Abstract
Diabetes mellitus is becoming increasingly prevalent worldwide and needs effective clinical treatment methods. β-Cell replacement therapy has become a safe alternative for diabetes treatment in recent years, and encapsulation methods have been proposed to facilitate this type of therapy. Here, we used coaxial microfluidic electrospray technology to generate microcapsules allowing high cell viability (>90%) with porous alginate shells and β cell-containing cores in less than half an hour. Benefitting from microfluidic electrospray, the sizes of the generated microcapsules were adjustable. The biocompatible porous hydrogel shell not only protected β cells from immune rejection but also allowed the exchange of small molecular nutrients during transplantation, and the liquid core guaranteed the high viability of the encapsulated cells. This constructed living cell biosystem further demonstrated its potential as an artificial islet after transplantation into the omental pouches of diabetic mice to control blood glucose levels and thus treat diabetes. We consider that this system, with an elaborate structure and an abundance of highly viable encapsulated β cells to improve treatment performance, could be applied in a wide range of clinical situations.
Similar content being viewed by others
Introduction
Diabetes is a global disease that has caused great problems1. Recently, oral agents, insulin injections, and β cell transplantation have become available for diabetes mellitus treatment2,3. The long-term use of oral agents and insulin increases the burden on patients with diabetes while direct implantation of β cells causes immunological rejection as well as graft apoptosis4. Therefore, biocompatible hydrogels have been introduced to prevent transplanted cells from immune rejection5,6. Three main methods have been proposed for cell encapsulation, including utilizing porous devices, hydrogel encapsulation, and thin polymer coating7,8,9,10,11,12. Although each has received clear and pleasant success, these approaches lead to either a fibrotic response or unsatisfactory viability of the encapsulated cells13. In addition, the resultant materials with common homogeneous structures lack the ability to exchange metabolites, such as oxygen, glucose, and insulin, making the long-term performance of encapsulated cells difficult14,15. Therefore, a novel encapsulation approach that guarantees cell viability for treatment is urgently needed.
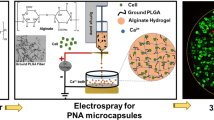
In this paper, we present novel porous microcapsules encapsulating β cells for diabetes treatment by using coaxial microfluidic electrospray technology, as schemed in Fig. 1. Microfluidic electrospray is an advanced technology used for the mass production of materials with controllable morphologies and desired features due to its ability to simultaneously operate multiple kinds of fluids in small channels16,17,18,19,20. A multitude of functional materials have been created through this amazing technology, such as microspheres, capsules, fibers, and rod-shaped hydrogels21,22. These materials have found important applications in three-dimensional (3D) cell culture, drug delivery, and biomaterials23,24,25,26. Although there have been many successes, most microfluidic electrospray methods focus on the direct encapsulation of cells, where cell viability usually fails to satisfy the needs of the application because the hydrogel framework restricts the growth and migration of cells as well as intercellular communication27,28,29. In addition, the compact shell prevents the inner cells from receiving small molecular nutrients, limiting the long-term viability of these cells and the expected outcomes in diabetes treatment30. Thus, microcapsules encapsulating highly viable cells for diabetes treatment are desired.
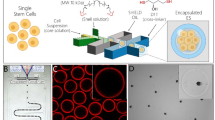
a Porous microcapsules fabricated via the microfluidic electrospray system. b The β cell-encapsulated microcapsules isolate immune cells, release insulin, and exchange oxygen as well as glucose. c The β cell-encapsulated porous microcapsules were applied to treat diabetes in diabetic mice after omentum transplantation.
