Abstract
The mosaic theory of hypertension was advocated by Irvine Page ~80 years ago and suggested that hypertension resulted from the close interactions of different causes. Increasing evidence indicates that hypertension and hypertensive end-organ damage are not only mediated by the proposed mechanisms that result in hemodynamic injury. Inflammation plays an important role in the pathophysiology and contributes to the deleterious consequences of arterial hypertension. Sodium intake is indispensable for normal body function but can be detrimental when it exceeds dietary requirements. Recent data show that sodium levels also modulate the function of monocytes/macrophages, dendritic cells, and different T-cell subsets. Some of these effects are mediated by changes in the microbiome and metabolome due to high-salt intake. The purpose of this review is to propose a revised and extended version of the mosaic theory by summarizing and integrating recent advances in salt, immunity, and hypertension research. Salt and inflammation are placed in the middle of the mosaic because both factors influence each of the remaining pieces.
Similar content being viewed by others
Introduction
Arterial hypertension, which is commonly defined as blood pressure (BP) > 140/90 mmHg, affects more than a quarter of the global population, which is more than one billion people worldwide [1,2,3]. Due to multiple cardiovascular, renal, ocular and cognitive complications, arterial hypertension is a leading contributor to the global disease burden [1]. The limit of 140/90 mmHg was chosen according to multiple randomized controlled clinical trials that showed clear benefits of antihypertensive treatment relative to potential treatment-associated adverse effects in response to the primary preventative treatment of BP > 140/90 mmHg [3,4,5]. To account for the importance of BP elevations that are less than the usual classification of arterial hypertension, the American Heart Association and American College of Cardiology decided to reclassify arterial hypertension as BP > 130/80 mmHg in their latest guidelines in 2017 [6]. Under this definition, the prevalence of arterial hypertension in the United States has soared from 32 to 46% [6]. Despite the importance of arterial hypertension due to its high prevalence and immense impact on health worldwide, many details of the underlying pathological mechanisms leading to arterial hypertension remain unclear. This gap in knowledge is even more relevant when focusing on current antihypertensive treatment options, which mainly treat symptoms or have incompletely understood mechanisms of action. A better understanding of the pathogenesis of hypertension and the finely tuned interplay of key factors will lead to more precisely targeted therapeutic options with better global health outcomes and reduced side effects.
In the present review, we propose a revised, extended, and updated version of the mosaic theory that considers recent and exciting discoveries of the interactions among salt, inflammation, and hypertension. In this revised version of the mosaic theory, we place salt and inflammation in the middle of the mosaic because both factors influence each of the remaining tesserae.
The multifactorial etiology of hypertension
The multitude and interconnectedness of factors that influence BP were first recognized 80 years ago by Irvin Page, who postulated the impact of blood volume and viscosity, renal perfusion, vasculature, humoral factors and the central nervous system on BP. He realized the reciprocal influence of individual contributors on one another, composing a network of hypertension-inducing factors that is famously known as the mosaic theory of hypertension [7, 8]. Although several later revisions and many details have been added since its initial description, the principal idea of a complex system with interdependence among the individual components instead of simple cause-effect relationships of solitary sources of hypertension holds true today. An update of the mosaic theory was recently presented by Harrison et al. [9]. To underscore the influence of salt and inflammation on every piece of the mosaic, we would like to propose a further revised mosaic theory highlighting the central roles of salt and inflammation in the pathogenesis of arterial hypertension, as depicted in Fig. 1. The revised mosaic theory places salt and inflammation in its center; both factors influence all other mosaic pieces that have been proposed and are added by us. Notably, salt also influences innate and adaptive immune cells and vice versa. This extended mosaic will serve as a thread to discuss some of the individual key components. Another highly interesting and recent finding is the hypertensive response due to dehydration [10, 11]. However, further work needs to be done to add this interesting and fascinating theory to the mosaic.
The individual mosaic pieces are ordered clockwise according to the historical acknowledgment of their role in hypertension. The kidney was one of the first pieces of the mosaic to be recognized as crucial for BP regulation. Guyton developed the concept of pressure salt diuresis, establishing the link between BP, renal salt excretion and body fluid homeostasis [12]. Vascular dysfunction in arterial hypertension includes structural alterations in small and large arteries with increased vasoconstriction, endothelial dysfunction with impaired vasodilatation, and arterial wall thickening and stiffening by modifying the extracellular matrix and vascular smooth muscle cells [9, 13, 14]. We know that high-salt intake and inflammatory cells cause vascular stiffening [15, 16]. Sympathetic nerve (over)activity contributes to hypertension in several ways, including catecholamine-mediated vasoconstriction, vascular remodeling, adrenergic stimulation of renin production and secretion in juxtaglomerular cells, and increased sodium reabsorption and renal inflammation [17, 18]. The renin–angiotensin–aldosterone system (RAAS) is a crucial endocrine cascade that regulates water and electrolyte homeostasis, vascular tone, renal perfusion and cardiac remodeling, and its tight association with arterial hypertension was first noted in the 19th century and elaborated in the following decades [8, 19,20,21,22]. Recently, the influence the RAAS on immune cells was discovered [23]. The RAAS is discussed later in this review, with a particular focus on the role of the mineralocorticoid and Ang II type 1 (AT1) receptors in immune cells. The pleiotropic effects of vascular oxidation-induced reactive oxygen species (ROS) and oxidative stress on BP involving the vasculature, kidney, nervous system and immune activation have been extensively reviewed recently [24, 25]. Genetic polymorphisms in genome-wide association studies, familial hypertension and monogenetic disorders associated with a hypertensive phenotype underscore the importance of complex genetic influences on BP, which involve mutations in ion channels or receptor molecules that are important for renal sodium and potassium handling in the context of single gene mutations [9]. Almost all known monogenic blood pressure disorders of affect kidney salt metabolism. Open questions and the future (therapeutic) potential of hypertension genetics are elaborated elsewhere [26, 27]. Finally, the recently recognized role of monocytes/macrophages (M/Ms) in cutaneous salt storage and the response of the gut metabolome to salt yet another link between salt, systemic inflammation, and BP regulation and will be discussed further in this review.
The immune system and hypertension
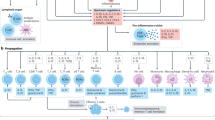
In recent years, increasing evidence of the immune pathogenesis of hypertension has emerged [28]. To review the complex connections between the immune system and hypertension, we will discuss the relevant individual components of the innate and adaptive immune systems, as visualized in Fig. 2.
Overview of immune cells that influence BP- and hypertension-mediated end-organ damage. PMNs polymorphonuclear neutrophils, M/Ms monocytes/macrophages, MDSCs myeloid-derived suppressor cells, DCs dendritic cells, NKT cells natural killer T cells, NK cells natural killer cells, CD4+ cluster of differentiation 4-positive cells, CD8+ cluster of differentiation 8-positive cells, Tregs regulatory T cells
The innate immune system and hypertension
The innate immune system, which is characterized by rapid defense mechanisms against invading pathogens, consists of cellular and humoral components. The main cellular mediators are neutrophils, M/Ms, mast cells and innate lymphoid cells (ILCs, including killer-ILCs, which were formerly called natural killer cells and helper-like ILCs). Dendritic cells (DC), which are antigen presenting cells (APC), serve as a link between the innate and adaptive immune systems by processing foreign antigens and presenting the resulting antigenic peptides to T cells for their activation. Humoral factors of the innate arm of the immune system include the defensin and complement systems [29, 30].
The complement system
The complement system is a network of fluid-phase and membrane-bound proteins that detect pathogens, resulting in opsonization and the elimination of pathogens, as well as chemotaxis induced by anaphylatoxins to attract immune cells to the site of infection and induce a general inflammatory reaction. Cascade activation of these proteins triggers a highly amplified, instantaneous immune response that leads to the activation of C3- and C5-convertase complexes, which ultimately terminate in the formation of the membrane attack complex, which consists of the complement factors C5b to C9. Remarkable similarities in clinical and histopathological presentations of hypertension-induced malignant nephrosclerosis and atypical hemolytic uremic syndrome, which are complement-driven diseases, suggest a role of complement activation in the development and maintenance of hypertension. Elevated serum C3 and C5 levels in hypertensive patients and animal models of hypertension provide another hint of the role of the complement system in the etiology of hypertension. For further elaboration, the reader is referred to recent reviews with a particular focus on complement and hypertension [31,32,33].
Neutrophils
Polymorphonuclear neutrophils are part of the front-line defense of the innate immune system by rapidly eliminating microbes through phagocytosis, the release of antimicrobial proteins by degranulation and the generation of neutrophil extracellular traps. However, their role in the etiology of hypertension remains unclear. Epidemiological and animal data show an association between neutrophils, their activity and increased BP: epidemiological studies showed that the blood neutrophil-to-lymphocyte ratio, which is often used as a marker of systemic inflammation, correlates with the risk of developing hypertension and could serve as a predictor of hypertension [29, 34]. An animal model of spontaneously hypertensive rats exhibited significant associations between markers of neutrophil activity (myeloperoxidase activity and superoxide generation) and high BP [35]. However, these associations do not prove causality, and adoptive transfer of neutrophils did not restore the hypertensive response in myeloid cell-depleted mice [31]. Thus, neutrophils may not induce hypertension on their own, and their involvement with other cells that influence BP and factors that lead to hypertension remains to be further elucidated.
Monocytes/macrophages
Monocytes/macrophages (M/Ms), which are cells of the myeloid lineage, contribute to host defense through phagocytosis induced by pattern recognition receptors, antibodies or complement-mediated opsonization, as well as cytokine production and antigen presentation. Their role in the generation of hypertension was established using osteopetrotic mice that were deficient for macrophage colony-stimulating factor and subsequently had impaired M/Ms function. These mice exhibited reduced hypertension and vascular injury upon angiotensin II (Ang II) infusion or deoxycorticosterone acetate (DOCA) salt treatment [36, 37]. Wenzel et al. found attenuated infiltration of the vascular wall after Ang II treatment in mice with selective ablation of lysozyme M-positive myelomonocytic cells, accompanied by reductions in oxidative stress, vascular dysfunction and hypertension [38]. This attenuation was reversible upon the adoptive transfer of monocytes. Renal infiltration and the proliferation of macrophages is associated with interleukin (IL)-6 expression and can be reduced by anti-IL-6 treatment [39]. Furthermore, monocytes, particularly proinflammatory M1-like macrophages, express angiotensin-converting enzyme (ACE), indicating another direct way these cells can influence BP by activating the RAAS [40, 41]. For further elaboration on the role of macrophage polarization in hypertension, please refer to Harwani et al. and Wenzel [42, 43].
IL-6 is a pivotal cytokine associated with innate immunity that plays a proatherogenic role in cardiovascular disease, and IL-6 inhibition may be a novel strategy to exert cardiovascular protection. IL-6 signaling and anti-IL-6 treatment have recently been reviewed by Ridker and Rane [44]. Ziltivekimab, a novel IL-6 ligand antibody, has been developed specifically for use in atherosclerotic diseases and is poised to be tested in a large-scale cardiovascular outcome trial focused on individuals with high atherothrombotic and inflammatory risk due to chronic kidney disease and elevated levels of CRP [44, 45].
Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of myeloid progenitor cells and immature myeloid cells with immunosuppressive capacities [29, 46, 47]. These cells are characterized by the expression of the myeloid markers CD11b and Gr1 (in mice) and can suppress T-cell activation and TH17 and TH1 cells [47, 48]. Established mechanisms by which MDSCs suppress T-cell activity include the enzymes arginase 1 (which depletes the nonessential amino acid l-arginine, whose depletion in turn inhibits T-cell proliferation) and inducible nitric oxide synthase (which generates NO, which inhibits T-cell function via the Janus kinase JAK3 and signal transducer and activator of transcription (STAT5), as well as MHC II expression and apoptosis induction), as well as powerful oxidants such as ROS and peroxynitrite [47]. Furthermore, these cells can induce the differentiation of regulatory T cells (Tregs) by producing interleukin 10 (IL-10) [48]. Through their anti-inflammatory effects, MDSCs play a protective role and counteract the development of hypertension: different mouse models of hypertension showed increased numbers of CD11b+Gr1+ myeloid cells, and the depletion of these MDSCs enhanced renal inflammation and BP. Adoptive transfer of MDSCs from hypertensive but not normotensive mice reduced Ang II-induced hypertension [49]. These antihypertensive effects were mediated at least partially by ROS generation, and MDSCs are an interesting new addition to the story of how the immune system and ROS influence and classically increase BP.
Dendritic cells
DCs contribute to the inflammatory response associated with hypertension via T-cell activation. This effect is induced by antigen presentation and costimulation of T cells by B7 ligands (cluster of differentiation (CD)-80 and CD86) interacting with the T-cell costimulation receptor CD28 (Fig. 3). Mice with experimentally induced hypertension due to Ang II infusion or DOCA salt treatment exhibit increased numbers of DCs in secondary lymphatic tissues. DCs secrete cytokines such as IL-1, IL-6, and IL-23 and can activate CD8+ and CD4+ T cells in renal lymph nodes or other tissues, and these activated T cells can then migrate into the kidney and vasculature. Inhibition of B7-induced costimulation by CTLA4-Ig (abatacept) reduces vascular T-cell infiltration and activation, leading to marked alleviation of hypertension in these mouse model [50]. In knockout mice lacking B7, Ang II infusion was incapable of inducing hypertension [50]. To rule out the influence of B7 on endothelial cells, these knockout mice underwent wild-type bone marrow transplantation, which reconstituted Ang II-induced increase in BP [50]. Hevia et al. confirmed these findings by showing that specific ablation of CD11c-expressing APCs, which characterizes DCs, prevented the development of hypertension after Ang II infusion on a high-salt diet and improved natriuresis [51]. The researchers found a decrease in anti-inflammatory effects, as measured by decreased cardiac and renal transcription of IL-10 and the master transcription factor of regulatory T cells, forkhead box P3 (FoxP3), and this effect was prevented by DC ablation. Furthermore, the researchers showed an increase in CD86 in splenic and renal DCs due to Ang II and salt treatment. In vitro, these DCs induced a stronger proinflammatory response characterized by increased T-cell proliferation and differentiation toward CD8+/IFN-γ cells than those from control mice without Ang II infusion and with a regular salt diet [51]. However, given the expression of CD11c in other cell types, such as macrophages, further studies with specific DC-knockout could improve our understanding of the influence of different APC or DC subtypes on BP [52]. Lu et al. additionally showed that mice lacking renal DCs had increased sodium chloride cotransporter expression and enhanced natriuresis and diuresis, as well as reduced urinary oxidative stress markers compared to wild-type mice with Ang II and salt treatment [53].
In monocytes/macrophages, high-salt enters proinflammatory cells via NCX1, causing NFAT5 expression and subsequent NO production by NOS2. In addition, proinflammatory cytokines such as IL-1β, IL-6, and TNF-α are released in response to high salt. In T cells, sodium enters the cell through the NKCC1 transporter, resulting in the upregulation of NFAT5 and its downstream target SGK1. Upregulation of the TH17 transcription marker RORγt mediates the transcription and translation of IL-23R, which is essential for TH17 induction and the secretion of IL-17a. NCX1 sodium calcium exchanger 1, NKCC1 sodium potassium chloride cotransporter, NOS2 nitric oxide synthase, NO nitric oxide, IL interleukin, TNF tumor necrosis factor, SGK1 serum glucocorticoid-regulated kinase, NFAT5 nuclear factor of activated T cells, RORγt retinoic-acid receptor-related orphan nuclear receptor gamma, M/M monocyte/macrophage
However, the influence of DCs and hypertension is bidirectional, resulting in a self-enhancing interplay: DCs not only exacerbate hypertension, but hypertension enhances DC activity and activation. Hypertensive mechanical trauma might produce neoantigens due to cell death and the subsequent exposure of intracellular antigens, which are then presented to T cells by DCs. Furthermore, increased oxidative stress due to arterial hypertension results in lipid oxidation with the formation of isoketal adducts of various self-proteins. Highly reactive isoketals can ligate to the lysine residues of proteins in DCs, forming protein adducts that are recognized as nonself. These proteins then act as damage-associated molecular patterns and stimulate the production of cytokines, including IL-1β, IL-6, and IL-23; these proteins are then processed into peptides and presented to T cells. The resulting T-cell activation stimulates IFN-γ and IL-17A production and exacerbates hypertension, which was shown by transferring isoketal-activated DCs into wild-type mice, leading to a rise in BP [54]. The control group of Rag1−/− mice lacking T cells did not develop hypertension. Consistently, treatment with isoketal scavengers prevented hypertension and associated renal damage in animal studies [54]. Thus, DCs mediate the development of hypertension through proinflammatory T-cell activation and enhance oxidative stress, as well as fluid and sodium retention.
γδ T cells
The unconventional and rather small subgroup of γδ T cells has receptors composed of γ- and δ-chains and plays a role in hypertension by producing IL-17A and IFN-γ (for further elaboration of the hypertensive effects of IL-17A and IFN-y, see CD4+ T cells). Caillon et al. showed that Ang II-induced hypertension was paralleled by an increase in the number and activation of γδ T cells and that knockout or antibody-mediated γδ T deficiency prevented Ang II-induced hypertension, endothelial dysfunction and splenic T-cell activation in mice [55]. In humans, there was an association between blood γδ T-cell frequency and (rising) BP. γδT cells are also involved in the inflammatory infiltration of the hypertensive heart, and γδT-cell depletion prevented Ang II-induced cardiac injury in mice [56].
Natural killer T cells
Although further studies are necessary, the first evidence from CD1d-knockout mice suggested a protective role of natural killer T cells (NKTCs) in the development of hypertension. Cd1d knockout in APCs leads to impaired NKTC activation and subsequent reductions in NKTC activity. Wang et al. showed increases in Ang II-induced hypertension, cardiac inflammation, hypertrophy and fibrosis in mice with impaired NKTC activity and IL-10 production [57]. Adoptive transfer of CD1d-knockout bone marrow to wild-type mice confirmed these findings. Activating NKTCs specifically with α-galactosylceramide or recombinant murine IL-10 improved Ang II-induced hypertension, cardiac function and unfavorable remodeling.
Natural killer and innate lymphoid cells
Natural killer (NK) cells have cytotoxic functions and a large array of activating and inhibitory receptors are distinct from ILCs, which are the most recently discovered family of innate immune cells. The latter are primarily tissue-resident cells with innate and adaptive features that respond to pathogenic tissue damage by secreting signaling molecules and cytokines. These properties may predispose ILCs to play roles in the development of hypertension, which has not been studied thus far. In comparison, a proinflammatory effect of classic NK cells in hypertensive cells has already been suggested; Ang II-induced the proliferation and migration of NK cells in vitro and their recruitment to the aortic wall, causing endothelial dysfunction in vivo, and this effect was blunted by the presence of anti-NK cell antibodies [29, 58]. Additional studies are needed to further characterize the role of NK cells in hypertension and to elucidate the effects of other ILC types on BP and the cardiovascular system.
The adaptive immune system and hypertension
The first conclusive evidence for the role of the adaptive immune system in the pathogenesis of arterial hypertension was provided by Guzik et al. and showed that the increase in BP caused by Ang II infusion was significantly blunted in mice lacking T and B cells [59]. This finding was confirmed in several other laboratories and genetic models [60,61,62,63]. Surprisingly, we did not observe resistance to the effects of Ang II in B6.Rag1−/− mice, which do not have mature T and B cells [64]. The Sandberg laboratory also reported that Jackson B6.Rag1−/− mice lost their resistance to Ang II-induced hypertension later on [65]. While it is clear that lymphocytes play a critical role in hypertension, the negative data illustrate that there are important but unidentified confounders. Possible confounders range from the loss of phenotype due to compensatory mechanisms in the immune system over generations of laboratory breeding to undiscovered environmental factors, which might change over time and between different laboratories, such as regular chow and/or alterations in the microbiota [63, 66, 67]. Under the hypothesis of compensatory mechanisms, one could even consider on the possibly of even greater importance of the immune system in BP regulation since it seems to be able to regain its effect on BP after initial knockout. Taken together, this ambiguity sparks even more interest in disentangling the complex relations among immune cells and arterial hypertension, which is why we will discuss the latest insights into how cells of the adaptive immune system regulate BP.
CD4+ T cells
Depending on antigen stimulation and costimulation, as well as exposure to specific cytokines, naïve CD4+ T-helper cells differentiate into different lineages, as determined by master transcription factors that are activated by signaling transducer and activator of transcription (STAT) signaling. The resulting subpopulations are TH1, TH2, and TH17 cells, Treg cells and follicular helper T cells, which have specific cytokine profiles and functions [68]. As early as 1976, Svendsen demonstrated the need for T cells ni the development of chronic hypertension and renal end-organ damage in response to DOCA salt treatment in athymic mice, and DOCA salt treatment induced a prolonged increase in BP only after thymus transplantation from wild-type mice [69]. This finding of the need for T cells in hypertension was confirmed by Guzik et al. in T- and B-cell-deficient mice, which developed Ang II-induced hypertension, endothelial dysfunction, vascular remodeling and increased oxidative stress only upon adoptive transfer of T cells [59]. Salt-sensitive rats showed a significant increase in renal T-cell infiltration and oxidative stress with increasing salt intake, which was blunted by immunosuppressive treatment with the calcineurin inhibitor (CNI) tacrolimus for 3 weeks [70]. Tacrolimus treatment also significantly reduced high-salt-induced mean arterial blood pressure and albumin excretion in this study, which serves as proof of concept that calcineurin inhibition antagonizes salt-induced hypertension. The clinical effects, however, are not as straightforward: data from several studies showed reductions, no changes or increases in blood pressure in response to CNI treatment [71]. These clinical effects were probably due to the long-term off-target effects of CNIs, which enhance hypertension by increasing sympathetic activity, endothelin production, renal afferent vasoconstriction, and salt retention [71]. In addition to oxidative stress, T cells seem to influence BP via cytokine-dependent regulation of renal sodium reabsorption [72]. Mice that are deficient in IFN-γ, the major cytokine of TH1 cells, showed differences in the abundance and phosphorylation of renal ion transporters after Ang II infusion compared to wild-type mice. In addition, these mice showed decreased levels of sodium-proton-exchanger (NHE) in the proximal tubule, sodium potassium chloride cotransporter (NKCC) in the thick ascending limb and sodium chloride cotransporter (NCC) in the distal tubulus, resulting in a blunted hypertensive responses [72]. NHE and the overall hypertensive response to Ang II were also reduced in IL-17-deficient mice [72]. Another study confirmed that IL-17A deficiency abolished the activation of NCC and the epithelial sodium channel (ENaC) and blunted glomerular and tubular injury in Ang II-infused mice. In vitro treatment of distal convoluted tubule cells with IL-17A increased NCC activity via the phosphorylation of serum glucocorticoid-regulated kinase 1 (SGK1), a downstream target of osmoregulatory and proinflammatory signaling pathways (also see Salt, immune cell activation, and antimicrobial defense). These effects were countered by SGK1 inhibition [73]. IL-17A, which is mainly produced by proinflammatory TH17 cells, is a key mediator of renal and vascular dysfunction in hypertensive mice, correlates with hypertension in humans and constitutes a promising target for new therapeutic approaches, such as IL-17 antibodies, as these agents are already approved for certain autoimmune diseases [74].
