Abstract
Macrophages are critical regulators of tissue homeostasis but are also abundant in the tumor microenvironment (TME). In both primary tumors and metastases, such tumor-associated macrophages (TAMs) seem to support tumor development. While we know that TAMs are the dominant immune cells in the TME, their vast heterogeneity and associated functions are only just being unraveled. In this review, we outline the various known TAM populations found thus far and delineate their specialized roles associated with the main stages of cancer progression. We discuss how macrophages may prime the premetastatic niche to enable the growth of a metastasis and then how subsequent metastasis-associated macrophages can support secondary tumor growth. Finally, we speculate on the challenges that remain to be overcome in TAM research.
Similar content being viewed by others
Introduction
Macrophages are tissue-resident immune cells that emerge from multiple waves of hematopoiesis during embryonic development to seed their organs of residency [1]. In mice, primitive myeloid progenitors arise in the yolk sac around embryonic Day E7-8 and give rise to microglia, which are self-maintained locally from this initial seeding, independent of circulating monocytes [2]. A second semi-definitive wave of hematopoiesis starts at E8.25 and gives rise to progenitors that transiently shelter in the fetal liver, differentiate into monocyte-like cells [3, 4] that subsequently colonize fetal tissues to give rise to these cells are known as resident tissue macrophages (RTMs). A final, third wave of hematopoietic precursors emerges from the aorta-gonado-mesonephros region at E10.5 and leads to the generation of hematopoietic stem cells that will later establish definitive hematopoiesis in the fetal liver and then in the bone marrow. Monocytes from this third wave are recruited to tissues from late embryonic stages to adulthood, thus somewhat diluting the preexisting embryonic RTMs in a tissue-dependent manner [5]. Thus, contrary to early consensus [6], many RTMs found in adult tissues are long-lived cells with embryonic origins.
During organogenesis, macrophages undergo tissue imprinting whereby embryonic progenitors first acquire a core macrophage differentiation program including pattern recognition and cytokine receptors [7]. Then, tissue-specific programs emerge during embryonic development with the differential activation of transcription factors and gene networks [7]. Such tissue imprinting is not an event restricted to embryonic development: as we draw on in this review, the relatively long lifespan of RTMs means that they are inevitably exposed to both non-homeostatic events, such as inflammation or infection, and systemic signals. The somewhat continuous imprinting that ensues as a result of a dynamically altered niche can lead to RTM dysregulation, which in turn might favor oncogenesis [8].
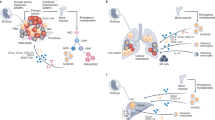
In the most basic sense, oncogenesis occurs as a result of an accumulation of mutations in oncogenes that permit normal cells to overcome restrictions on cellular replication such that they can grow without restraint to form a tumor. However, it is not only the tumor that has pathological consequences; other components that form the tumor microenvironment (TME) influence oncogenesis and cancer progression [9]. The TME comprises the blood and lymphatic vessels, extracellular matrix (ECM), and distinct host cells, including fibroblasts and immune cells, in the immediate ecosystem that surrounds the tumor, in addition to the tumor cells themselves [10]. Among the immune cellular components of the TME, macrophages have received particular attention. These so-called tumor-associated macrophages (TAMs) are typically the most abundant immune population within the TME, and their abundance is in fact now leveraged as a diagnostic marker, as it often correlates with prognosis [11,12,13]. In this review, we discuss the recent advances made in clarifying the roles of various populations of TAMs at key stages of cancer progression from tumor initiation to metastasis (Fig. 1).
Macrophages involved in tumor growth and metastasis. A In the primary tumor, tumor-associated macrophages favor tumor development. B Tissue-resident macrophages from distant tissues shape the pre-metastatic niche upon stimulation with tumor-derived factors such as extracellular vesicles to prepare cancer cell colonization. C As the cancer cells colonize the pre-metastatic niche, recruited monocytes give rise to metastatic-associated macrophages that fuel metastatic progression
Macrophage diversity in the cancer context
Macrophage diversity is broadly conceptualized through the prism of two antagonistic polarization states: pro-inflammatory M1 and anti-inflammatory M2 macrophages [14]. Upon the identification of TAMs in the TME, a similar paradigm was adopted with immunosuppressive TAMs clinically associated with a poor prognosis, considered M2 macrophages [15,16,17]. A therapeutic extension of this view has culminated in attempts to repolarize TAMs from the M2 to M1 state by acting on key modulators of macrophage biology, such as colony-stimulating factor 1 [18, 19] or IFN-γ [20].
Tumor-associated RTMs and monocyte-derived TAMs
Although TAMs undoubtedly exhibit immunosuppressive properties, the M1/M2 paradigm does not fully reflect the extent of macrophage and TAM heterogeneity [21] and the different states of macrophage activation uncovered as a result of recent studies using single-cell and lineage-tracing technologies [22,23,24,25,26,27] (Table 1). Concerning their ontogeny, most TAMs are derived from circulating monocytes [28] and can almost completely overcome the pre-existing population of embryonically derived RTMs. A small population of native embryonic macrophages can, however, persist in the TME and have distinct functions from TAMs, including specific remodeling of the extracellular matrix [29]. More strikingly, a differential location within the TME has been revealed for pre-existing tumor-associated RTMs and monocyte-derived TAMs in various cancers, including breast cancer [30], non-small cell lung carcinoma [31], and glioma [32]. In both lung cancer [31] and glioma [32], embryonic-derived RTMs are found preferentially at the periphery of the tumor, while monocyte-derived TAMs infiltrate the tumor core. Nevertheless, monocyte-derived HO-1+ TAMs have also been shown to preferentially localize at the invasive margins of primary tumors and metastases in the MN-MCA1 murine model of cancer [33], therefore arguing for disease-specific localization of ontogenically distinct TAM populations. Altogether, these results suggest that such distinction between tumor-associated RTMs and monocyte-derived TAMs should be made when considering TAM identities and functions.
TAM function in primary tumors
TAMs were originally considered remnants of an abortive immune response against the tumor [34]. However, in 2001, the Jeffrey Pollard group showed that mice with a recessive null mutation in the colony-stimulating factor 1 gene (Csf1op), the major macrophage growth factor, and genetically modified to develop mammary cancer had a delay in the development of metastatic carcinomas, therefore showing the involvement of macrophages in malignant progression of breast cancer [35]. These seminal findings have led to investigations into the mechanisms of these pro-tumoral roles of macrophages. We now know that within the TME, TAMs have several supporting functions that promote tumor development (Fig. 2), which we describe below.