Here, we used a simple microfluidic electrospray technique to fabricate porous hydrogel microcapsules encapsulating β cells. The porous hydrogel shell was made from a mixture of sodium alginate (ALG) and polyethylene oxide (PEO), where the PEO worked as a pore-making agent because it is not crosslinked during gelation of the alginate hydrogel. The inner liquid core consisted of a solution of β cells dispersed in sodium carboxymethyl cellulose (CMC). Taking advantage of the microfluidic technique, the produced microcapsules had a uniform structure that could be further tailored by tuning different parameters during the electrospray process. In addition to the liquid core of the microcapsule providing a 3D culture environment for the encapsulated β cells, the porous shell protected these cells from immunological rejection during transplantation. It should be noted that the porous shell structure allows the exchange of small molecules that are necessary for β cells. As a result, the cells showed good activity and function after their encapsulation. The application value of this constructed living cell system was further demonstrated by employing the microcapsules as artificial islets to control blood glucose levels and treat diabetes in mice. Thus, all of the stated features demonstrate that porous microcapsules encapsulating β cells show distinctive potential in diabetes treatment, making them promising candidates for further clinical research.
Experimental section
Materials, cell lines, and animals
ALG, CMC, and CaCl2 were purchased from Aladdin. The calcein-AM and propidium iodide staining kits was purchased from Invitrogen. Trypsin-EDTA solution and phosphate-buffered saline (PBS) were from Gibco (Grand Island, NY). MTT powders were purchased from J&K Scientific Ltd., Shanghai. Dimethyl sulfoxide (DMSO) was purchased from Sigma, USA. The insulin-producing β cell line was obtained from the American Type Culture Collection (ATCC). These cells were cultured in RPMI-1640 medium with L-glutamine (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA), 10 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 50 μmol/L β-mercaptoethanol, 1 mmol/L sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified 5% CO2 incubator. The medium was replaced every other day. Eight- to ten-week-old male C57BL/6 mice were obtained from Shanghai Sipo-Bikai Laboratory Animal Co., Ltd. (SCXK2018-0006). All animals were treated in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, USA. The experimental protocols and care of all animals were reviewed and approved by the Animal Investigation Ethics Committee of ZhongDa Hospital.
Microfluidic device design
The microfluidic electrospray device included a glass slide, two capillary tubes with different inner diameters, and two connectors used for fixing and connecting. The diameter of the outer glass capillary was 500 μm and the inner diameter was 300 μm, tapered by a capillary puller (P-97, Sutter Instrument) and sanded to the desired orifice diameter. The coaxial microfluidic electrospray system was assembled by inserting the tapered capillary into a cylindrical capillary. A needle with two crevices at the bottom was used at the connection between the inner and outer capillary channels. Afterward, a transparent epoxy resin (Devcon 5 min Epoxy) was used to seal where necessary.
Fabrication of the porous microcapsules via microfluidic electrospray and their characterization
Typically, ALG (0.2 g) was dissolved in 10 mL of ddH2O and stirred at 37 °C for 4 h to ensure full dissolution. Then, a 1% (w/v) PEO solution was added dropwise into the ALG solution at a volume ratio of 1:1. The mixture was stirred at 40 °C with a spinning speed of 400 rpm for 8 h. CMC (0.1 g) was dissolved in 10 mL of ddH2O and stirred at 37 °C for 2 h to ensure full dissolution. Two syringe pumps were used to push the core fluid of 1% (w/v) carboxymethyl cellulose sodium and shell fluid of 1% (w/v) alginate sodium with 0.5% (w/v) PEO through the concentric inner (300 μm) and outer (500 μm) lumens in the coaxial needle, respectively. Under an open electric field from a voltage generator, concentric drops of the two coaxial fluids at the needle tip were broken up into microdrops and sprayed into the gelling bath containing 100 mM calcium chloride to instantly gel the alginate in the shell fluid before the two fluids were mixed. Bright-field images of the porous microcapsules were observed by microscopy (OLYMPUS IX71) and recorded with CCD cameras (DP30BW). The prepared microcapsules were frozen overnight and then freeze-dried under the vacuum for 24 h. Then, the microstructures of the porous microcapsules were characterized by scanning electron microscopy (SEM; HITACHI, S3000N).