CD8+ T cells
Further evidence for a role of T-cell-driven inflammation in human hypertension stems from Youn et al. who observed a T-cell population with an increased fraction of immunosenescent, proinflammatory cytotoxic CD8+ T cells in the peripheral blood samples of patients with newly diagnosed hypertension [75]. CD8+ T cells constitute a major source of IFN-γ, and hypertensive patients showed increased numbers of CD8+ T cells expressing IFN-γ, granzyme B, perforin, and tumor necrosis factor alpha (TNF-α). Granzyme B, a serine protease, and perforin, a pore-forming protein, are involved in cell-mediated cytotoxicity. Granzyme B is further thought to be involved in matrix remodeling, fibrosis, and the disruption of endothelial barrier function. Serum granzyme B levels were elevated in hypertensive patients, and Shen et al. found that granzyme B deficiency protected against Ang II-induced cardiac end-organ damage, including hypertrophy, fibrosis, and inflammation [76, 77]. CD8+ deficiency in mice also prevented Ang II-induced endothelial dysfunction and renal vascular remodeling and rarefaction, leading to the alleviation of Ang II-induced hypertension [78]. CD8+-deficient mice showed superior renal sodium excretion upon artificial sodium loading, suggesting a role for cytotoxic CD8+ T cells in sodium retention. The previously mentioned effect of CD8+ T-cell-derived IFN-γ on renal sodium transporters, particularly NHE in the proximal tubule, NKCC in the thick ascending limb and NCC in the distal tubulus, might be one reason for this observation [72]. Another way CD8+ T cells can influence renal sodium resorption was observed by Liu et al.: direct contact of CD8+ T cells with cells of the distal convoluted tube stimulated the expression of NCC and the development of salt-sensitive hypertension in DOCA salt-treated mice [79]. In vitro, this upregulation was shown to involve ROS-induced activation of tyrosine-protein kinase Src (also called cSrc, cellular and sarcoma) and the subsequent upregulation and stimulation of potassium and chloride channels to facilitate increased sodium reabsorption. Spatial separation of CD8+ T cells and tubular epithelial cells abolished the observed effect, strongly supporting the necessity of direct physical contact [79].
B cells
Although the adoptive transfer of B cells alone was not able to ameliorate hypertension in T-cell- and B cell-deficient mice, accumulating evidence supports the role of B cells in the etiology of hypertension. Chen et al. demonstrated an increase in IgG and the accumulation of activated B cells, plasmablasts and plasma cells in the aortic wall upon Ang II infusion [80]. Mice with B-cell deficiency due to knockout of the B-cell activating factor receptor (Tnfrsf13c−/− mice) or the transcription factor Myb (c-mybh/h mice), as well as mice treated with anti-CD20 antibody therapy, were protected against Ang II-induced hypertension and showed reduced aortic macrophage infiltration, collagen deposition and vascular stiffening [80, 81]. In vitro treatment of macrophages with purified IgG from hypertensive mice induced the production of the profibrotic transforming growth factor (TGF)-β. The protective effect was reversed by adoptive transfer of wild-type B cells into Tnfrsf13c−/− mice [80]. Depletion of plasma cells by the proteasome inhibitor bortezomib showed similar beneficial results [82]. These results are consistent with long-standing findings that patients with essential and pregnancy-related hypertension have elevated circulating levels of IgG and IgM in comparison to normotensive individuals, and hypertensive patients are prone to develop circulating autoantibodies against a variety of autoantigens, including AT1 receptors, adrenoceptors and voltage-gated calcium channels [83,84,85,86]. Since B cell-targeted therapies are frequently used in clinical settings, the effect of anti-CD20 therapy on blood pressure in humans is of high interest. Data on the effects on blood pressure, however, are often confounded by polypharmacy. Studies without polypharmacy consistently showed a short-term reduction in BP in response to anti-CD20 treatment, but long-term results were inconsistent [71].
Regulatory T cells
Regulatory T cells (Tregs) are essential immunosuppressive regulators of the immune response and inflammation that induce and maintain immunological tolerance mainly through IL-10 production. Recent data suggest a protective role of Tregs in the etiology of hypertension, particularly by regulating renal and vascular inflammatory processes. The first evidence was provided by animal studies that showed an association between hypertension and decreased Treg counts in peripheral blood and the kidney [76, 87,88,89,90,91,92]. Although these findings were not reproducible in all mouse models of hypertension and in humans, the protective effects of Tregs against hypertension is underscored by several other findings [76, 93]. Vascular damage induced by Ang II infusion was exacerbated in Rag1−/− mice with T-cell deficiency and T-cell reconstitution from mice lacking Tregs due to a mutation in the master transcription factor of Tregs, FoxP3 [94]. Consistently, IL-10-deficient mice exhibited exacerbated endothelial dysfunction and increased superoxide production in response to Ang II [95]. Studies on the adoptive transfer of Tregs in Ang II- or aldosterone- and salt-treated mice yielded heterogeneous results regarding BP reductions, but a robust decrease in hypertension-induced end-organ damage, such as vascular stiffening and cardiac hypertrophy was observed [76]. Chen et al. further examined the relationship between Ang II-induced damage and Treg function and suggested a role of complement, particularly the binding of the anaphylatoxins C3a and C5a to their receptors on Tregs, in Ang II-mediated impairment of Treg function. The researchers found decreased expression of FoxP3 in response to Ang II treatment [88]. Knockout of C3a and C5a receptors alleviated Ang II-induced hypertension and renal and vascular damage. Adoptive transfer of C3a- and C5a-deficient Tregs protected against Ang II-induced hypertension [88]. Another Treg-dependent way to protect against hypertension and subsequent damage was demonstrated by Taylor et al. [96] and Majeed et al. [97] who showed that selective Treg expansion by infusion of low-dose IL-2 attenuated BP increases and renal damage in a mouse model of SLE, inhibited aortic collagen remodeling and resulted in vascular stiffening in Ang II-treated mice [96, 97]. Furthermore, cotreatment with IL-10 and IL-4 prevented preeclampsia in pregnant mice [98]. Taken together, these data strongly support a beneficial, anti-inflammatory role of Tregs in the etiology of hypertension and particularly in subsequent end-organ damage. Further elucidation of the underlying pathological mechanisms might provide new therapeutic approaches, including the expansion, stimulation or even application of Tregs and associated cytokines, which is a promising outlook, especially in light of currently expanding Treg-based therapies in the treatment of autoimmune diseases, transplant rejection and graft-versus-host disease [99].
The influence of the immune system on cardiovascular health involves not only the development of hypertension but also the chronic inflammation driving atherosclerosis and cardiovascular disease (CVD) [100]. Research on systemic anti-inflammatory treatment of CVD first targeted C-reactive protein in rats with acute myocardial infarction (MI) to limit infarct size and cardiac dysfunction (39). Further research now shows promising results in reducing cardiovascular morbidity and mortality. The monoclonal antibody canakinumab, which targets IL-1β, could reduce recurrent cardiovascular events in a high-risk population with previous MI and elevated CRP [101]. Similarly, continued colchicine treatment after MI decreased the risk of ischemic cardiovascular events [102]. However, this systemic anti-inflammatory treatment comes at the cost of increased susceptibility to (fatal) infectious complications [101]. To fully exploit the beneficial effects of immunosuppression on cardiovascular health while avoiding (infectious) side effects, a better understanding of the underlying pathological mechanisms and, ultimately, more specific anti-inflammatory treatment options are needed.
Salt
Salt plays a central role in the development and maintenance of hypertension, as highlighted in our revised version of the mosaic theory shown in Fig. 1. Consumption of the modern diet has led to a worldwide increase in sodium intake to levels above the biologically required and recommended limits. These eating habits can promote hypertension and cardiovascular disease [103, 104]. However, there is still a vigorous debate about optimal salt intake and the importance of dietary salt reductions, which were recently fueled by a global observational study that showed a positive correlation between salt intake and life expectancy and an inverse correlation with all-cause mortality [105]. Such an emotional discussion is rather unusual in scientific questions. Could the emotional energy surrounding the issue of salt be due to the cultural and historical significance of this substance over the last several thousand years [106, 107]?
Salt has a long history with mankind. The harvest of salt from the surface of Xiechi Lake near Yuncheng in Shanxi, China, is one of the oldest verifiable saltworks and dates back to 6000 B.C. [108]. The Latin terms for health and healthy, “salutem” and “salubris”, are derived from the word “sal” (salt). Approximately 2700 years B.C. The PENG-TZAO-KAN-MU was published in China. It is the earliest known treatise on pharmacology, and a large part was devoted to the discussion of more than 40 kinds of salt. This discussion included descriptions of salt extraction and manufacturing methods that show amazing similarity to processes used today [108]. Salt products were rare and expensive and brought wealth and power to its owners. It is thought that much of the construction of The Great Wall of China was paid by the salt tax [108]. Salt was used as money in many regions of the world, and salt money has recently been found in Africa and China [108].
Human evolution and salt intake
Hominid evolution began 30 million years ago in warm regions and was associated with a vegetarian diet and low salt intake. Adequate salt intake is important in tropical areas to enable acclimatization to intense sweating [109]. The development of salt saving mechanisms would thus have been favored by selection pressure, as an adequate amount of salt is indispensable for life. The consequences of salt consumption were of peculiar interest long ago. The famous Yellow Emperor wrote in ~3000 B.C.: “If too much salt is used in food, the pulse hardens, tears make their appearance and the complexion changes” [110]. However, only recently in the 19th century did the idea that too much salt intake could be harmful arise [109]. Since then, this harm has been investigated further. Excess salt ingestion can have profound health consequences, including hypertension and cardiovascular target organ damage. Several potential pathophysiological mechanisms relating a high-salt diet to cardiovascular disease have been characterized and include volume expansion and changes in the RAAS and ROS. Salt increases BP and induces damage in the target organ at least in part by polarizing adaptive and innate immune cells toward a proinflammatory phenotype, which is discussed in detail below.