Tumor-associated macrophages favor tumor development through different functions. TAMs have various roles in tumorigenesis and as such, interact closely with cancer cells and the TME. TAMs create a pro-tumoral immune environment by: inactivating cytotoxic T cells through PD-L1 expression; and producing various cytokines to recruit regulatory T cells (IL-6, IL-10 and TGF-ß) and create an inflammatory milieu (IL-6, IL- 1ß, CXCL8). TAMs shape the extracellular matrix by producing proteases such as matrix metalloproteinases or cathepsins that degrade collagen fibers and ensure their turnover. TAMs also produce cross-linking enzymes that modulate the stiffness of the extracellular matrix. TAM-secreted VEGF promotes angiogenesis that facilitates tumor progression as well as metastasis. TAMs migrate with cancer cells to blood vessel where they create openings known as “TMEM doorways”, allowing cancer cells to disseminate in the circulation. Finally, TAMs produce TGF-ß and CCL18 that have a role in epithelia to mesenchymal transition, allowing cancer cells to migrate. Mesenchymal cells promote TAM activation through GM-CSF production
Vascularization
TAMs promote vascularization to supply oxygen and nutrients to cancer cells in the well-described process of angiogenesis [36]. Numerous investigations into this process have led to the detection of angiogenesis-promoting molecules produced by TAMs, including vascular endothelial growth factor-A (VEGF-A) in the context of non-small cell lung cancer [37] and adrenomedullin in melanoma [38]. Of note, this angiogenesis-promoting property is also observed in macrophages across healthy embryonic development [39]; therefore, we might speculate that this feature represents a function acquired by macrophages early in evolution, which reappears in these two different yet comparable contexts [40]. As a consequence, VEGF/VEGF receptor-targeting compounds are emerging as very promising therapies and are starting to be used notably to treat non-small cell lung carcinomas [41].
Inflammation
Another prominent function of TAMs in primary tumors is their role in establishing and maintaining an inflammatory environment. Examples of such TAM-derived inflammatory factors favoring tumor development are multiple and include CXCL8 in endometrial cancer [42], IL-6 in breast cancer [43], and IL-1ß in pancreatic cancer [44]. While this proinflammatory profile is supposed to support an active immune response against tumors, the remarkable plasticity of TAMs makes them more often associated with immunosuppression. As such, TAMs have the capacity to promote regulatory T-cell (Treg) recruitment. This phenomenon has been highlighted in ovarian cancer [45], nasopharyngeal carcinoma [46], and liver cancer [47], where these Tregs can then deactivate cytotoxic T cells directed against tumor cells [48]. TAMs can also directly promote cytotoxic T-cell exhaustion [30, 49, 50], and many current immunotherapies aim to reactivate antitumoral cytotoxic T cells by inhibiting the PD-1/PD-L1 immune checkpoint pathway [51]. It is worth noting, however, that TAMs also express PD-1 [52] or PD-L1 [22] and could therefore be considered off-targets/second targets of current protocols using pembrolizumab or nivolumab. The impact of such indirect TAM targeting on patient responses to treatment is largely unknown but should be taken into consideration in future studies, particularly as the effectiveness of current immunotherapies is variable. Nevertheless, the apparent heterogeneity in patient responses to treatment is likely mediated, in part, by TAMs. For example, macrophage recruitment is enhanced in patients with prostate cancer treated with androgen blockade therapy, and this recruitment subsequently contributes to tumor development. Those administered with anti-CSF-1 antibody in parallel, however, show an improved response to treatment [53]. In a similar manner, macrophage depletion with an anti-CSF-1 antibody reduces tumor growth in a mouse model with mammary gland tumors treated with radiotherapy [54].
Epithelial to mesenchymal transition
TAMs also promote epithelial to mesenchymal transition (EMT), a process during which epithelial-like, early proliferating cancer cells lose the capacity for cell–cell adhesion and adopt a fibroblast-like phenotype with invasive and migratory properties [55, 56]. EMT ultimately later permits metastatic cell dissemination. At the molecular level, EMT is orchestrated by the transcription factors zinc-finger E-box binding homeobox factor 1 (ZEB1) [57, 58], Snail [59, 60] and Twist [61] (reviewed in [62]). TAMs can regulate these EMT-modulating factors through their secretome [55, 63]. For example, TAM-produced tumor necrosis factor (TNF)-α stabilizes Snail through NF-kB signaling [64], while TAM-produced TGF-β induces Snail and ZEB1 expression by activating the β-catenin pathways [65,66,67]. Moreover, mesenchymal cell production of GM-CSF induces TAM activation and CCL18 production and further promotes EMT in a positive feedback loop [68].
ECM remodeling
TAMs are also involved in active ECM remodeling, collaborating notably with cancer-associated fibroblasts (CAFs) to promote tumor cell intravasation [69]. Indeed, tumors often display a dense ECM that notably impairs drug penetration, limiting treatment efficacy and resulting in more metastases [70, 71]. TAMs express and secrete various membrane-associated proteases that degrade ECM collagen fibers, such as matrix metalloproteinases (MMPs) [72, 73], secreted protein acidic and rich in cysteine [74], and cathepsins [69, 73]. Once degraded, TAMs mediate collagen fragment turnover via phagocytosis and degradation in the lysosome by cathepsins [69]. TAMs, by producing cross-linking enzymes from the lysyl hydroxylase (LH) family, such as LH2 in triple-negative breast cancer [75], also increase ECM stiffness, which promotes tumor progression and metastasis by mechanical forces [76]. In addition, in models of lung adenocarcinoma and breast cancer, a subset of TAMs expressing fibroblast activating protein (FAP)-α, which acts both as a signaling protein for CAFs and as a collagenase, and heme oxygenase (HO)-1 was found to be associated with ECM remodeling [77, 78]. Altogether, these observations suggest that similar mechanisms are involved in both wound healing and tumor formation, in line with the famous statement that tumors are “wounds that do not heal” [79].
Intravasation
EMT and ECM remodeling precede the intravasation of tumor cells into the circulation and their subsequent dissemination to distal organs. This key event in metastasis formation occurs at sites known as “tumor microenvironment metastasis (TMEM) doorways”, characterized by the dynamic association between one endothelial cell, one TAM and one cancer cell [80,81,82]. TAMs from the TMEM doorway arise from recruited monocytes that become CXCR4+ TAMs upon TGF-β stimulation in the TME. Attracted by fibroblast-derived CXCL12, these TAMs migrate toward the vascular niche, where they adopt a perivascular TAM phenotype and disrupt the junctions between endothelial cells, which allow tumor cells to intravasate into the circulation [83,84,85]. Of note, TMEM density in tumors has been linked with increased metastatic burden and could be used as a tool for predicting the occurrence of metastasis [80, 86].