Biocompatibility of the porous microcapsules
The viability and proliferation of β cells with or without the porous microcapsules were evaluated with a [3-(4,5)-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. First, the β cells were transferred to clean unused 96-well plates (Thermo, USA) at a density of 2 × 104 cells/well. The porous microcapsules were prepared, and those with uniform size were selected under the microscope. Microcarriers (1 mg) were added to each well for one group, while the other group served as a control group. Briefly, the microcapsules were cocultured with β cells in the experimental group, and β cells without microcapsules were cultured as a control for 3 days. Every group had three parallels. Bright-field images of the β cells in the two groups were recorded by microscopy every day. The MTT solution was prepared at a concentration of 5 mg/mL in PBS. Then, the culture medium with 10% MTT was added to each of the two groups of cells. After incubation at 37 °C for 3–4 h, the medium was removed, and 100 µL of DMSO was added to dissolve the MTT formazan crystals. Then, the corresponding OD value was measured with a microplate reader (SYNERGY HTX) at a wavelength of 570 nm. Cell viability in both groups from Day 1 to Day 3 was determined by MTT assay.
Cells-encapsulated porous microcapsules prepared via microfluidic electrospray
CMC (1%, w/v) was mixed with β cells (2 × 106 cells/ml) to make microcapsules with a liquid core. The shell fluid consisted of 1% (w/v) sodium alginate with 0.5% (w/v) PEO. The gelling bath contained 100 mM calcium chloride. All solutions were filtered through a 0.22 μm micron filter before being used for encapsulation. The chip and pipes were washed with and soaked in alcohol and penicillin–streptomycin solution. After encapsulation, the microcapsules containing β cells in the gelling solution were washed with medium three times and suspended in β cell medium after calcium removal for further culture.
Porous microcapsule permeability test
To more intuitively determine whether insulin could penetrate through the porous microcapsules, isothiocyanate-labeled insulin (insulin-FITC, final concentration of 1.5 mg/mL) was used with CMC solution as the inner phase, and ALG solutions with different concentrations were used as the outer phase. Then, the release of fluorescence at 0, 30, and 60 min was recorded with a fluorescence microscope (Carl Zeiss, Germany). Different molecular weight substances, such as fluorescein sodium salt fluorescein (NaFI), fluorescein isothiocyanate-labeled insulin (insulin-FITC), and fluorescein isothiocyanate-labeled bovine serum albumin (BSA-FITC), were used with the microcapsules. Approximately 50 microcapsules were placed into culture dishes, and fixed amounts of NaFI, insulin-FITC, or BSA-FITC solution was added (final concentration of 2 mg/mL). Finally, the green fluorescence of the microcapsules was observed and recorded by fluorescence microscopy at 0, 5, 10, 20, 40, 60, and 90 min.
Cell viability, proliferation, and function
The viability of the encapsulated β cells was determined using a live/dead assay kit. The proliferation of the encapsulated β cells was monitored by observing the formation of cell aggregates in the microcapsule core. The whole-cell experiment lasted for seven days. A total of 2 μL of calcein-AM and 2 μL of propidium iodide solution were added to 1 mL of cell medium containing the β cells microcapsules. After 20 min of incubation at 37 °C in the dark, the β cells in the microcapsules were imaged under a fluorescence microscope (Carl Zeiss, Germany). Then, by staining the β cell aggregates that had formed in the liquid core after culturing for 1, 3, 5, and 7 days with calcein-AM and propidium iodide, we obtained fluorescence images of the cells in the microcapsules. The function of the aggregated β cells on Day 7 was examined based on basic insulin secretion in low-glucose states. After preconditioning in a low glucose medium for 1 h, the β cells were washed twice with PBS and cultured in a low glucose medium for another 1 h. The supernatant was retained to detect the insulin concentration by RIA as described previously31.