New salt-associated concepts: Salt, inflammatory cells, and hypertension
Salt, which is a central component of the mosaic theory of hypertension, was long known to be an important factor in the etiology of hypertension; early concepts of renal hypertension suggested that insufficient renal sodium excretion led to the expansion of extracellular fluid volume during high-salt intake and a subsequent rise in BP due to increased cardiac output and an adaptive increase in peripheral resistance [9, 12]. Guyton noted the increase in salt excretion with increased BP [12]. Additionally, salt sensitivity in arterial hypertension was discovered early in salt feeding experiments with rats and was later shown to affect ~25% of normotensive and 50% of hypertensive humans, with an increased prevalence in the elderly population [9, 111, 112]. Salt sensitivity is characterized by the enhanced dependence of BP on dietary salt intake of affected individuals, and an increase in dietary salt results in a parallel rise in BP [112]. Overreactivity of the RAAS and sympathetic nervous system, vascular dysfunction with increased peripheral resistance and endothelial stiffness in response to high sodium, insufficient negative tubuloglomerular feedback of the macula densa and atrial natriuretic peptide are among the reasons for impaired renal sodium excretion in salt-sensitive hypertension [112]. For further details, the reader is referred to the recent review by Ellison and Welling on salt handling and blood pressure [113]. Accordingly, epidemiological genomic studies showed a moderate heritability of salt sensitivity and the associations of different genetic variants affecting the RAAS, ion channels involved in sodium and water regulation, endothelial components, and gene products involved in the generation of NO and ROS [114]. The multitude of associated genetic variants hints at the complexity with which salt influences BP. Over the last decades, our understanding of the complex influence of sodium on BP has been broadened, and many new and fascinating details have been illuminated further.
An important finding in recent years is that salt can directly modulate the phenotypes of myeloid cells and different subsets of T cells, as reviewed recently by Rucker et al. and Wilck et al. [103, 115]. Whether salt-induced immunomodulation leads to beneficial or harmful effects on the body might depend on the specific immunological context and is explained in more detail below.
Cutaneous salt storage
One new approach to explain salt sensitivity and its increase with age is an impaired or exhausted ability to balance total body sodium over time. Sodium is the most abundant cation in extracellular fluid, which consists of the interstitial and intravascular fluids. Contrary to the initial assumption of iso-osmolarity in those two fluid compartments, the idea of hypertonic sodium storage in interstitial fluid in the skin, which is controlled and drained into the intravascular fluid by lymphatic vessels, emerged, since rodents fed high sodium exhibited hypertonic interstitial volume retention [116]. This finding triggered some interest to improve understanding of the effect of altered ion availability on the biology of immune cells in maintaining inflammatory, infectious, and homeostatic processes [117,118,119]. High sodium concentrations in turn are recognized as chemotactic stimuli by macrophages in a dose-dependent manner, as depicted in Fig. 3 [120]. Macrophages can sense high sodium concentrations via sodium calcium exchanger 1 (NCX1), resulting in macrophage activation and the subsequent activation of the osmoprotective transcription factor nuclear factor of activated T cells 5 (NFAT5, also called TonEBP) [121]. NFAT5 leads to increased production of NO by nitric oxide synthase (NOS)-2 and the production of proinflammatory cytokines such as IL-1β, IL-6 and TNF-α, which are released in response to high salt [122]. Furthermore, NFAT5 triggers the secretion of vascular endothelial growth factor (VEGF)-C [116, 121]. VEGF-C signaling in turn results in VEGF receptor (VEGFR) 3-dependent hyperplasia of the lymph capillary network, which enhances interstitial sodium clearance by improving the drainage of interstitial fluid and electrolytes into the intravascular compartment. VEGF-C also stimulates the expression of endothelial nitric oxide synthase (eNOS) via the VEGFR2 receptor, increasing NO production and acting as a direct compensatory vasodilatory mechanism to buffer the increase in BP due to excess extracellular volume [116]. Furthermore, a disturbance in macrophage infiltration or VEGF-C signaling increases BP in rats fed a high-salt diet [116].
In addition to the central role of macrophage and dendritic cell signaling in osmotic homeostasis, the importance of glycosaminoglycans (GAGs) in the regulation of interstitial sodium storage was postulated by Titze et al. [123]. A hypertonic, osmotically inactive (e.g., water-free) sodium concentration of up to 190 mmol/l was found to be associated with skin levels of GAG, which might be responsible for binding sodium via its negative charge [123]. Changing the skin levels of GAG by inhibiting or stimulating GAG chain polymerization might be a means of regulating skin as a potential sodium reservoir [123]. Accordingly, 23Na-MRI visualization of skin sodium levels showed increased sodium accumulation with age, as well as in patients with refractory hypertension, and skin biopsies of healthy volunteers revealed an increase in skin sodium concentrations that paralleled the increase in dietary salt intake [124, 125].
In T cells, sodium enters the cell by the NKCC1 transporter, which can be inhibited by furosemide. Sodium upregulates the expression of NFAT5 and its downstream target SGK1. SGK1 phosphorylation subsequently enables retinoic-acid receptor-related orphan receptor gamma t (RORγt)-mediated transcription of the IL-23 receptor, which is essential for TH17 polarization (Fig. 3) [103].
Salt, immune cell activation, and antimicrobial defense
In addition to its role in BP regulation, locally increased sodium concentrations in the skin were thought to serve another purpose; Jantsch et al. found local sodium accumulation in skin infection sites in humans and mice, strengthening antimicrobial host defense by activating macrophages via NFAT5, enhancing NO production and targeting pathogens to autolysosomes [122, 126]. Ultimately, a high-salt diet improved antimicrobial control and healing in mice infected with Leishmania major [122]. Accordingly, sodium chloride treatment of macrophages followed by activation via lipopolysaccharide (LPS) or CD4+ T-cell interactions in vitro stimulated a proinflammatory phenotype with enhanced production of the proinflammatory cytokines TNF-α and interleukin 12 (IL-12), promoted the differentiation of “M1-like” macrophages and reduced M2-like regulatory macrophage activity to suppress T-cell proliferation [127, 128]. The downside of this salt-induced macrophage boost lies in macrophage-driven autoimmunity. Mice with experimental autoimmune encephalomyelitis (EAE, a mouse model of multiple sclerosis) on a high-salt diet exhibited increased macrophage infiltration and activation within the central nervous system, and transferring macrophages that were treated ex vivo with excess amounts of sodium chloride exacerbated the disease state [127]. In addition, glucocorticoids are increased during a high-salt diet in mice and humans. This effect is a direct consequence of the downregulation of aldosterone synthase, which causes the accumulation of corticosterone, which is an aldosterone precursor with glucocorticoid functionality [129].
Consistent with macrophage infiltration and activation, Kleinewietfeld et al. and Wu et al. showed increased induction and infiltration of TH17 cells in the central nervous system in mice with EAE that were fed a high-salt diet [130, 131]. Exposure to excess sodium chloride in vitro enhanced the polarization of naïve CD4+ cells to TH17 cells and upregulated the production of the proinflammatory cytokines IL-17A, granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α and IL-2 [131]. This induction of TH17 cells is mediated by the osmoprotective transcription factor NFAT5 and its downstream target SGK1, which deactivates the transcription factor forkhead box protein O1 (FOXO1). Together with the upregulation of the TH17 transcription marker RORγt, this leads to enhanced transcription and translation of the IL-23 receptor and subsequent TH17 cell activation by IL-23 [130]. Biochemical or genetic ablation of NFAT5 or SGK1 abolishes salt-induced TH17 differentiation [131]. Furthermore, high sodium chloride conditions compromise regulatory T-cell function by reducing IFN-γ production via SGK1 [132]. SGK1 is also involved in osmoregulation as a target of mineralocorticoid receptor (MR) signaling. Indeed, SGK1 is responsible for the mineralocorticoid-induced increase in renal epithelial sodium channel ENaC activity by slowing its degradation. Therefore, SGK1 is essential for antinatriuresis under low salt conditions, and urinary sodium loss and decreases in BP and the glomerular filtration rate have lethal consequences in SGK1-knockout mice under salt restriction [133].
A high sodium concentration can further promote T-cell stimulation and increased IFN-γ and IL-17A production by boosting DC activation: DCs sense environmental sodium via amiloride-sensitive sodium channels, specifically NHE1 and ENaC [134]. Sodium influx elicits calcium influx by plasma membrane-bound NCX1, and calcium in turn activates protein kinase C (PKC) to phosphorylate NADPH oxidase (NOX) 2 subunits. The subsequent increased production of superoxide promotes the processing and presentation of self-antigens by DCs, resulting in autoimmune-like inflammation and dysfunction in the kidney and vasculature. Transferring high sodium-treated DCs into naïve mice and infusing subpressor doses of Ang II resulted in hypertension, and this effect was not seen after the transfer of DCs exposed to normal sodium concentrations [134].
An effect of sodium on the complement system was discovered when renin-mediated cleavage of C3 into C3b and C3a linked sodium-dependent RAAS activity to complement activation [135]. The detailed role of the complement system in arterial hypertension itself remains unclear and is comprehensively reviewed elsewhere [31, 32].
Salt and vascular dysfunction
As mentioned previously, vascular dysfunction, including endothelial dysfunction with impaired vasodilatation, arterial stiffening, hypertrophy, and the proliferation of VSMCs, plays an important role in the etiology of hypertension. High-salt levels can contribute to these adverse mechanisms. In addition, there is circumstantial evidence that sodium can accumulate around endothelial cells [136]. Sodium enters endothelial cells through endothelial sodium channels (EnNaC), where it stabilizes actin filaments within the actin network beneath the plasma membrane, causing mechanical stiffening of the endothelial cell [137]. Additionally, high sodium concentrations increase the abundance of EnNaC in the endothelial cell membrane. EnNaC in turn is thought to further stabilize actin filaments through direct biomechanical interactions, as was shown for the ENaC alpha subunit [138]. Stiff endothelial cells are less deformable by blood stream alterations and subsequently release less NO. High intracellular sodium further impairs NO production by inhibiting endothelial NO synthase. Reduced NO release from endothelial cells enhances VSMC-mediated vasoconstriction and all arterial stiffening. High sodium concentrations also impact the endothelial glycocalyx (eGC). The eGC works as a shear stress sensor with subsequent inward cell signaling and as a sodium buffer, attracting the positively charged sodium ion to negatively charged proteoglycans [137]. In response to prolonged salt overload, the sodium buffer capacity of the eGC diminishes, and structural changes in heparan sulfate residues damage the eGC structure, impairing the endothelial sodium barrier and increasing sodium absorption into the cell [139]. Damage and conformational changes in the eGC further influence intracellular signaling and contribute to sodium-induced mechanical changes in endothelial cells. Chronic changes in mechanical properties and reduced NO release ultimately lead to the transition from endothelial function to dysfunction, increasing TGF-β1-mediated fibrogenesis, arterial stiffness and the development of hypertension [137, 140]. These salt-induced changes in the endothelium can be prevented by EnNaC inhibition, either directly by amiloride or indirectly by spironolactone [137].