In addition, activation of a paracrine loop also allows cancer cells that produce CSF-1 and TAMs that produce EGF to migrate together toward TMEM doorways. Thus, blocking CSF-1 or EGF receptors reduces cancer cell migration and invasiveness in breast cancer rodent models [87]. Furthermore, IL-4-producing TH2-CD4+ T cells stimulate EGF production by TAMs, and depletion of CD4 + T cells or IL-4-neutralizing antibody treatment reduces the metastatic burden [88]. Collectively, these examples demonstrate the crucial role of macrophages in initiating the metastatic process by favoring the migration and intravasation of cancer cells into the blood circulation.
Roles of RTMs in shaping the pre-metastatic niche
In the late 19th century, Paget proposed the “seed and soil” theory of metastasis [89] in which tumor cells (the “seeds”) can only grow in a hospitable environment (the “soil”). While the nature of the “hospitable” environment remains to be defined, this theory suggests that changes occur in distant tissues before the arrival of cancer cells to ensure that the environment favors metastatic growth. These changes constitute the development of a “pre-metastatic niche” (Fig. 3). As key mediators of inflammation, macrophages produce various cytokines that directly prime naive tissue to welcome disseminated tumor cells [90].
Pre-metastatic niche formation. Exosomes from the primary tumor reach distant tissues by trafficking through blood vessels. Once they arrive at the tissue site, they are engulfed by tissue resident macrophages (RTMs) cells, which triggers pre-metastatic niche formation. Activated RTMs are then able to activate fibroblasts, which in turn promote immune-cell recruitment and natural killer cell suppression. Activated RTMs also help establish a pro-tumoral environment by promoting inflammation and suppressing adaptive immune responses, thus creating a favorable environment for disseminating cancer cells. Activated RTMs maintain this favorable environment for metastatic growth when cancer cells start to colonize the pre-metastatic niche. Cancer cells favor monocyte recruitment that become metastasis-associated macrophages (MAMs). MAMs in turn favor metastasis progression through their role in cancer cell extravasation and T-cell suppression
Influence of macrophage origin
Investigations are ongoing to understand how macrophages shape the premetastatic niche and whether their origins have a differential impact. This latter question has been approached using the Cx3cr1CreERT2-based fate mapping mouse model challenged with ovarian cancer cells, which has the capacity to form metastases in the omenta [91]. In this context, a specific subset of embryonic TIM4+ CD163+ omentum macrophages were shown to favor metastatic dissemination from ovaries to omenta, and their depletion resulted in reduced ascitic volume and metastatic invasion of this organ [91]. To date, the molecular mechanisms by which embryonic macrophages preferentially shape the pre-metastatic niche remain to be clarified, but their documented very long half-life could be a key parameter.
Macrophage imprinting and extracellular vesicles
Mechanistically, pre-metastatic niche development and macrophage imprinting within that niche have been shown to be dependent on extracellular vesicles (EVs) that originate from the primary tumor and circulate within the blood. Of note, EVs have been classified based on their specific size and biogenesis and encompass microvesicles (150–1000 nm) arising from membrane budding, which are involved in local communication [92], and smaller exosomes (30–150 nm), which are derived from late endosomes and circulate over longer distances between tissues [92, 93]. EVs transport various cargos, such as RNAs, lipids, metabolites, or proteins, that they can transfer to other cell types to modulate their phenotype and functions. Through their distinct cargos, EVs impact the pre-metastatic niche through immune cell modulation, ECM remodeling and angiogenesis [94]. Findings derived from a seminal study from the group of David Lyden showed that integrins on the surface of tumor exosomes drive metastatic organotropism, as their patterns correlated with metastatic sites [93]. In the liver, for example, ITGαvβ5+ exosomes bind specifically to liver-resident macrophages (known as Kupffer cells), whereas ITGα6β4+ and ITGα6β1+ exosomes recognize lung fibroblasts and epithelial cells.
The mechanisms of action of tumor-derived EVs are only beginning to be elucidated. For example, macrophage migratory inhibiting factor (MIF) produced by primary pancreatic tumors and delivered by EVs remotely induces liver Kupffer cell production of TGF-β [95]. This process subsequently activates hepatic stellate cells (HSCs), which initiate liver fibrosis through fibronectin production, inducing the recruitment of inflammatory cells such as neutrophils and monocytes. Furthermore, activated HSCs also express CXCL12, which induces the quiescence of natural killer (NK) cells [96] and excludes CD8+ cytotoxic T cells from the pre-metastatic niche [97, 98]. The overall effect of this pathway is to reduce immunosurveillance of the pre-metastatic niche. Interestingly, monocyte-derived macrophages but not embryonically derived macrophages in the hepatic pre-metastatic niche secrete granulin, which serves to maintain HSC activation and liver fibrosis [99]. Further studies are needed to understand this observation, but as addressed earlier, it seems that macrophage origin affects the roles these cells play in determining metastasis.
In the liver, EV lipid cargo is handled by a specific subset of CD206+ Kupffer cells [100, 101], leading to upregulation of their expression of the fatty acid transporter CD36 and polarization toward an anti-inflammatory phenotype [102]. This phenotype favors immunosuppressive CD8+ T cells and improves the growth potential of disseminated tumor cells. Others have shown that EVs from lung adenocarcinoma notably induce upregulation of CD206, PD-L1 and GLUT1 by lymph node CD68+ macrophages. GLUT1 expression by macrophages increases their glucose uptake, and this glycolytic shift favors the establishment of the pre-metastatic niche [103]. In line with this, myeloid cells, including TAMs, have been shown to have the greatest capacity to take up glucose in the TME, a notably greater capacity than cancer cells [104], redefining the well-described Warburg effect. Coupled with the notion of the heterogeneity of TAM metabolic features [105], these findings argue that premetastatic niche priming relies on the metabolic capabilities of macrophages and promise groundbreaking discoveries with the increase in immunometabolism-related research.
These emerging findings, which place macrophages at the forefront of pre-metastatic niche establishment, can also be envisaged in the context of the macrophage network between distal tissues, which has been demonstrated to play a notable role in the context of myocardial infarction [106]. Indeed, it has been shown that macrophages from unrelated tissues such as lungs are activated after a heart-restricted challenge. The molecular mechanisms remain to be deciphered, but further studies could identify the actors involved in this phenomenon and assess their relevance in the context of cancer.
Finally, it should be noted that while EVs are scrutinized for their role in the priming of premetastatic niches, tumor cells can also prime distal macrophages via their release of free enzymes such as lysyl oxidase (LOX) [107].