Animal study of the porous microcapsules encapsulating β cells
Finally, an in vivo experiment was conducted to investigate the practical value of these microcapsules in streptozotocin (STZ, 150 mg/kg)-induced diabetic mice (C57BL/6 mice) on the basis of their desirable biocompatibility, good molecular release and immune isolation32. Twenty-four mice were included in the experiment. Type 1 diabetes was successfully induced in 18 mice by a single i.p. injection of STZ. The diabetic mice were randomly divided into three groups: the control group, β cells group, and β cells-Mi group. For omental pouch transplantation, a median abdominal incision was made, and the greater omentum was spread out onto wet gauze33. The control group was treated as a sham-operated diabetes group, whereas the β cells group received 2 × 106 β cells and the β cells-Mi group received microcapsules encapsulating 2 × 106 β cells in the omental pouch. Thereafter, the body weights and blood glucose levels of the mice were recorded every other day with a weight scale and blood glucose meter. Mice were intraperitoneally administered glucose injection one week after transplantation. Mice then fasted for 12 h, and the diabetic mice in each group were treated with an intraperitoneal injection of 15% glucose. Blood glucose levels were measured at 0, 15, 30, 60, 90, and 120 min. The area under the curve between 0 and 120 min was calculated to indicate the maintenance of glucose homeostasis in the different groups. Mice were sacrificed after 28 days of treatment. The omentums and pancreases of the mice were removed and fixed for 24–48 h. Then hematoxylin and eosin (H&E) and immunofluorescent stainings were carried out. Representative immunofluorescence staining for insulin (β cell marker) and glucagon (α cell marker) in the pancreas were carried out to indicate the destruction of islet β cells in diabetic mice, using normal mouse islets as a control.
Statistical analysis
All statistical analyses were conducted with SPSS 20.0. The data are presented as the mean ± standard deviation. The statistical significance of the differences was determined by t-test. P < 0.05 was considered significant.
Results and discussion
Synthesis of the porous microcapsules
In this experiment, porous microcapsules were fabricated with a coaxial microfluidic electrospray system. Because of the hydrodynamic focusing effect and low Reynolds number, the inner liquid formed laminar flow and was sheathed by the outer stream of alginate at the merging point of the two fluids (Fig. 2a and Fig. S1, Supplementary Materials). Finally, the coaxial fluid was broken up into droplets under the outer electric field and sprayed into the calcium chloride (CaCl2) solution. The fast diffusion of Ca2+ instantly solidified the ALG to form microcapsules. After further gelation of the alginate in the calcium chloride collection pool, the core-shell structured microcapsules were obtained. A large number of CMC-ALG microcapsules showed smooth surfaces and core-shell structures (Fig. 2d–f). In detail, scanning electron microscopy (SEM) images of the porous microcapsules displayed their porous shell and inner core (Fig. 2b, c). The porous structure of the shell was attributed to PEO, which was not crosslinked during the reaction between ALG and calcium ions. In addition, by dynamically regulating the parameters of the microfluidic electrospray, the size and the core-shell structure of the microcapsule was precisely controlled to endow microcapsules with different sizes (Fig. 2d–f and Fig. S2, Supplementary Materials). With increasing collection distance, flow rate of CMC, and concentration of ALG, the size of the microcapsules increased. However, when the concentration of ALG increased to 2%, the resulting microcapsules were unstable because the solution was too viscous. A negative correlation was observed between microcapsule size and electric field strength. Notably, due to the advanced and precise control of these parameters, the microcapsules had a narrow size distribution (Fig. 2g–i).
a The formation of CMC-ALG droplets during the microfluidic electrospray process. b, c Scanning electron microscopy (SEM) images of the microcapsules showing the core and shell structures. d–f Bright-field microscopic images of the CMC-ALG core-shell microcapsules with different device orifice diameters. g–i Corresponding diameter distributions of the CMC-ALG porous microcapsules; 100 microcapsules were measured for each map. Scale bars are 200 μm.