Salt and (extra)renal RAAS
The sodium concentration in the distal tubulus is sensed by tubular epithelial cells of the macula densa via ion channels, particularly NKCC2 and potentially NHE2 [141]. Low sodium concentrations lead to the formation of prostanoids such as prostaglandin E2 (PGE2) and prostacyclin (PGI2), NO and adenosine triphosphate (ATP), which stimulate renin secretion by juxtaglomerular cells of the afferent arteriole and thereby initiate RAAS-mediated increases in BP [141]. The BP increase induced by RAAS activation is mediated by an increase in renal sodium reabsorption, among other pleiotropic effects. While high sodium intake suppresses renin secretion and the systemic RAAS under healthy conditions, a study with a mouse model of chronic kidney disease on a high-salt diet showed upregulation due to mechanisms other than systemic RAAS [141, 142]. On a high-salt diet, 5/6 nephrectomized mice exhibited activation of intrarenal RAAS stemming from renal tissue other than juxtaglomerular cells and increased expression of angiotensin-converting enzyme (ACE)-1, Ang II, and AT1 receptors. Additionally, neuronal expression of Ang II and AT1 receptors in brain regions that are important for cardiovascular regulation, including the organum vasculosum of the lamina terminalis, was enhanced [142]. Independent of preexisting kidney disease, sodium also affects neuronal activity within the organum vasculosum directly through sodium concentrations in cerebrospinal fluid; elevated sodium concentrations in the organum vasculosum of Sprague-Dawley rats increased sympathetic nerve activity and BP in a concentration-dependent manner [143].
Salt and oxidative stress
Oxidative stress and ROS influence the cardiovascular system, including the vasculature, heart, kidney, and nervous system, through pleiotropic effects, altering oxidative posttranslational modifications and redox signaling. ROS directly inactivate NO and subsequently inhibit vasodilatation. This disturbance in NO-dependent relaxation of VSMCs is further impaired by high sodium concentrations, which inhibit the activity of endothelial NO synthase. Additionally, high sodium intake enhances NAD(P)H oxidase activity and decreases superoxide dismutase expression in the renal cortex in rats, increasing local ROS generation [144]. Increased ROS can activate neuronal signaling in renal afferents and subsequently induce further peripheral vasoconstriction [9]. Accordingly, salt-sensitive rats show increased renal medullary oxidative stress due to excess superoxide generated by NOX, and NOX4 is especially important for salt-induced renal injury and hypertension [24, 145]. Recently, there has been emerging evidence that ROS contribute to immune activation in hypertension [24, 25].
Salt, the microbiome, and inflammation
By interacting with the external environment and internal metabolism, the gut microbiome plays an important role in priming the immune system. Furthermore, the gut microbiome is essential for the digestion of food, as well as the production of vitamins and other bioactive metabolites. Altogether, this leads to the suggestion that the gut microbiome is an endocrine organ and has crucial effects on other organs and complex systems within the body [146]. Understanding the individual components and bioactive metabolites of the gut microbiota in health and disease holds great potential for the development of new drug targets and (nutritive) interventions for several diseases. Studies have shown reduced microbial diversity in patients and mice with arterial hypertension, which is known as dysbiosis [146]. Partially controversial data even showed an association between colonization with certain microbial species and high BP [146]. Moreover, stool transplantation from hypertensive patients to germ-free mice led to a significant increase in BP in these mice compared to mice transplanted with fecal microbiotas from normotensive humans [147]. However, after comparing germ-free and conventionally raised mice, Karbach et al. found a reduction in the upregulation of RORγt, the signature transcription factor for IL-17 synthesis, and the attenuation of blood pressure increases in response to Ang II in germ-free mice [148]. This finding suggests that the gut microbiota facilitates Ang II-induced hypertension by IL-17-driven inflammation [148]. The link between the gut microbiome and hypertension was elegantly investigated by Wilck et al. who demonstrated the depletion of Lactobacillus murinus induced by high-salt intake in mice and in humans [149]. The reduction in L. murinus led to a reduced production of indoles. Indoles were shown to influence the differentiation of CD4+ cells into TH17 cells in a dose-dependent manner; more indoles were associated with the less polarization into TH17 cells. Accordingly, TH17 cells were expanded among lymphocytes in the intestinal lamina propria in mice that were fed a high-salt diet and had depleted L. murinus and reduced indole production, and this effect was absent in germ-free mice. Furthermore, treatment with L. murinus in mice fed a high-salt diet attenuated these changes and reduced salt-sensitive hypertension and autoimmune encephalomyelitis by modulating the TH17 cell population [149]. Expansion of TH17 cells due to a high-salt diet and a concurrent reduction in L. murinus could also affect other immune-mediated diseases. The exacerbation of experimentally induced colitis by high-salt intake was associated with a decrease in L. murinus, an increase in TH17 cells in the mesenteric lymph nodes and enhanced expression of proinflammatory genes related to IL-17 in immune cells of the lamina propria in the colon [150]. Furthermore, intestinal levels of the short-chain fatty acid butyrate were reduced; this bacterial metabolite is known to have an anti-inflammatory effect on intestinal macrophages, induce the differentiation of Treg cells in the colon and influence T-cell differentiation in vitro by inhibiting TH17 cell polarization [151,152,153]. Transferring butyrate-exposed T cells into mice led to the development of an attenuated form of colitis compared to the transfer of nonbutyrate-exposed T cells [151, 153]. Additionally, Faraco et al. showed IL-17-dependent endothelial and cognitive dysfunction in mice fed a high-salt diet [154]. Accordingly, the short-chain fatty acid propionate significantly attenuated systemic inflammation, cardiac hypertrophy, fibrosis, vascular dysfunction, and arterial hypertension in Ang II-infused wild-type and apolipoprotein E-knockout mice [155]. This effect was abolished in Ang II-infused mice that lacked Tregs, suggesting that Tregs mediate these anti-inflammatory effects [155].
An important and interesting observation was recently made by Rosshart et al. [156]. The mammalian phenotype is driven by the combination of the host genome and the microbial genome (microbiome), which is referred to as the metagenome. However, the repertoire of microbes in the wild has not been replicated in the laboratory. This difference can substantially distort the development and function of the immune system under laboratory conditions, leading to false translational assumptions of how our own immune system works. Thus, laboratory mice are too far from natural environmental conditions to faithfully mirror the physiology of humans. To address this shortcoming, embryos of laboratory mice can be transferred into wild mice to generate wildlings that resemble the natural mammalian metaorganism while preserving the research benefits of the tractable genetics of laboratory mice [146]. To our knowledge, hypertension has not yet been examined in wildlings. More work needs to be done to clarify the role of gut bacteria in hypertension.
Impact of salt: outlook
The importance of daily salt intake was underscored recently by the results of the Salt Substitute and Stroke Study in China, which examined the effect of substituting table salt, and the researchers examined 100% sodium chloride and substitutes containing 75% sodium chloride and 25% potassium chloride over a period of 5 years [157]. The authors found a reduction in systolic BP by approximately 3.3 mmHg and decreased risks of stroke, severe cardiovascular events, and overall mortality by ~14%, 13% and 12%, respectively [157]. High potassium intake may lower blood pressure and reduce salt sensitivity. Accordingly, a prospective cohort study assessed sodium and potassium intake that was measured in 24 h urine samples and showed a dose-dependent association between higher sodium and lower potassium intakes with an increased cardiovascular risk [158]. To explain this finding, a potassium switch in the distal nephron was proposed recently that permits homeostasis despite variations in sodium intake [113]. A new aspect of high sodium intake and its impact on water homeostasis has been discussed recently [159]. Increased renal salt excretion and water conservation in response to high dietary salt intake results in katabolic metabolism. Sodium excretion in parallel with water reabsorption requires extrarenal urea production in the liver and skeletal muscles, as well as urea recycling in the kidneys; both processes require high energy consumption [160]. To provide for the increased tubular energy consumption, endogenous protein sources such as skeletal muscle are exploited or increased food intake is needed [161]. Overall, salt plays a pivotal role in the etiology of arterial hypertension and cardiovascular disease.
Aldosterone, mineralocorticoid receptor, inflammatory cells, and hypertension
The history of aldosterone and the mineralocorticoid receptor (MR) can be divided into three phases. First, aldosterone and the MR are mainly known for their classic effects: promoting renal sodium and water reabsorption by inducing epithelial sodium channels (ENaC) in epithelial cells in the distal nephron in response to aldosterone binding, thereby regulating BP and salt homeostasis, as shown in Fig. 4A [162]. However, in the last decade, it has been shown that MR is present on virtually all cells of the cardiovascular system, such as endothelial cells, VSMCs and cardiomyocytes [162, 163] (Fig. 4B). Experimental studies using cell type-specific gene targeting of the MR in mice have revealed the importance of this extrarenal aldosterone signaling in cardiomyocytes, endothelial cells and VSMCs in hypertension and CVD. MR activation in vascular smooth muscle cells (Fig. 4B) directly contributes to vascular oxidative stress, vasoconstriction, and arterial hypertension, as McCurley et al. showed by selectively knocking out MR in vascular smooth muscle cells in mice [164]. Finally, in the third phase, it became clear that MR also plays an important and proinflammatory role in the innate and adaptive immune systems. The effect of mineralocorticoid is conveyed via the ligand-activated translocation of cytosolic MR into the nucleus, where it binds to a specific DNA sequence known as the hormone response element and initiates proinflammatory gene expression (Fig. 4C).
A The classic effect of the MR is to promote renal sodium reabsorption by increasing the abundance of the epithelial sodium channel (ENaC) on the apical membrane of epithelial cells in the collecting duct after binding to its classic ligand aldosterone. B Second, it became clear that MR signaling in endothelial cells, VSMCs and cardiomyocytes promotes hypertension and fibrosis. C Third and more recently, it was discovered that MR signaling in innate and adaptive immune cells can cause hypertension and hypertensive end-organ damage. The effect of mineralocorticoid is conveyed via the ligand-activated translocation of cytosolic MR into the nucleus, where it binds to a specific DNA sequence known as the hormone response element (HRE) and initiates proinflammatory gene expression, inducing an M1 cascade with increased generation of ROS and M1 markers, as well as the secretion of MMPs and cytokines. In T cells, MR interacts with NFAT1 and AP-1 and promotes differentiation toward IFN-γ- and IL-17A-producing T-cell populations. ENaC epithelial sodium channel, ROMK renal outer medullary potassium channel, MR mineralocorticoid receptor, VSMC vascular smooth muscle cell, M/Ms monocytes/macrophages, MMP matrix metalloprotease, HRE hormone response element, ROS reactive oxygen species, TGF transforming growth factor, TNF tumor necrosis factor, INF interferon, IL interleukin, AP-1 activator protein 1, NFAT1 nuclear factor of activated T cells 1
The MR in innate immune cells
The presence of the MR in monocytes/macrophages (M/Ms) has been shown at the mRNA and protein levels [165,166,167]. Aldosterone infusion in rodents promotes renal macrophage infiltration and macrophage differentiation toward the proinflammatory M1-like phenotype and enhances the expression of markers associated with M1-like activation, such as TNF-α and inducible nitric oxide synthase (iNOS) [41, 162, 168]. This aldosterone-induced M1-like activation is reduced by aldosterone antagonists [168]. MR signaling in M/Ms increases the expression of markers linked to M1-like activation, ROS formation and the secretion of inflammatory cytokines and matrix metalloproteases (MMPs) (Fig. 4C) [165, 169]. In contrast, myeloid-specific knockout of MR or MR antagonist treatment skews cells toward an M2-like anti-inflammatory phenotype and induces specific M2-like markers such as IL-10, Arg1 and peroxisome proliferator-activated receptor (PPAR-γ). The role of the myeloid MR has been studied in different models of hypertension. Knockout of MR in monocytes and macrophages uniformly decreased vascular and cardiac fibrosis [165, 170], independent of the model of hypertension used. Cardiac hypertrophy and fibrosis induced by aortic constriction were also significantly attenuated in myeloid MR-deficient mice. Macrophage infiltration in the heart was inhibited, and the expression of inflammatory genes was decreased in myeloid MR-deficient mice. In addition, aortic fibrosis and inflammation were attenuated [171]. MR-knockout mice showed a smaller increase in BP and cardiac fibrosis than wild-type mice after 8 weeks of DOCA salt treatment. In summary, these studies clearly demonstrate a role for MR in macrophages in hypertension and hypertensive cardiovascular remodeling [67].