Roles of macrophages in maintaining metastases
Macrophages continue to support metastasis development after tumor cell migration has occurred. This is evident based on the finding that inhibiting TAM recruitment to a metastatic site results in a lower metastatic burden, as shown for lung [108,109,110] and liver [111] metastasis murine models. Specifically, in the liver, macrophages produce hepatic growth factor that binds to c-Met at the surface of migrating tumor cells [112], stopping their circulation and promoting their extravasation within the liver. In the lungs, a similar phenomenon occurs but is mediated by interactions between VCAM-1 at the surface of migrating tumor cells and integrin α4 at the surface of lung macrophages [109]. In addition, this interaction triggers the Ezrin-PI3K/Akt pathway in tumor cells, which confers some protection against proapoptotic cytokines [108].
Metastasis-associated macrophages (MAMs)
Once the secondary tumor is established, macrophages deemed metastasis-associated macrophages (MAMs) in the literature [113] maintain immunosuppression by impairing cytotoxic T-cell activation. Specifically, and as mentioned earlier, EV-mediated priming of lung macrophages leads to a metabolic switch in these cells toward glycolytic respiration that produces lactate as a byproduct [103]. Lactate subsequently upregulates PD-L1 expression, blocking T-cell activation due to PD-1 engagement. Meanwhile, in the liver, macrophages induce systemic loss of T cells by triggering their apoptosis through the FAS-L pathway [114].
Many studies have described the recruitment of CCR2-expressing monocytes to the metastatic niche upon CCL2 production by stromal cells, which gives rise to MAMs [115, 116]. These monocytes might have different roles compared to RTMs present from the inception of the pre-metastatic niche. In MMTV-PyMT breast tumor-bearing mice, for example, monocyte-derived MAMs have a crucial role in cancer cell extravasation in the lung by producing VEGF-A [116], which can bind to the VEGF receptor on endothelial cells, thus inducing the remodeling of blood vessels at the metastatic site [117]. Monocyte-derived MAMs also seem to impact tumor-infiltrating lymphocytes in liver metastases of colorectal carcinoma. Specifically, a study in which colorectal cancer cells (MC38) were injected into the spleen of wild-type or CCR2 knockout mice showed that the knockout mice had a higher abundance of CD8+ and CD4+ lymphocytes and a reduced metastatic burden [115].
Kupffer cells promote liver metastases
The liver is the most common site for metastasis, likely due to its dense blood vessel architecture, with the portal vein supplying a large amount of blood and hepatic sinusoids offering a secondary network with lower pressure and thus more time for migrating tumor cells to attach to the organ [118]. As shown in rats, Kupffer cells within the sinusoids limit these events through phagocytosis, clearing 90% of circulating tumor cells [119]. When Kupffer cells are overloaded, however, tumor cells can extravasate into the liver [120]. As mentioned earlier, Kupffer cells can also favor metastasis by activating HSCs and creating a fibrotic and inflammatory pre-metastatic niche that sustains tumor cell invasion [95, 96]. Kupffer cells also act as key drivers of liver metastatic tropism through their specific engulfment of tumor-derived exosomes [93]. Accordingly, depletion of Kupffer cells before the induction of liver metastasis resulted in an increased metastatic burden, while depletion of KCs after metastatic establishment reduced metastatic growth [120]. Of note, other populations of macrophages also populate the liver, such as capsular or lipid-associated macrophages (LAMs) [121,122,123]. These cells have only recently been described, and their role in cancer has not yet been fully characterized, although LAM accumulation in metastases has been reported [124].
Lung macrophages modulate lung metastases
After the liver, the lungs constitute the second most frequent site of metastases. Exosomal priming of lung macrophages promotes the development of the pre-metastatic niche by inducing T-cell suppression [103] and neutrophil recruitment [125]. Lung macrophages also promote metastatic invasion by serving as anchors for circulating tumor cells, allowing their extravasation [108,109,110]. Again, heterogeneous macrophage populations with different features inhabit the lungs [126] and could have various roles in the metastasis of different primary tumors to this organ. For example, interstitial macrophages evolve over time in the metastatic niche, first exhibiting an antitumoral phenotype and later a protumoral phenotype, likely due to signals received from the stroma [103]. Alveolar macrophages also play a role in metastasis development, and notably, a subpopulation of lipid-laden Trem2+ macrophages display metabolic, immunosuppressive and matrix remodeling features that accumulate in metastases [127].
Limits and future perspectives in TAM research
In this review, we have highlighted various facets of tumor-associated macrophage biology that influence different steps of cancer development. The versatility of TAM functions is evident; thus, it is difficult to identify one unified target that might be of clinical benefit [128,129,130]. The very limited efficacy of global approaches such as those targeting the CSF-1/CSF-1R or CCL2/CCR2 pathways illustrates the challenges faced. Therefore, refinement of our strategies is needed and is on-going, as exemplified by the recent results suggesting efficacy of a combination of a TREM2-specific antibody with the widely used anti-PD-1 antibody in different cancer models [131].
To argue for this improved consideration of TAM heterogeneity, we have discussed the extent of TAM heterogeneity, with TAMs actually encompassing spatiotemporally unrelated macrophage populations within primary tumors, distal healthy tissues and metastasis sites. It remains to be fully deciphered how fundamental determinants of macrophage biology, such as their origin, their local environment and the time spent in the tissues, differentially influence tumor progression in these three different contexts [132].
To tackle these fundamental questions, our methodology needs to evolve. Many studies have relied on mouse models of cancer thus far, but we should acknowledge the inherent limitations of these systems. Orthotopic models, such as the widely used canonical B16 melanoma model, are convenient and easily combinable with knock-in or knock-out animals; unfortunately, this type of model is quite different from the natural disease course of cancer. Indeed, while the primary TME can be more-or-less recapitulated depending on the models, these systems completely bypass the key step of pre-metastatic niche priming owing to their fast-developing nature. This feature disconnects these models from patient contexts in which, as previously stated, metastasis remains the main cause of death. Genetic models closer to what is observed in patients do exist but are usually less convenient due to their lower penetrance and often asynchronous tumor emergence, limiting reproducibility and the establishment of robust conclusions. In contrast, patient biopsies represent invaluable samples and are extensively used; however, disease genesis is difficult to determine from one end-point sample from one location, either the primary tumor or metastasis, and only limited information can be extracted from the analysis.
Considering these issues, meaningful alternatives are needed to better understand the roles of macrophages in every step of the disease process. There are many avenues to be explored, and the recent increase in single-cell omics technologies offering snapshots of tissue activity at an unprecedented resolution will no doubt enable the precise identification of targets during disease development. These approaches now need to be coupled with models that consider disease dynamics, from the initial acquisition of oncogenic mutations to metastasis and multiorgan failure. The most recently developed spatial transcriptomic technologies allow for the identification of pathways that are activated in TAMs but also in all the other cells from the TME while conserving its architecture. These technologies can even be applied to fixed samples, allowing the retrospective analysis of hundreds of thousands of samples from cancer patients stored in hospitals worldwide. The increase in immunometabolism research should also reveal novel insights into macrophage activity within the TME, which could lead to the development of a new generation of metabolite-targeted therapies to reprogram TAMs into anti-tumor cells. It is up to us to make fruitful use of this wealth of information to generate knowledge that will inform the precise design of innovative TAM-related immunotherapies.