Preparation of the cell-laden porous microcapsules and in vitro culture
To investigate their biomedical application potential, the biocompatibility of these porous microcapsules was first estimated. The viabilities of the β cells cultured with (microcapsule group) or without (control group) the microcapsules were evaluated using the [3-(4,5)-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. During 3 days of cultivation, cell viability in the microcapsule group was not significantly different from that in the control group; both groups of cells grew well, as observed from the microscope images and the results of the MTT assay (Fig. S3, Supplementary Materials). These results proved the good biocompatibility of the porous microcapsules, which laid the foundation for subsequent cell encapsulation and in vivo diabetes treatment. Then, cell-laden porous microcapsules were made by dispersing a sufficient number of β cells into the inner phase. To acquire vivid images of the cells encapsulated in the porous microcapsules, calcein-AM and propidium iodide were used to stain the cells. The cells showed good viability and proliferation during cultivation (Fig. 3a). These observations were further demonstrated by statistical analysis of cell viability and fluorescence intensity, the former of which suggested that over 90% of the encapsulated cells had good viability and the latter showed an increase in fluorescence intensity, which indicated an increasing number of cells (Fig. 3b, c). All of these results demonstrated that β cells encapsulated in the porous microcapsules could maintain good viability and basic activity.
a Microscopic images and representative fluorescence microscopic images of β cells at 1, 3, 5, and 7 days after microencapsulation (green: live, red: dead). Scale bars are 200 μm. b Corresponding statistical graph of relative cell viability (live and dead). c Green fluorescence intensity of the β cells in the porous microcapsules after different lengths of cell cultivation.
In addition to good biocompatibility, porous microcapsules are believed to allow the exchange of small molecules that are necessary for β cells growth. To examine whether insulin could penetrate through the shell of the porous microcapsules, isothiocyanate-labeled insulin (insulin-FITC) was added to the CMC solution to form the core structure, and different concentrations of ALG were chosen to prepare the shell. After generation, the microcapsules were observed at different time points by fluorescence microscopy (Fig. 4a). It was inferred that the microcapsules showed good insulin permeability at different ALG concentrations. Moreover, with increasing ALG concentration, the fluorescence intensity showed a corresponding enhancement, indicating the slower release of insulin. As the concentration of the shell solution increased, the ability of the same substance to pass through the shell decreased. This phenomenon could be ascribed to the increase in ALG concentration increasing the cross-linking density and narrowing the molecular gap. In addition to the release ability, the permeability of the microcapsules was also investigated. Fluorescein sodium salt fluorescein (NaFI), isothiocyanate-labeled insulin (insulin-FITC) and bovine serum albumin (BSA-FITC) were used to treat the microcapsules to observe the permeability of substances with different molecular weights by fluorescence microscopy (Fig. S4, Supplementary Materials). The fluorescence intensities of the different groups at different time points were measured, and the results are shown in the chart in Fig. 4b. NaFI (MW 376 Da) penetrated the porous microcapsules easily, but BSA (MW 66.0 kDa) encountered more difficulty than insulin (MW 5.8 kDa) due to its relatively larger molecular weight. Furthermore, the insulin secreted from β cells after permeation through the alginate shell was examined on the 7th day (Fig. 4c). The encapsulated cells released the same amount of insulin as the unencapsulated cells under low glucose conditions, possibly because insulin was easily released from the sodium alginate shell.
a Fluorescence images of different concentrations (0.5%, 1%, 1.5% w/v) of ALG at the same time points: t1, t2, and t3 (0, 30, and 60 min), respectively. b Corresponding fluorescence intensity statistics of the microcapsules tested for membrane permeability using fluorescein sodium salt (MW 376 Da; NaFI), insulin-FITC (MW 5.8 kDa; insulin-FITC), and bovine serum albumin (MW 66.0 kDa; BSA). c The amount of insulin secreted from encapsulated β cells, unencapsulated β cells, and empty microcapsules as a control on the 7th day. The scale bar is 200 μm.