The MR in adaptive immune cells
MR expression in lymphocytes has been shown by Armanini et al. by radioreceptor assays [172]. Moreover, the expression of MR in T cells has been demonstrated by FACS analysis, PCR and Western blotting [173]. Therefore, convincing data exist that MR is expressed in T cells. Li et al. and Sun et al. found an important role for MR in T cells in two studies. First, the researchers showed that T-cell-specific MR knockout in mice resulted reduced cardiac hypertrophy, fibrosis and dysfunction compared to those in control mice after abdominal aortic constriction [171]. Correspondingly, the mice exhibited reduced cardiac inflammation, which was illustrated by decreased accumulation of myeloid cells and reduced expression of inflammatory cytokines. Flow cytometry showed that T-cell-specific MR-knockout mitigated the Ang II-induced accumulation of IFN-γ-producing T cells, particularly the CD8+ population, in both the kidneys and aorta. At the molecular level, MR interacted with NFAT1 and activator protein-1 (AP-1) in T cells (Fig. 4C) [173]. Barbaro, Kirabo and Harrison proposed in response to these data that MR signaling is a so called signal 3 in T cells. [174]. T cells require three signals for activation, called signals 1, 2, and 3 (Fig. 5). T-cell receptor recognition of specific antigenic peptides presented on APCs constitutes signal 1. Signal 2 is costimulation, which involves interactions between CD80 and CD86 on APCs and CD28 on T cells. Signal 3 involves the stimulation of receptors outside of the immunologic synapse by hormones, cytokines, and danger signals present in the inflammatory milieu [174]. Therefore, MR signaling is clearly a new signal 3 for lymphocytes in arterial hypertension [67].
MR activation as a third signal in the immunological synapse in T-cell activation. T cells require three signals for activation: signals 1, 2, and 3. Signal 1 is T-cell receptor recognition of specific antigenic peptides presented on APCs. Signal 2 is costimulation, which involves interactions between CD80 and CD86 on APCs and CD28 on T cells. Signal 3 involves the stimulation of receptors outside of the immunologic synapse by hormones, cytokines, and danger signals present in the inflammatory milieu. MR signaling is a new signal 3 for lymphocytes associated with arterial hypertension. MR on T cells stimulates the production of IFN-γ, resulting in hypertension and cardiovascular fibrosis. DC dendritic cell, MHC major histocompatibility complex, TCR T-cell receptor, CD cluster of differentiation, AP-1 activator protein 1, NFAT1 nuclear factor of activated T cells 1, IL interleukin, IFN interferon
Type 1 angiotensin receptor in immune cells
The most important part of the RAAS is undoubtedly the AT1 receptor. Although classically known for its role in regulating circulatory homeostasis, Ang II acts as a powerful proinflammatory mediator by stimulating the AT1 receptor. The binding of Ang II to its receptor results in nuclear factor (NF)-κB and ROS activation. This activation increases vascular permeability and upregulates adhesion molecules, chemokines and inflammatory cytokines such as TNF-α and IL-6 [175]. The AT1 receptor is expressed on almost all cells in the body. However, very little data are available on the role of the AT1 receptor in inflammatory cells in hypertension. The AT1 receptor is expressed on myeloid cells and lymphocytes. Surprisingly, activation of the AT1 receptor in these cells has immunologic effects that diverge from AT1 receptor stimulation in the kidney, heart, or vasculature. AT1 receptors on immune cells appear to play a protective role, as reviewed by Crowley and Rudemiller and summarized in Fig. 6 [23].
In an Ang II infusion model of hypertension, AT1 receptor–deficient bone marrow chimeras have exaggerated BP elevation, albuminuria, and accumulation of T cells and macrophages in the kidney [176]. In the same Ang II infusion model, mice lacking myeloid AT1 receptors have a preserved hypertensive response but more severe renal tubular injury and interstitial fibrosis than controls [177]. The capacity of macrophage AT1 receptors to mitigate kidney fibrosis was similarly evident in a normotensive model of kidney fibrosis known as the ureteral obstruction model [177]. Activating the AT1 receptor on macrophages suppresses their proinflammatory M1 polarization and reduces TNF-α and IL-1β expression.
Ang II-induced hypertension in T-cell-specific AT1 receptor-knockout mice resulted in similar BP responses but augmented albuminuria and exaggerated perivascular accumulation of CD4+ T lymphocytes in the hypertensive kidney, as reviewed in [23]. CD4+ T cells lacking the AT1 receptor were isolated from the hypertensive kidney or spleen and expressed increased levels of the proinflammatory cytokines IFN-γ and TNF-α and the TH1 transcription factor T-bet (Tbx21), which drives the expression of these cytokines in T cells [178]. Thus, AT1 receptor stimulation in T cells suppresses T-bet-dependent differentiation of CD4+ T-helper cells toward the proinflammatory TH1 cell lineage (Fig. 6).
Thus, based on bone marrow chimera and conditional gene targeting studies, AT1 receptors on myeloid and lymphoid populations play an immunomodulatory role that tempers the pathogenic actions of AT1 receptors in the kidney and vasculature during hypertension [23]. Interestingly, immune amplification by AT1 receptor blockade resulted in a severe, autoimmune-mediated enteropathy associated with olmesartan treatment in rare cases [179, 180]. The reason for the opposite effects of MR and AT1 receptor activation in inflammatory cells remains unclear. One possible advantage of this counterintuitive finding was postulated by Crowley and Rudemiller [23]: the protective effect of AT1 receptors on immune cells may provide a feedback mechanism to alleviate or limit the proinflammatory effects of AT1 receptor activation in target organs. Once immune cells are recruited into tissue that is damaged by AT1 receptor activation, these cells can dampen inflammation and limit the severity of injury.
Conclusion
Approximately 80 years have passed since Page proposed the mosaic theory of arterial hypertension. Since then, several new, exciting, and sometimes surprising findings and connections have been added. These findings prompted us to propose a revised version of the mosaic theory that is shown in Fig. 1. Although immune cells and high-salt intake are important for protecting against invading pathogens, their interaction and consequent overactivation may lead to tissue damage and high BP. Clearly, salt and inflammation play a central role in almost every aspect of hypertension and therefore deserve a place in the center of the mosaic. In humans, verification of the experimental evidence suggesting a relationship between salt intake, immune cells, inflammation and hypertension is a major challenge, but an improved understanding of how salt and immune cells influence hypertension is crucial and clinically relevant for identifying therapeutic interventions. Research in wildling mice, which have diverse microbial compositions compared to clean laboratory mice, may mimic human hypertension better than current experimental models and may have more translational relevance. A better and deeper understanding of the immune mechanisms of hypertension will allow for more targeted and personalized therapeutic approaches. The aim of investigating immunity in hypertension is to develop therapies that can be tested in vivo. Novel technologies, such as targeted nanoparticles, cell-specific siRNA or nanobodies, may be able to inhibit or overexpress certain factors in specific subsets of immune cells, thus delivering precise and targeted therapies without the risks of global immunosuppression [76]. In this context, MR (but not the AT1 receptor) in myeloid or lymphoid cells could be a target of interest. There are remaining knowledge gaps to be addressed before immunomodulatory therapies might be applied to even a subset of patients with hypertension. In contrast, reducing salt intake or substituting sodium with potassium are easy to achieve and successful in reducing BP and hypertensive end-organ damage [157]. This reduction in BP and morbidity is predicted by the revised mosaic theory that places salt and inflammation in the center of hypertension.
References
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23.
Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165–82.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;2018:3021–104.
Brunstrom M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28–36.
Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens. 2014;32:2285–95.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–5.
Page IH. Pathogenesis of arterial hypertension. J Am Med Assoc. 1949;140:451–8.
Page IH. The nature of arterial hypertension. Arch Intern Med. 1963;111:103–15.
Harrison DG, Coffman TM, Wilcox CS. Pathophysiology of hypertension: the mosaic theory and beyond. Circ Res. 2021;128:847–63.
Kovarik JJ, Morisawa N, Wild J, Marton A, Takase-Minegishi K, Minegishi S, et al. Adaptive physiological water conservation explains hypertension and muscle catabolism in experimental chronic renal failure. Acta Physiol. 2021;232:e13629.
Wild J, Jung R, Knopp T, Efentakis P, Benaki D, Grill A, et al. Aestivation motifs explain hypertension and muscle mass loss in mice with psoriatic skin barrier defect. Acta Physiol. 2021;232:e13628.
Guyton AC, Coleman TG, Cowley AW Jr., Liard JF, Norman RA Jr. Manning RD, Jr. Systems analysis of arterial pressure regulation and hypertension. Ann Biomed Eng. 1972;1:254–81.
Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, et al. Interaction between hypertension and arterial stiffness. Hypertension. 2018;72:796–805.
Taddei S, Virdis A, Ghiadoni L, Sudano I, Salvetti A. Endothelial dysfunction in hypertension. J Cardiovasc Pharmacol. 2001;38:S11–4.
Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, et al. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res. 2014;114:616–25.
Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KRC, Xiao L, et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Investig. 2016;126:50–67.
Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, et al. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension. 2016;68:1415–23.
DeLalio LJ, Sved AF, Stocker SD. Sympathetic nervous system contributions to hypertension: updates and therapeutic relevance. Can J Cardiol. 2020;36:712–20.
Tigerstedt R, Bergman PQ. Niere und Kreislauf. Skandinavisches Archiv Für. Physiologie. 1898;8:223–71.
Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental Hypertension: I. The Production of persistent elevation of systolic blood pressure by means of renal Ischemia. J Exp Med. 1934;59:347–79.
Braun-Menendez E, Fasciolo JC, Leloir LF, Muñoz JM. The substance causing renal hypertension. J Physiol. 1940;98:283–98.
Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. J Exp Med. 1940;71:29–42.
Crowley SD, Rudemiller NP. Immunologic effects of the renin-angiotensin system. J Am Soc Nephrol. 2017;28:1350–61.
Griendling KK, Camargo LL, Rios FJ, Alves-Lopes R, Montezano AC, Touyz RM. Oxidative stress and hypertension. Circ Res. 2021;128:993–1020.
Loperena R, Harrison DG. Oxidative stress and hypertensive diseases. Med Clin North Am. 2017;101:169–93.
Magavern EF, Warren HR, Ng FL, Cabrera CP, Munroe PB, Caulfield MJ. An academic clinician’s road map to hypertension genomics. Hypertension. 2021;77:284–95.