References
Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–44. https://doi.org/10.1016/j.cell.2008.01.025
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5. https://doi.org/10.1126/science.1194637
Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–78. https://doi.org/10.1016/j.immuni.2015.03.011
Johnson GR, Moore MA. Role of stem cell migration in initiation of mouse foetal liver haemopoiesis. Nature. 1975;258:726–8. https://doi.org/10.1038/258726a0
Ginhoux F, Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44:439–49. https://doi.org/10.1016/j.immuni.2016.02.024
van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46:845–52.
Mass E, Ballesteros I, Farlik M, Halbritter F, Günther P, Crozet L, et al. Specification of tissue-resident macrophages during organogenesis. Science 2016;353. https://doi.org/10.1126/science.aaf4238
Park MD, Silvin A, Ginhoux F, Merad M. Macrophages in health and disease. Cell. 2022;185:4259–79. https://doi.org/10.1016/j.cell.2022.10.007
Ho WW, Pittet MJ, Fukumura D, Jain RK. The local microenvironment matters in preclinical basic and translational studies of cancer immunology and immunotherapy. Cancer Cell. 2022;40:701–2. https://doi.org/10.1016/j.ccell.2022.05.016
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24:541–50. https://doi.org/10.1038/s41591-018-0014-x
Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–90. https://doi.org/10.1016/s0092-8674(00)00139-2
Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992;6:2591–9. https://doi.org/10.1096/fasebj.6.8.1592209
Prehn RT. The immune reaction as a stimulator of tumor growth. Science. 1972;176:170–1. https://doi.org/10.1126/science.176.4031.170
Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. https://doi.org/10.4049/jimmunol.164.12.6166
Wang L, Zhang C, Zhang Z, Han B, Shen Z, Li L, et al. Specific clinical and immune features of CD68 in glioma via 1,024 samples. Cancer Manag Res. 2018;10:6409–19. https://doi.org/10.2147/CMAR.S183293
Chen X, Chen J, Zhang W, Sun R, Liu T, Zheng Y, et al. Prognostic value of diametrically polarized tumor-associated macrophages in multiple myeloma. Oncotarget. 2017;8:112685–96. https://doi.org/10.18632/oncotarget.22340
DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. https://doi.org/10.1158/2159-8274.CD-10-0028
Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–59. https://doi.org/10.1016/j.ccr.2014.05.016
Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72. https://doi.org/10.1038/nm.3337
Shields CW, Evans MA, Wang LL, Baugh N, Iyer S, Wu D, et al. Cellular backpacks for macrophage immunotherapy. Sci Adv. 2020;6:eaaz6579 https://doi.org/10.1126/sciadv.aaz6579
Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17:34–40. https://doi.org/10.1038/ni.3324
Mulder K, Patel AA, Kong WT, Piot C, Halitzki E, Dunsmore G, et al. Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity. 2021;54:1883–1900. https://doi.org/10.1016/j.immuni.2021.07.007. e1885
Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. 2021;184:792–809. https://doi.org/10.1016/j.cell.2021.01.010.e723
Peng J, Sun BF, Chen CY, Zhou JY, Chen YS, Chen H, et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 2019;29:725–38. https://doi.org/10.1038/s41422-019-0195-y
Giladi A, Amit I. Single-Cell Genomics: A Stepping Stone for Future Immunology Discoveries. Cell. 2018;172:14–21. https://doi.org/10.1016/j.cell.2017.11.011
Regev A, Teichmann SA, Lander ES, Amit I, Benoist C, Birney E, et al. The Human Cell Atlas. Elife 2017; 6. https://doi.org/10.7554/eLife.27041
Chung W, Eum HH, Lee HO, Lee KM, Lee HB, Kim KT, et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat Commun. 2017;8:15081 https://doi.org/10.1038/ncomms15081
Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–5. https://doi.org/10.1126/science.1252510
Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, et al. Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity. 2017;47:323–38. https://doi.org/10.1016/j.immuni.2017.07.014.e326
Nalio Ramos R, Missolo-Koussou Y, Gerber-Ferder Y, Bromley CP, Bugatti M, Núñez NG, et al. Tissue-resident FOLR2(+) macrophages associate with CD8(+) T cell infiltration in human breast cancer. Cell. 2022;185:1189–207. https://doi.org/10.1016/j.cell.2022.02.021.e1125-1207
Casanova-Acebes M, Dalla E, Leader AM, LeBerichel J, Nikolic J, Morales BM, et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature. 2021;595:578–84. https://doi.org/10.1038/s41586-021-03651-8
Müller S, Kohanbash G, Liu SJ, Alvarado B, Carrera D, Bhaduri A, et al. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 2017;18:234 https://doi.org/10.1186/s13059-017-1362-4
Consonni FM, Bleve A, Totaro MG, Storto M, Kunderfranco P, Termanini A, et al. Heme catabolism by tumor-associated macrophages controls metastasis formation. Nat Immunol 2021;22:595–606. https://doi.org/10.1038/s41590-021-00921-5
Fidler IJ, Schroit AJ. Recognition and destruction of neoplastic cells by activated macrophages: discrimination of altered self. Biochim Biophys Acta. 1988;948:151–73. https://doi.org/10.1016/0304-419x(88)90009-1
Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–40. https://doi.org/10.1084/jem.193.6.727
Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–6. https://doi.org/10.1056/NEJM197111182852108
Hwang I, Kim JW, Ylaya K, Chung EJ, Kitano H, Perry C, et al. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J Transl Med. 2020;18:443 https://doi.org/10.1186/s12967-020-02618-z
Chen P, Huang Y, Bong R, Ding Y, Song N, Wang X, et al. Tumor-associated macrophages promote angiogenesis and melanoma growth via adrenomedullin in a paracrine and autocrine manner. Clin Cancer Res. 2011;17:7230–9. https://doi.org/10.1158/1078-0432.CCR-11-1354
Tay H, Du Cheyne C, Demeyere K, De Craene J, De Bels L, Meyer E, et al. Depletion of Embryonic Macrophages Leads to a Reduction in Angiogenesis in the Ex Ovo Chick Chorioallantoic Membrane Assay. Cells 2020,10. https://doi.org/10.3390/cells10010005
Sharma A, Bleriot C, Currenti J, Ginhoux F. Oncofetal reprogramming in tumour development and progression. Nat Rev Cancer. 2022;22:593–602. https://doi.org/10.1038/s41568-022-00497-8
Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27:1345–56. https://doi.org/10.1038/s41591-021-01450-2
Tong H, Ke JQ, Jiang FZ, Wang XJ, Wang FY, Li YR, et al. Tumor-associated macrophage-derived CXCL8 could induce ERalpha suppression via HOXB13 in endometrial cancer. Cancer Lett. 2016;376:127–36. https://doi.org/10.1016/j.canlet.2016.03.036
Radharani N, Yadav AS, Nimma R, Kumar T, Bulbule A, Chanukuppa V, et al. Tumor-associated macrophage derived IL-6 enriches cancer stem cell population and promotes breast tumor progression via Stat-3 pathway. Cancer Cell Int. 2022;22:122 https://doi.org/10.1186/s12935-022-02527-9
Arima K, Komohara Y, Bu L, Tsukamoto M, Itoyama R, Miyake K, et al. Downregulation of 15-hydroxyprostaglandin dehydrogenase by interleukin-1beta from activated macrophages leads to poor prognosis in pancreatic cancer. Cancer Sci. 2018;109:462–70. https://doi.org/10.1111/cas.13467
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. https://doi.org/10.1038/nm1093
Wang J, Huang H, Lu J, Bi P, Wang F, Liu X, et al. Tumor cells induced-M2 macrophage favors accumulation of Treg in nasopharyngeal carcinoma. Int J Clin Exp Pathol. 2017;10:8389–401.