Transplantation of the cell-laden porous microcapsules into diabetic mice
The potential of these porous microcapsules encapsulating β cells for diabetes treatment was finally investigated by employing them as artificial islets to control blood glucose levels in diabetic mice. First, a diabetic mouse model was constructed in C57BL/6 mice by administration of streptozotocin (STZ). The mice were randomly divided into three groups, β cells group, β cells-Mi group, and the control group, referring to the groups receiving β cells, β cell microcapsules, and sham surgery, respectively. The process of implanting the β cells microcapsules is shown in Fig. 5a and Fig. S5, Supplementary Materials. Then, the blood glucose levels and body weights of the mice were recorded (Fig. 5b, c). The control group mice maintained a high blood glucose level and displayed significant weight loss, which was consistent with the diabetic status. The β cells group exhibited partial reversal of the hyperglycemic conditions within 3 days after transplantation, but hyperglycemia later returned during Days 3–7, and the mice lost more bodyweight than the mice in the other groups. In contrast, the β cells-Mi group of mice that received an equal number of β cells encapsulated in microcapsules experienced normoglycemia and gained some weight after transplantation. These results demonstrated that direct transplantation of β cells may lead to immune rejection, resulting in weight loss in mice, while β cells encapsulated in microcapsules mitigated immune rejection to steadily lower blood glucose to almost normal levels through the shielding effect of the hydrogel shell. In addition, the intraperitoneal glucose tolerance test showed that the mice in the β cells-Mi group had decreased blood glucose levels after 30 min compared to the other groups (Fig. 5d). Additionally, the area under the curve values presented a significant difference between the β cells-Mi group and the control group at 2 h post-glucose challenge, and there was also a significant difference between the β cells-Mi group and the β cells group (Fig. 5e). These results illustrated that the mice treated with β cells microcapsules had good glucose tolerance, which should be ascribed to the sustainable release of insulin from the porous microcapsules.
a Schematic diagram of the effect of transplantation therapy. b, c Blood glucose levels and body weights after transplantation. *p < 0.05, **p < 0.01, ***p < 0.001. d Glucose tolerance test in diabetic mice in different groups 2 h post-administration. e Responsiveness was calculated based on the area under the curve (AUC) at 120 min.
To intensively study diabetes treatment performance, histological evaluations were also carried out. H&E staining of the omentum and immunofluorescence staining of the pancreas (glucagon: α cell, insulin: β cell) were performed. The diabetic mice showed extensive destruction of islet β cells in the pancreas compared to normal mice (Fig. S6, Supplementary Materials). The H&E images of the omentum tissue of the control group mice were normal, and adipose tissue and blood vessels were clearly observed (Fig. 6a). A large number of cells and severe adhesions between tissues were found in the β cells group (Fig. 6b). In the β cells-Mi group, β cell-laden microcapsules were discovered, as indicated by the red arrow in the picture (Fig. 6c). This result indicated that the β cells were completely encapsulated in the microcapsules and survived. The H&E results of omentum tissue from the mice in the β cells-Mi group were almost the same as those of the control group without transplantation. However, the β cells group displayed extensive cellular infiltration into the omentum tissue, which may be caused by an inflammatory response.
Conclusion
In conclusion, we proposed novel porous microcapsules encapsulating β cells for diabetes treatment by using microfluidic electrospray technology. During preparation, a coaxial capillary device was employed to fabricate the microcapsules with porous hydrogel shells and liquid cores containing β cells. The porous hydrogel shell was made from a mixture of ALG and PEO, and the inner liquid core was a cell suspension with CMC. The co-flow was broken into droplets driven by an outer electric field and fell into a CaCl2 solution. Because the gelation of ALG occurred quickly, the porous shell of the microcapsules was instantly obtained, protecting the encapsulated cells from immune cell attack during transplantation and allowing the exchange of small molecules necessary for β cells survival and β cell secretions. Moreover, the inner liquid core provided β cells in a 3D culture environment in which the encapsulated cells maintained good activity and function. Taking advantage of the microfluidic electrospray method, the porous microcapsules had tunable morphologies allowing the intelligent, controllable, and programmable release of insulin and other kinds of small molecules. With a biomedically relevant dose, the released insulin showed satisfactory anti-diabetic functions. The practical value of the resulting porous microcapsules encapsulating β cells also showed improvements in blood glucose levels and body weights among treated mice. These features indicate that porous microcapsules encapsulating β cells are efficient for diabetes treatment, and thus, we believe that this method will be widely used in the clinic based on its excellent capabilities.
References
IDFATLAS Ninth edition IDFATLAS9e-final-web (2020).