Ng FL, Warren HR, Caulfield MJ. Hypertension genomics and cardiovascular prevention. Ann Transl Med. 2018;6:291.
Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. 2018;215:21–33.
Higaki A, Caillon A, Paradis P, Schiffrin EL. Innate and innate-like immune system in hypertension and vascular injury. Curr Hypertens Rep. 2019;21:4.
Wenzel U, Turner JE, Krebs C, Kurts C, Harrison DG, Ehmke H. Immune mechanisms in arterial hypertension. J Am Soc Nephrol. 2016;27:677–86.
Wenzel UO, Kemper C, Bode M. The role of complement in arterial hypertension and hypertensive end organ damage. Br J Pharmacol. 2021;178:2849–62.
Ruan C-C, Gao P-J. Role of complement-related inflammation and vascular dysfunction in hypertension. Hypertension. 2019;73:965–71.
Wenzel UO, Bode M, Kohl J, Ehmke H. A pathogenic role of complement in arterial hypertension and hypertensive end organ damage. Am J Physiol Heart Circ Physiol. 2017;312:H349–54.
Liu X, Zhang Q, Wu H, Du H, Liu L, Shi H, et al. Blood neutrophil to lymphocyte ratio as a predictor of hypertension. Am J Hypertens. 2015;28:1339–46.
Zhang R, Inagawa H, Kazumura K, Tsuchiya H, Miwa T, Morishita N, et al. Evaluation of a hypertensive rat model using peripheral blood neutrophil activity, phagocytic activity and oxidized LDL evaluation. Anticancer Res. 2018;38:4289–94.
Ciuceis CD, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2106–13.
Ko EA, Amiri F, Pandey NR, Javeshghani D, Leibovitz E, Touyz RM, et al. Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am J Physiol Heart Circ Physiol. 2007;292:H1789–95.
Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–81.
Hashmat S, Rudemiller N, Lund H, Abais-Battad JM, Van Why S, Mattson DL. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Ren Physiol. 2016;311:F555–61.
Ulrich C, Heine GH, Garcia P, Reichart B, Georg T, Krause M, et al. Increased expression of monocytic angiotensin-converting enzyme in dialysis patients with cardiovascular disease. Nephrol Dial Transplant. 2006;21:1596–602.
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20.
Harwani SC. Macrophages under pressure: the role of macrophage polarization in hypertension. Transl Res. 2018;191:45–63.
Wenzel P. Monocytes as immune targets in arterial hypertension. Br J Pharmacol. 2019;176:1966–77.
Ridker PM, Rane M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ Res. 2021;128:1728–46.
Ridker PM, Devalaraja M, Baeres FMM, Engelmann MDM, Hovingh GK, Ivkovic M, et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021;397:2060–9.
Wenzel UO, Ehmke H, Bode M. Immune mechanisms in arterial hypertension. Recent advances. Cell Tissue Res. 2021;385:393–40.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74.
Park MJ, Lee SH, Kim EK, Lee EJ, Baek JA, Park SH, et al. Interleukin-10 produced by myeloid-derived suppressor cells is critical for the induction of Tregs and attenuation of rheumatoid inflammation in mice. Sci Rep. 2018;8:3753.
Shah KH, Shi P, Giani JF, Janjulia T, Bernstein EA, Li Y, et al. Myeloid suppressor cells accumulate and regulate blood pressure in hypertension. Circ Res. 2015;117:858–69.
Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, et al. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–37.
Hevia D, Araos P, Prado C, Fuentes Luppichini E, Rojas M, Alzamora R, et al. Myeloid CD11c(+) antigen-presenting cells ablation prevents hypertension in response to angiotensin II plus high-salt diet. Hypertension. 2018;71:709–18.
Brahler S, Zinselmeyer BH, Raju S, Nitschke M, Suleiman H, Saunders BT, et al. Opposing roles of dendritic cell subsets in experimental GN. J Am Soc Nephrol. 2018;29:138–54.
Lu X, Rudemiller NP, Privratsky JR, Ren J, Wen Y, Griffiths R, et al. Classical dendritic cells mediate hypertension by promoting renal oxidative stress and fluid retention. Hypertension. 2020;75:131–8.
Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Investig. 2014;124:4642–56.
Caillon A, Mian MOR, Fraulob-Aquino JC, Huo KG, Barhoumi T, Ouerd S, et al. gammadelta T cells mediate angiotensin II-induced hypertension and vascular injury. Circulation. 2017;135:2155–62.
Li Y, Wu Y, Zhang C, Li P, Cui W, Hao J, et al. γδT Cell-derived interleukin-17A via an interleukin-1β-dependent mechanism mediates cardiac injury and fibrosis in hypertension. Hypertension. 2014;64:305–14.
Wang HX, Li WJ, Hou CL, Lai S, Zhang YL, Tian C, et al. CD1d-dependent natural killer T cells attenuate angiotensin II-induced cardiac remodelling via IL-10 signalling in mice. Cardiovasc Res. 2019;115:83–93.
Kossmann S, Schwenk M, Hausding M, Karbach SH, Schmidgen MI, Brandt M, et al. Angiotensin II-induced vascular dysfunction depends on interferon-γ-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler Thrombo Vasc Biol. 2013;33:1313–9.
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–60.
Ji Q, Cheng G, Ma N, Huang Y, Lin Y, Zhou Q, et al. Circulating Th1, Th2, and Th17 levels in hypertensive patients. Dis Mark. 2017;2017:7146290.
Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–7.
Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol. 2013;304:R407–14.
Madhur MS, Kirabo A, Guzik TJ, Harrison DG. From rags to riches: moving beyond RAG1 in studies of hypertension. Hypertension. 2020;75:930–4.
Seniuk A, Thiele JL, Stubbe A, Oser P, Rosendahl A, Bode M, et al. B6.Rag1 knockout mice generated at the Jackson laboratory in 2009 show a robust wild-type hypertensive phenotype in response to ang II (Aangiotensin II). Hypertension. 2020;75:1110–6.
Ji H, Pai AV, West CA, Wu X, Speth RC, Sandberg K. Loss of resistance to angiotensin II-induced hypertension in the Jackson laboratory recombination-activating gene null mouse on the C57BL/6J background. Hypertension. 2017;69:1121–7.
Rios FJ, Montezano AC, Touyz RM. Lessons learned from RAG-1-deficient mice in hypertension. Hypertension. 2020;75:935–7.
van der Heijden Cdcc BM, Riksen NP, Wenzel UO. MR in inflammation and immunity. Br J Pharmacol. 2021. https://doi.org/10.1111/bph.15782. [Online ahead of print].
Zhu JT. Helper cell differentiation, heterogeneity, and plasticity. Cold Spring Harb Perspect Biol. 2018;10:a030338.
Svendsen UG. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol Microbiol Scand A. 1976;84:523–8.
De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Ren Physiol. 2011;300:F734–42.
Murray EC, Nosalski R, MacRitchie N, Tomaszewski M, Maffia P, Harrison DG, et al. Therapeutic targeting of inflammation in hypertension: from novel mechanisms to translational perspective. Cardiovasc Res. 2021;117:2589–609.
Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, et al. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-gamma-/- and interleukin-17A-/- mice. Hypertension. 2015;65:569–76.
Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, et al. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension. 2016;68:167–74.
Davis GK, Fehrenbach DJ, Madhur MS. Interleukin 17A: key player in the pathogenesis of hypertension and a potential therapeutic target. Curr Hypertens Rep. 2021;23:13.
Youn J-C, Yu HT, Lim BJ, Koh MJ, Lee J, Chang D-Y, et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126–33.
Madhur MS, Elijovich F, Alexander MR, Pitzer A, Ishimwe J, Van Beusecum JP, et al. Hypertension: do inflammation and immunity hold the key to solving this epidemic? Circ Res. 2021;128:908–33.
Shen Y, Cheng F, Sharma M, Merkulova Y, Raithatha SA, Parkinson LG, et al. Granzyme B deficiency protects against angiotensin II-induced cardiac fibrosis. Am J Pathol. 2016;186:87–100.
Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, et al. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension. 2014;64:1108–15.
Liu Y, Rafferty TM, Rhee SW, Webber JS, Song L, Ko B, et al. CD8(+) T cells stimulate Na-Cl co-transporter NCC in distal convoluted tubules leading to salt-sensitive hypertension. Nat Commun. 2017;8:14037.
Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, et al. Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension. 2015;66:1023–33.
Mathis KW, Wallace K, Flynn ER, Maric-Bilkan C, LaMarca B, Ryan MJ. Preventing autoimmunity protects against the development of hypertension and renal injury. Hypertension. 2014;64:792–800.
Taylor EB, Barati MT, Powell DW, Turbeville HR, Ryan MJ. Plasma cell depletion attenuates hypertension in an experimental model of autoimmune disease. Hypertension. 2018;71:719–28.
Chan CT, Lieu M, Toh BH, Kyaw TS, Bobik A, Sobey CG, et al. Antibodies in the pathogenesis of hypertension. Biomed Res Int. 2014;2014:504045.
Kristensen BO, Andersen PL, Wiik A. Autoantibodies and vascular events in essential hypertension: a five-year longitudinal study. J Hypertens. 1984;2:19–24.
Ebringer A, Doyle AE. Raised serum IgG levels in hypertension. Br Med J. 1970;2:146–8.
Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol. 2019;19:517–32.
Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–76.
Chen XH, Ruan CC, Ge Q, Ma Y, Xu JZ, Zhang ZB, et al. Deficiency of complement C3a and C5a receptors prevents angiotensin II-induced hypertension via regulatory T cells. Circ Res. 2018;122:970–83.
Chen Z-Y, Chen F, Wang Y-G, Wang D-H, Jang L-L, Cheng L-X. Down-regulation of Helios expression in Tregs from patients with hypertension. Curr Med Sci. 2018;38:58–63.
Emmerson A, Trevelin SC, Mongue-Din H, Becker PD, Ortiz C, Smyth LA, et al. Nox2 in regulatory T cells promotes angiotensin II–induced cardiovascular remodeling. J Clin Investig. 2018;128:3088–101.
Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, et al. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension. 2012;59:324–30.
Katsuki M, Hirooka Y, Kishi T, Sunagawa K. Decreased proportion of Foxp3+CD4+ regulatory T cells contributes to the development of hypertension in genetically hypertensive rats. J Hypertension. 2015;33:773–83.
Krebs CF, Lange S, Niemann G, Rosendahl A, Lehners A, Meyer-Schwesinger C, et al. Deficiency of the interleukin 17/23 axis accelerates renal injury in mice with deoxycorticosterone acetate+angiotensin II-induced hypertension. Hypertension. 2014;63:565–71.
Mian MO, Barhoumi T, Briet M, Paradis P, Schiffrin EL. Deficiency of T-regulatory cells exaggerates angiotensin II-induced microvascular injury by enhancing immune responses. J Hypertens. 2016;34:97–108.
Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II–induced vascular dysfunction. Hypertension. 2009;54:619–24.
Taylor EB, Sasser JM, Maeda KJ, Ryan MJ. Expansion of regulatory T cells using low-dose interleukin-2 attenuates hypertension in an experimental model of systemic lupus erythematosus. Am J Physiol Ren Physiol. 2019;317:F1274–84.