Ren L, Yu Y, Wang L, Zhu Z, Lu R, Yao Z. Hypoxia-induced CCL28 promotes recruitment of regulatory T cells and tumor growth in liver cancer. Oncotarget. 2016;7:75763–73. https://doi.org/10.18632/oncotarget.12409
Liu C, Chikina M, Deshpande R, Menk AV, Wang T, Tabib T, et al. Treg Cells Promote the SREBP1-Dependent Metabolic Fitness of Tumor-Promoting Macrophages via Repression of CD8(+) T Cell-Derived Interferon-gamma. Immunity. 2019;51:381–97. https://doi.org/10.1016/j.immuni.2019.06.017
Nixon BG, Kuo F, Ji L, Liu M, Capistrano K, Do M, et al. Tumor-associated macrophages expressing the transcription factor IRF8 promote T cell exhaustion in cancer. Immunity. 2022;55:2044–58. https://doi.org/10.1016/j.immuni.2022.10.002.e2045
Kersten K, Hu KH, Combes AJ, Samad B, Harwin T, Ray A, et al. Spatiotemporal co-dependency between macrophages and exhausted CD8(+) T cells in cancer. Cancer Cell. 2022;40:624–38. https://doi.org/10.1016/j.ccell.2022.05.004.e629
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–7. https://doi.org/10.1073/pnas.192461099
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–9. https://doi.org/10.1038/nature22396
Escamilla J, Schokrpur S, Liu C, Priceman SJ, Moughon D, Jiang Z, et al. CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Res. 2015;75:950–62. https://doi.org/10.1158/0008-5472.CAN-14-0992
Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM. TH2-Polarized CD4(+) T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer Immunol Res. 2015;3:518–25. https://doi.org/10.1158/2326-6066.CIR-14-0232
Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61. https://doi.org/10.1016/j.cell.2011.11.025
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. https://doi.org/10.1016/j.cell.2009.11.007
Sánchez-Tilló E, Lázaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, et al. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;29:3490–3500. https://doi.org/10.1038/onc.2010.102
Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M, et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–88. https://doi.org/10.1038/sj.onc.1210508
Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. https://doi.org/10.1038/35000025
Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. https://doi.org/10.1038/35000034
Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12:5369–76. https://doi.org/10.1158/1078-0432.CCR-05-2722
Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. https://doi.org/10.1038/s41580-018-0080-4
Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525:256–60. https://doi.org/10.1038/nature14897
Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102:639–44. https://doi.org/10.1038/sj.bjc.6605530
Ramachandran A, Vizán P, Das D, Chakravarty P, Vogt J, Rogers KW, et al. TGF-beta uses a novel mode of receptor activation to phosphorylate SMAD1/5 and induce epithelial-to-mesenchymal transition. Elife. 2018, 7. https://doi.org/10.7554/eLife.31756
Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC Cancer. 2012;12:35 https://doi.org/10.1186/1471-2407-12-35
Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–72. https://doi.org/10.1038/cr.2009.5
Su S, Liu Q, Chen J, Chen J, Chen F, He C, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605–20. https://doi.org/10.1016/j.ccr.2014.03.021
Madsen DH, Jürgensen HJ, Siersbæk MS, Kuczek DE, Grey Cloud L, Liu S, et al. Tumor-Associated Macrophages Derived from Circulating Inflammatory Monocytes Degrade Collagen through Cellular Uptake. Cell Rep. 2017;21:3662–71. https://doi.org/10.1016/j.celrep.2017.12.011
Laklai H, Miroshnikova YA, Pickup MW, Collisson EA, Kim GE, Barrett AS, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med. 2016;22:497–505. https://doi.org/10.1038/nm.4082
Chaudhuri O, Koshy ST, Branco da Cunha C, Shin JW, Verbeke CS, Allison KH, et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater. 2014;13:970–8. https://doi.org/10.1038/nmat4009
Bahr JC, Li XY, Feinberg TY, Jiang L, Weiss SJ. Divergent regulation of basement membrane trafficking by human macrophages and cancer cells. Nat Commun. 2022;13:6409 https://doi.org/10.1038/s41467-022-34087-x
Afik R, Zigmond E, Vugman M, Klepfish M, Shimshoni E, Pasmanik-Chor M, et al. Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med. 2016;213:2315–31. https://doi.org/10.1084/jem.20151193
Sangaletti S, Di Carlo E, Gariboldi S, Miotti S, Cappetti B, Parenza M, et al. Macrophage-derived SPARC bridges tumor cell-extracellular matrix interactions toward metastasis. Cancer Res. 2008;68:9050–9. https://doi.org/10.1158/0008-5472.CAN-08-1327
Maller O, Drain AP, Barrett AS, Borgquist S, Ruffell B, Zakharevich I, et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat Mater. 2021;20:548–59. https://doi.org/10.1038/s41563-020-00849-5
Fattet L, Jung HY, Matsumoto MW, Aubol BE, Kumar A, Adams JA, et al. Matrix Rigidity Controls Epithelial-Mesenchymal Plasticity and Tumor Metastasis via a Mechanoresponsive EPHA2/LYN Complex. Dev Cell. 2020;54:302–16. https://doi.org/10.1016/j.devcel.2020.05.031.e307
Muliaditan T, Caron J, Okesola M, Opzoomer JW, Kosti P, Georgouli M, et al. Macrophages are exploited from an innate wound healing response to facilitate cancer metastasis. Nat Commun. 2018;9:2951 https://doi.org/10.1038/s41467-018-05346-7
Arnold JN, Magiera L, Kraman M, Fearon DT. Tumoral immune suppression by macrophages expressing fibroblast activation protein-alpha and heme oxygenase-1. Cancer Immunol Res. 2014;2:121–6. https://doi.org/10.1158/2326-6066.CIR-13-0150
Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl J Med. 1986;315:1650–9. https://doi.org/10.1056/NEJM198612253152606
Robinson BD, Sica GL, Liu YF, Rohan TE, Gertler FB, Condeelis JS, et al. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res. 2009;15:2433–41. https://doi.org/10.1158/1078-0432.CCR-08-2179
Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–56. https://doi.org/10.1158/0008-5472.CAN-06-1823
Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, et al. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer Discov. 2015;5:932–43. https://doi.org/10.1158/2159-8290.CD-15-0012