Bluestone, J. A., Herold, K. & Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464, 1293–1300 (2010).
Vantyghem, M.-C., de Koning, E. J. P., Pattou, F. & Rickels, M. R. Advances in β-cell replacement therapy for the treatment of type 1 diabetes. Lancet 394, 1274–1285 (2019).
McCoy, R., Van Houten, H., Ziegenfuss, J., Shah, N., Wermers, R. & Smith, S. J. D. C. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 35, 1897–1901 (2012).
Bose, S., Volpatti, L. R., Thiono, D., Yesilyurt, V., McGladrigan, C. & Tang, Y. et al. A retrievable implant for the long-term encapsulation and survival of therapeutic xenogeneic cells. Nat. Biomed. Eng. 4, 814–826 (2020).
Mitragotri, S., Anderson, D., Chen, X., Chow, E., Ho, D. & Kabanov, A. et al. Accelerating the translation of nanomaterials in biomedicine. ACS Nano. 9, 6644–6654 (2015).
Leijten, J., Rouwkema, J., Zhang, Y. S., Nasajpour, A., Dokmeci, M. R. & Khademhosseini, A. Advancing tissue engineering: a tale of nano-, micro-, and macroscale integration. Small 12, 2130–2145 (2016).
Kobayashi, T., Aomatsu, Y., Iwata, H., Kin, T., Kanehiro, H. & Hisanaga, M. et al. Indefinite islet protection from autoimmune destruction in nonobese diabetic mice by agarose microencapsulation without immunosuppression. Transplantation 75, 619–625 (2003).
Jeyhani, M., Mak, S. Y., Sammut, S., Shum, H. C., Hwang, D. K. & Tsai, S. S. H. Controlled electrospray generation of nonspherical alginate microparticles. Chemphyschem 19, 2113–2118 (2018).
Zhao, X. & Cui, W. Disease-triggered hydrogel therapy. Mater. Today 18, 56–57 (2015).
Wang, L., Liu, H., Zhang, F., Li, G. & Wang, S. Smart thin hydrogel coatings harnessing hydrophobicity and topography to capture and release cancer cells. Small 12, 4697–4701 (2016).
Yang, J., Zhang, Y. S., Yue, K. & Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 57, 1–25 (2017).
Ryan, A. J., O’Neill, H. S., Duffy, G. P. & O’Brien, F. J. Advances in polymeric islet cell encapsulation technologies to limit the foreign body response and provide immunoisolation. Curr. Opin. Pharmacol. 36, 66–71 (2017).
Jacobs-Tulleneers-Thevissen, D., Chintinne, M., Ling, Z., Gillard, P., Schoonjans, L. & Delvaux, G. et al. Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia 56, 1605–1614 (2013).
Zhang, Y. S. & Khademhosseini, A. Advances in engineering hydrogels. Science. 356, 500 (2017).
Zhao, S., Agarwal, P., Rao, W., Huang, H., Zhang, R. & Liu, Z. et al. Coaxial electrospray of liquid core–hydrogel shell microcapsules for encapsulation and miniaturized 3D culture of pluripotent stem cells. Integr. Biol. 6, 874–884 (2014).
Chen, G., Y. Yu, X. Wu, G. Wang, G. Gu, F. Wang. et al. Microfluidic electrospray niacin metal-organic frameworks encapsulated microcapsules for wound healing. Research. 6175398 (2019).
Li, Z., Mak, S. Y., Sauret, A. & Shum, H. C. Syringe-pump-induced fluctuation in all-aqueous microfluidic system implications for flow rate accuracy. Lab Chip. 14, 744–749 (2014).
Liu, D., Zhang, H., Fontana, F., Hirvonen, J. T. & Santos, H. A. Microfluidic-assisted fabrication of carriers for controlled drug delivery. Lab Chip. 17, 1856–1883 (2017).
Zhao, C., Chen, G., Wang, H., Zhao, Y. & Chai, R. Bio-inspired intestinal scavenger from microfluidic electrospray for detoxifying lipopolysaccharide. Bioact. Mater. 6, 1653–1662 (2021).
