org Chem. 2016;81:6281–91.
Budhiraja A, Kadian K, Kaur M, Aggarwal V, Garg A, Sapra S, Nepali K, P. Suri O, Dhar KL. Synthesis and biological evaluation of naphthalene, furan and pyrrole based chalcones as cytotoxic and antimicrobial agents. Med Chem Res. 2011;21:2133–40.
Bellier Q, Dalier F, Jeanneau E, Maury O, Andraud C. Thiophene-substituted aza-BODIPY as a strategic synthon for the design of near-infrared dyes. New J Chem. 2012;36:768–73.
Yamane H, Ohtani S, Tanaka K, Chujo Y. Synthesis of furan-substituted aza-bodipys having strong near-infrared emission. Tetrahedron Lett. 2017;58:2989–92.
Chen C-P, Chan S-H, Chao T-C, Ting C, Ko B-T. Low-bandgap poly(thiophene-phenylene-thiophene) derivatives with broaden absorption spectra for use in high-performance bulk-heterojunction polymer solar cells. J Am Chem Soc. 2008;130:12828–33.
Acknowledgements
This work was partially supported by the Mitsubishi Foundation (for KT) and a Grant-in-Aid for Scientific Research on Innovative Areas “New Polymeric Materials Based on Element-Blocks (No.2401)” (JSPS KAKENHI Grant Number JP24102013).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yamane, H., Tanaka, K. & Chujo, Y. Synthesis of a near-infrared light-absorbing polymer based on thiophene-substituted Aza-BODIPY. Polym J 50, 271–275 (2018). https://doi.org/10.1038/s41428-017-0014-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-017-0014-6