Borriello L, Coste A, Traub B, Sharma VP, Karagiannis GS, Lin Y, et al. Primary tumor associated macrophages activate programs of invasion and dormancy in disseminating tumor cells. Nat Commun. 2022;13:626 https://doi.org/10.1038/s41467-022-28076-3
Sharma VP, Tang B, Wang Y, Duran CL, Karagiannis GS, Xue EA, et al. Live tumor imaging shows macrophage induction and TMEM-mediated enrichment of cancer stem cells during metastatic dissemination. Nat Commun. 2021;12:7300 https://doi.org/10.1038/s41467-021-27308-2
Arwert EN, Harney AS, Entenberg D, Wang Y, Sahai E, Pollard JW, et al. A Unidirectional Transition from Migratory to Perivascular Macrophage Is Required for Tumor Cell Intravasation. Cell Rep. 2018;23:1239–48. https://doi.org/10.1016/j.celrep.2018.04.007
Sparano JA, Gray R, Oktay MH, Entenberg D, Rohan T, Xue X, et al. A metastasis biomarker (MetaSite Breast Score) is associated with distant recurrence in hormone receptor-positive, HER2-negative early-stage breast cancer. NPJ Breast Cancer. 2017;3:42 https://doi.org/10.1038/s41523-017-0043-5
Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9. https://doi.org/10.1158/0008-5472.CAN-04-1449
DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. https://doi.org/10.1016/j.ccr.2009.06.018
Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–3.
Oishi Y, Manabe I. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech Dis. 2016;2:16018 https://doi.org/10.1038/npjamd.2016.18
Etzerodt A, Moulin M, Doktor TK, Delfini M, Mossadegh-Keller N, Bajenoff M, et al. Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer. J Exp Med. 2020; 217. https://doi.org/10.1084/jem.20191869.
Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. https://doi.org/10.1038/s41556-018-0250-9
Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–35. https://doi.org/10.1038/nature15756
Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: Communication from a distance. Dev Cell. 2019;49:347–60. https://doi.org/10.1016/j.devcel.2019.04.011
Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–26. https://doi.org/10.1038/ncb3169
Correia AL, Guimaraes JC, Auf der Maur P, De Silva D, Trefny MP, Okamoto R, et al. Hepatic stellate cells suppress NK cell-sustained breast cancer dormancy. Nature. 2021;594:566–71. https://doi.org/10.1038/s41586-021-03614-z
Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res. 2014;2:187–93. https://doi.org/10.1158/2326-6066.CIR-14-0002
Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20212–7. https://doi.org/10.1073/pnas.1320318110
Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18:549–60. https://doi.org/10.1038/ncb3340
De Simone G, Andreata F, Bleriot C, Fumagalli V, Laura C, Garcia-Manteiga JM, et al. Identification of a Kupffer cell subset capable of reverting the T cell dysfunction induced by hepatocellular priming. Immunity. 2021;54:2089–2100. https://doi.org/10.1016/j.immuni.2021.05.005. e2088
Blériot C, Barreby E, Dunsmore G, Ballaire R, Chakarov S, Ficht X, et al. A subset of Kupffer cells regulates metabolism through the expression of CD36. Immunity. 2021;54:2101–16. https://doi.org/10.1016/j.immuni.2021.08.006.e2106
Yang P, Qin H, Li Y, Xiao A, Zheng E, Zeng H, et al. CD36-mediated metabolic crosstalk between tumor cells and macrophages affects liver metastasis. Nat Commun. 2022;13:5782 https://doi.org/10.1038/s41467-022-33349-y
Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C, et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 2021;33:2040–58. e2010
Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593:282–8. https://doi.org/10.1038/s41586-021-03442-1
Geeraerts X, Fernández-Garcia J, Hartmann FJ, de Goede KE, Martens L, Elkrim Y, et al. Macrophages are metabolically heterogeneous within the tumor microenvironment. Cell Rep. 2021;37:110171 https://doi.org/10.1016/j.celrep.2021.110171
Hoyer FF, Naxerova K, Schloss MJ, Hulsmans M, Nair AV, Dutta P, et al. Tissue-specific macrophage responses to remote injury impact the outcome of subsequent local immune challenge. Immunity. 2019;51:899–914. https://doi.org/10.1016/j.immuni.2019.10.010
Kitamura T, Doughty-Shenton D, Cassetta L, Fragkogianni S, Brownlie D, Kato Y, et al. Monocytes Differentiate to Immune Suppressive Precursors of Metastasis-Associated Macrophages in Mouse Models of Metastatic Breast Cancer. Front Immunol. 2017;8:2004 https://doi.org/10.3389/fimmu.2017.02004
Rodriguez-Tirado C, Entenberg D, Li J, Qian BZ, Condeelis JS, Pollard JW. Interleukin 4 Controls the Pro-Tumoral Role of Macrophages in Mammary Cancer Pulmonary Metastasis in Mice. Cancers (Basel). 2022;14:4336 https://doi.org/10.3390/cancers14174336
Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20:538–49. https://doi.org/10.1016/j.ccr.2011.08.025
Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562 https://doi.org/10.1371/journal.pone.0006562
Auguste P, Fallavollita L, Wang N, Burnier J, Bikfalvi A, Brodt P. The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. Am J Pathol. 2007;170:1781–92. https://doi.org/10.2353/ajpath.2007.060886
Kitamura T, Kato Y, Brownlie D, Soong DYH, Sugano G, Kippen N, et al. Mammary tumor cells with high metastatic potential are hypersensitive to macrophage-derived HGF. Cancer Immunol Res. 2019;7:2052–64. https://doi.org/10.1158/2326-6066.CIR-19-0234
Cassetta L, Pollard JW. A timeline of tumour-associated macrophage biology. Nat Rev Cancer. 2023;23:238–57. https://doi.org/10.1038/s41568-022-00547-1
Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T-cell elimination. Nat Med. 2021;27:152–64. https://doi.org/10.1038/s41591-020-1131-x
Grossman JG, Nywening TM, Belt BA, Panni RZ, Krasnick BA, DeNardo DG, et al. Recruitment of CCR2(+) tumor associated macrophage to sites of liver metastasis confers a poor prognosis in human colorectal cancer. Oncoimmunology. 2018;7:e1470729 https://doi.org/10.1080/2162402X.2018.1470729
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. https://doi.org/10.1038/nature10138
Follain G, Osmani N, Gensbittel V, Asokan N, Larnicol A, Mercier L, et al. Impairing flow-mediated endothelial remodeling reduces extravasation of tumor cells. Sci Rep. 2021;11:13144 https://doi.org/10.1038/s41598-021-92515-2
Kumar A, Sharma P, Sarin SK. Hepatic venous pressure gradient measurement: time to learn! Indian J Gastroenterol. 2008;27:74–80.