Dong, R., Liu, Y., Mou, L., Deng, J. & Jiang, X. Microfluidics-based biomaterials and biodevices. Adv. Mater. 31, e1805033 (2019).
Chao, Y. & Shum, H. C. Emerging aqueous two-phase systems: from fundamentals of interfaces to biomedical applications. Chem. Soc. Rev. 49, 114–142 (2020).
Huang, G., Li, F., Zhao, X., Ma, Y., Li, Y. & Lin, M. et al. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem. Rev. 117, 12764–12850 (2017).
Lin, K., Zhang, D., Macedo, M. H., Cui, W., Sarmento, B. & Shen, G. Advanced collagen-based biomaterials for regenerative biomedicine. Adv. Funct. Mater. 29, 1804943 (2019).
Facklam, A. L., Volpatti, L. R. & Anderson, D. G. Biomaterials for personalized cell therapy. Adv. Mater. 32, e1902005 (2020).
Gao, B., Yang, Q., Zhao, X., Jin, G., Ma, Y. & Xu, F. 4D bioprinting for biomedical applications. Trends Biotechnol. 34, 746–756 (2016).
Wu, D., Yu, Y., Zhao, C., Shou, X., Piao, Y. & Zhao, X. et al. NK-cell-encapsulated porous microspheres via microfluidic electrospray for tumor immunotherapy. ACS Appl Mater. Interfaces 11, 33716–33724 (2019).
Zhao, C., Yu, Y., Zhang, X., Wu, X., Ren, J. & Zhao, Y. Biomimetic intestinal barrier based on microfluidic encapsulated sucralfate microcapsules. Sci. Bull. 64, 1418–1425 (2019).
Nie, M., Chen, G., Zhao, C., Gan, J., Alip, M. & Zhao, Y. et al. Bio-inspired adhesive porous particles with human MSCs encapsulation for systemic lupus erythematosus treatment. Bioact. Mater. 6, 84–90 (2021).
Ying, G. L., Jiang, N., Maharjan, S., Yin, Y. X., Chai, R. R. & Cao, X. et al. Aqueous two-phase emulsion bioink-enabled 3D bioprinting of porous hydrogels. Adv. Mater. 30, e1805460 (2018).
Meng, Z. X., Sun, J. X., Ling, J. J., Lv, J. H., Zhu, D. Y. & Chen, Q. et al. Prostaglandin E2 regulates Foxo activity via the Akt pathway: implications for pancreatic islet beta cell dysfunction. Diabetologia 49, 2959–2968 (2006).
Furman, B. L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 70, 5.47.1–5.47.20 (2015).
Ibarra, V., Appel, A. A., Anastasio, M. A., Opara, E. C. & Brey, E. M. This paper is a winner in the Undergraduate category for the SFB awards: evaluation of the tissue response to alginate encapsulated islets in an omentum pouch model. J. Biomed. Mater. Res A. 104, 1581–1590 (2016).
Acknowledgements
This study was supported by Professor Ling Li in the Department of Endocrinology, Zhongda Hospital, School of Medicine, Southeast University with grants from the National Natural Science Foundation of China (Nos. 81570739, 81970717, 82170845), the Key Research and Development Program of Jiangsu Province (No. BE2018742), and the Joint Key Project funded by the Southeast University and Nanjing Medical University (No. 2242019K3DN07). All funders had a role in the design of the study, analysis, interpretation of the data, and writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Q.W., Y.J.Z., S.J.P., and L.L. conceived the idea and designed the experiments; X.Y.L. conducted the experiments and data analysis; X.Y.L., Y.R.Y., and Y.J.Z. wrote the manuscript; and D.C.L., J.B.L., and J.S. contributed to scientific discussions regarding this article.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, X., Yu, Y., Liu, D. et al. Porous microcapsules encapsulating β cells generated by microfluidic electrospray technology for diabetes treatment. NPG Asia Mater 14, 39 (2022). https://doi.org/10.1038/s41427-022-00385-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41427-022-00385-5