Timmers M, Vekemans K, Vermijlen D, Asosingh K, Kuppen P, Bouwens L, et al. Interactions between rat colon carcinoma cells and Kupffer cells during the onset of hepatic metastasis. Int J Cancer. 2004;112:793–802. https://doi.org/10.1002/ijc.20481
Wen SW, Ager EI, Christophi C. Bimodal role of Kupffer cells during colorectal cancer liver metastasis. Cancer Biol Ther. 2013;14:606–13. https://doi.org/10.4161/cbt.24593
Remmerie A, Martens L, Thoné T, Castoldi A, Seurinck R, Pavie B, et al. Osteopontin Expression Identifies a Subset of Recruited Macrophages Distinct from Kupffer Cells in the Fatty Liver. Immunity. 2020;53:641–57. https://doi.org/10.1016/j.immuni.2020.08.004. e614
Bleriot C, Ginhoux F. Understanding the heterogeneity of resident liver macrophages. Front Immunol. 2019;10:2694 https://doi.org/10.3389/fimmu.2019.02694
Sierro F, Evrard M, Rizzetto S, Melino M, Mitchell AJ, Florido M, et al. A Liver Capsular Network of Monocyte-Derived Macrophages Restricts Hepatic Dissemination of Intraperitoneal Bacteria by Neutrophil Recruitment. Immunity. 2017;47:374–88. https://doi.org/10.1016/j.immuni.2017.07.018.e376
Donadon M, Torzilli G, Cortese N, Soldani C, Di Tommaso L, Franceschini B, et al. Macrophage morphology correlates with single-cell diversity and prognosis in colorectal liver metastasis. J Exp Med. 2020, 217. https://doi.org/10.1084/jem.20191847.
Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, et al. Tumor Exosomal RNAs Promote Lung Pre-metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell. 2016;30:243–56. https://doi.org/10.1016/j.ccell.2016.06.021
Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J, et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 2019; 363. https://doi.org/10.1126/science.aau0964.
Huggins DN, LaRue RS, Wang Y, Knutson TP, Xu Y, Williams JW, et al. Characterizing macrophage diversity in metastasis-bearing lungs reveals a lipid-associated macrophage subset. Cancer Res. 2021;81:5284–95. https://doi.org/10.1158/0008-5472.CAN-21-0101
Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21:799–820. https://doi.org/10.1038/s41573-022-00520-5
DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–82. https://doi.org/10.1038/s41577-019-0127-6
Cassetta L, Pollard JW. Targeting macrophages: Therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17:887–904. https://doi.org/10.1038/nrd.2018.169
Molgora M, Esaulova E, Vermi W, Hou J, Chen Y, Luo J, et al. TREM2 modulation remodels the tumor myeloid landscape enhancing Anti-PD-1 immunotherapy. Cell. 2020;182:886–900. https://doi.org/10.1016/j.cell.2020.07.013.e817
Bleriot C, Chakarov S, Ginhoux F. Determinants of resident tissue macrophage identity and function. Immunity. 2020;52:957–70. https://doi.org/10.1016/j.immuni.2020.05.014
Ma RY, Black A, Qian BZ. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 2022;43:546–63. https://doi.org/10.1016/j.it.2022.04.008
Zhao H, Teng Y, Hao W, Li J, Li Z, Chen Q, et al. Single-cell analysis revealed that IL4I1 promoted ovarian cancer progression. J Transl Med. 2021;19:454 https://doi.org/10.1186/s12967-021-03123-7
Wu SZ, Al-Eryani G, Roden DL, Junankar S, Harvey K, Andersson A, et al. A single-cell and spatially resolved atlas of human breast cancers. Nat Genet. 2021;53:1334–47. https://doi.org/10.1038/s41588-021-00911-1
Pombo Antunes AR, Scheyltjens I, Lodi F, Messiaen J, Antoranz A, Duerinck J, et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci. 2021;24:595–610. https://doi.org/10.1038/s41593-020-00789-y
Che LH, Liu JW, Huo JP, Luo R, Xu RM, He C, et al. A single-cell atlas of liver metastases of colorectal cancer reveals reprogramming of the tumor microenvironment in response to preoperative chemotherapy. Cell Discov. 2021;7:80 https://doi.org/10.1038/s41421-021-00312-y
Haensel D, Daniel B, Gaddam S, Pan C, Fabo T, Bjelajac J, et al. Skin basal cell carcinomas assemble a pro-tumorigenic spatially organized and self-propagating Trem2+ myeloid niche. Nat Commun. 2023;14:2685 https://doi.org/10.1038/s41467-023-37993-w
Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50:1317–34. https://doi.org/10.1016/j.immuni.2019.03.009. e1310
Timperi E, Gueguen P, Molgora M, Magagna I, Kieffer Y, Lopez-Lastra S, et al. Lipid-associated macrophages are induced by cancer-associated fibroblasts and mediate immune suppression in breast cancer. Cancer Res. 2022;82:3291–306. https://doi.org/10.1158/0008-5472.CAN-22-1427
Sharma A, Seow JJW, Dutertre CA, Pai R, Blériot C, Mishra A, et al. Onco-fetal Reprogramming of Endothelial Cells Drives Immunosuppressive Macrophages in Hepatocellular Carcinoma. Cell. 2020;183:377–94. https://doi.org/10.1016/j.cell.2020.08.040.e321
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bied, M., Ho, W.W., Ginhoux, F. et al. Roles of macrophages in tumor development: a spatiotemporal perspective. Cell Mol Immunol 20, 983–992 (2023). https://doi.org/10.1038/s41423-023-01061-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-023-01061-6
Keywords
This article is cited by
-
ALOX5 acts as a key role in regulating the immune microenvironment in intrahepatic cholangiocarcinoma, recruiting tumor-associated macrophages through PI3K pathway
Journal of Translational Medicine (2023)
-
Immunological modulation in health and disease
Cellular & Molecular Immunology (2023)