Majeed B, Tawinwung S, Eberson LS, Secomb TW, Larmonier N, Larson DF. Interleukin-2/anti-interleukin-2 immune complex expands regulatory T cells and reduces angiotensin II-induced aortic stiffening. Int J Hypertens. 2014;2014:126365.
Chatterjee P, Chiasson VL, Seerangan G, Tobin RP, Kopriva SE, Newell-Rogers MK, et al. Cotreatment with interleukin 4 and interleukin 10 modulates immune cells and prevents hypertension in pregnant mice. Am J Hypertens. 2015;28:135–42.
Raffin C, Vo LT, Bluestone JA. Treg cell-based therapies: challenges and perspectives. Nat Rev Immunol. 2020;20:158–72.
Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–6.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31.
Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–505.
Wilck N, Balogh A, Marko L, Bartolomaeus H, Muller DN. The role of sodium in modulating immune cell function. Nat Rev Nephrol. 2019;15:546–58.
Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–34.
Messerli FH, Hofstetter L, Syrogiannouli L, Rexhaj E, Siontis GCM, Seiler C, et al. Sodium intake, life expectancy, and all-cause mortality. Eur Heart J. 2020;42:2103–12.
Wenzel UO, Bode M, Kurts C, Ehmke H. Salt, inflammation, IL-17 and hypertension. Br J Pharmacol. 2019;176:1853–63.
Ritz E. The history of salt—aspects of interest to the nephrologist. Nephrol Dial Transplant. 1996;11:969–75.
McDowell L. Mineral nutrition history: the early years. Sarasota, FL, USA: First Edition Design Publishing; 2017.
Círillo M, Capasso G, Leo VAD, Santo NGD. A history of salt. Am J Nephrol. 1994;14:426–31.
Luft FC, Rankin LI, Bloch R, Weyman AE, Willis LR, Murray RH, et al. Cardiovascular and humoral responses to extremes of sodium intake in normal black and white men. Circulation. 1979;60:697–706.
Dahl LK, Heine M, Tassinari L. Role of genetic factors in susceptibility to experimental hypertension due to chronic excess salt ingestion. Nature. 1962;194:480–2.
Balafa O, Kalaitzidis RG. Salt sensitivity and hypertension. J Hum Hypertens. 2021;35:184–92.
Ellison DH, Welling P. Insights into salt handling and blood pressure. N Engl J Med. 2021;385:1981–93.
Liu Y, Shi M, Dolan J, He J. Sodium sensitivity of blood pressure in Chinese populations. J Hum Hypertens. 2020;34:94–107.
Rucker AJ, Rudemiller NP, Crowley SD. Salt, hypertension, and immunity. Annu Rev Physiol. 2018;80:283–307.
Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–52.
Schatz V, Neubert P, Schroder A, Binger K, Gebhard M, Muller DN, et al. Elementary immunology: Na(+) as a regulator of immunity. Pediatr Nephrol. 2017;32:201–10.
Jobin K, Müller DN, Jantsch J, Kurts C. Sodium and its manifold impact on our immune system. Trends Immunol. 2021;42:469–79.
Müller DN, Wilck N, Haase S, Kleinewietfeld M, Linker RA. Sodium in the microenvironment regulates immune responses and tissue homeostasis. Nat Rev Immunol. 2019;19:243–54.
Muller S, Quast T, Schroder A, Hucke S, Klotz L, Jantsch J, et al. Salt-dependent chemotaxis of macrophages. PLoS ONE. 2013;8:e73439.
Neubert P, Homann A, Wendelborn D, Bar AL, Krampert L, Trum M, et al. NCX1 represents an ionic Na+ sensing mechanism in macrophages. PLoS Biol. 2020;18:e3000722.
Jantsch J, Schatz V, Friedrich D, Schroder A, Kopp C, Siegert I, et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 2015;21:493–501.
Titze J, Shakibaei M, Schafflhuber M, Schulze-Tanzil G, Porst M, Schwind KH, et al. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Heart Circ Physiol. 2004;287:H203–8.
Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Muller DN, et al. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61:635–40.
Selvarajah V, Maki-Petaja KM, Pedro L, Bruggraber SFA, Burling K, Goodhart AK, et al. Novel mechanism for buffering dietary salt in humans: effects of salt loading on skin sodium, vascular endothelial growth factor C, and blood pressure. Hypertension. 2017;70:930–7.
Neubert P, Weichselbaum A, Reitinger C, Schatz V, Schroder A, Ferdinand JR, et al. HIF1A and NFAT5 coordinate Na(+)-boosted antibacterial defense via enhanced autophagy and autolysosomal targeting. Autophagy. 2019;15:1899–916.
Hucke S, Eschborn M, Liebmann M, Herold M, Freise N, Engbers A, et al. Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J Autoimmun. 2016;67:90–101.
Binger KJ, Gebhardt M, Heinig M, Rintisch C, Schroeder A, Neuhofer W, et al. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J Clin Investig. 2015;125:4223–38.
Jobin K, Stumpf NE, Schwab S, Eichler M, Neubert P, Rauh M, et al. A high-salt diet compromises antibacterial neutrophil responses through hormonal perturbation. Sci Transl Med. 2020;12:eaay3850.
Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–7.
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–22.
Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Investig. 2015;125:4212–22.
Wulff P, Vallon V, Huang DY, Volkl H, Yu F, Richter K, et al. Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Investig. 2002;110:1263–8.
Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, et al. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 2017;21:1009–20.
Bekassy ZD, Kristoffersson AC, Rebetz J, Tati R, Olin AI, Karpman D. Aliskiren inhibits renin-mediated complement activation. Kidney Int. 2018;94:689–700.
Olde Engberink RHG, Selvarajah V, Vogt L. Clinical impact of tissue sodium storage. Pediatr Nephrol. 2020;35:1373–80.
Kusche-Vihrog K, Schmitz B, Brand E. Salt controls endothelial and vascular phenotype. Pflug Arch. 2015;467:499–512.
Mazzochi C, Bubien JK, Smith PR, Benos DJ. The carboxyl terminus of the alpha-subunit of the amiloride-sensitive epithelial sodium channel binds to F-actin. J Biol Chem. 2006;281:6528–38.
Oberleithner H, Peters W, Kusche-Vihrog K, Korte S, Schillers H, Kliche K, et al. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflug Arch. 2011;462:519–28.
Lang F. Stiff endothelial cell syndrome in vascular inflammation and mineralocorticoid excess. Hypertension. 2011;57:146–7.
Castrop H, Hocherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiol Rev. 2010;90:607–73.
Cao W, Li A, Wang L, Zhou Z, Su Z, Bin W, et al. A salt-induced reno-cerebral reflex activates renin-angiotensin systems and promotes CKD progression. J Am Soc Nephrol. 2015;26:1619–33.
Kinsman BJ, Simmonds SS, Browning KN, Stocker SD. Organum vasculosum of the lamina terminalis detects NaCl to elevate sympathetic nerve activity and blood pressure. Hypertension. 2017;69:163–70.
Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, et al. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol. 2003;14:2775–82.
Taylor NE, Glocka P, Liang M, Cowley AW. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 2006;47:692–8.
Avery EG, Bartolomaeus H, Maifeld A, Marko L, Wiig H, Wilck N, et al. The gut microbiome in hypertension. Circulation Res. 2021;128:934–50.
Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14.
Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016;5:e003698.
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–9.
Miranda PM, De Palma G, Serkis V, Lu J, Louis-Auguste MP, McCarville JL, et al. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome. 2018;6:57.
Chen L, Sun M, Wu W, Yang W, Huang X, Xiao Y, et al. Microbiota metabolite butyrate differentially regulates Th1 and Th17 cells’ differentiation and function in induction of colitis. Inflamm Bowel Dis. 2019;25:1450–61.
Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111:2247–52.
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50.
Faraco G, Brea D, Garcia-Bonilla L, Wang G, Racchumi G, Chang H, et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat Neurosci. 2018;21:240–9.
Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139:1407–21.
Rosshart SP, Herz J, Vassallo BG, Hunter A, Wall MK, Badger JH, et al. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science. 2019;365:eaaw4361.
Neal B, Wu Y, Feng X, Zhang R, Zhang Y, Shi J, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067–77.
Ma Y, He FJ, Sun Q, Yuan C, Kieneker LM, Curhan GC, et al. 24-Hour urinary sodium and potassium excretion and cardiovascular risk. N Engl J Med. 2022;386:252–63.
Rossitto G, Delles C. Mechanisms of sodium-mediated injury in cardiovascular disease: old play, new scripts. FEBS J. 2021. https://doi.org/10.1111/febs.16155 [Online ahead of print].
Kitada K, Daub S, Zhang Y, Klein JD, Nakano D, Pedchenko T, et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J Clin Investig. 2017;127:1944–59.
Rossitto G, Maiolino G, Lerco S, Ceolotto G, Blackburn G, Mary S, et al. High sodium intake, glomerular hyperfiltration, and protein catabolism in patients with essential hypertension. Cardiovasc Res. 2021;117:1372–81.
van der Heijden C, Deinum J, Joosten LAB, Netea MG, Riksen NP. The mineralocorticoid receptor as a modulator of innate immunity and atherosclerosis. Cardiovasc Res. 2018;114:944–53.
Bene NC, Alcaide P, Wortis HH, Jaffe IZ. Mineralocorticoid receptors in immune cells: emerging role in cardiovascular disease. Steroids. 2014;91:38–45.
McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–33.
Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54:537–43.
Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schütz G, et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Investig. 2010;120:3350–64.
Montes-Cobos E, Schweingruber N, Li X, Fischer HJ, Reichardt HM, Lühder F. Deletion of the mineralocorticoid receptor in myeloid cells attenuates central nervous system autoimmunity. Front Immunol. 2017;8:1319.
Martin-Fernandez B, Rubio-Navarro A, Cortegano I, Ballesteros S, Alia M, Cannata-Ortiz P, et al. Aldosterone induces renal fibrosis and inflammatory M1-macrophage subtype via mineralocorticoid receptor in rats. PLoS ONE. 2016;11:e0145946.
Bienvenu LA, Morgan J, Rickard AJ, Tesch GH, Cranston GA, Fletcher EK, et al. Macrophage mineralocorticoid receptor signaling plays a key role in aldosterone-independent cardiac fibrosis. Endocrinology. 2012;153:3416–25.
Shen ZX, Chen XQ, Sun XN, Sun JY, Zhang WC, Zheng XJ, et al. Mineralocorticoid receptor deficiency in macrophages inhibits atherosclerosis by affecting foam cell formation and efferocytosis. J Biol Chem. 2017;292:925–35.
Li C, Zhang YY, Frieler RA, Zheng XJ, Zhang WC, Sun XN, et al. Myeloid mineralocorticoid receptor deficiency inhibits aortic constriction-induced cardiac hypertrophy in mice. PLOS ONE. 2014;9:e110950.
Armanini D, Endres S, Kuhnle U, Weber PC. Parallel determination of mineralocorticoid and glucocorticoid receptors in T- and B-lymphocytes of human spleen. Acta Endocrinol. 1988;118:479–82.
Sun XN, Li C, Liu Y, Du LJ, Zeng MR, Zheng XJ, et al. T-cell mineralocorticoid receptor controls blood pressure by regulating interferon-gamma. Circ Res. 2017;120:1584–97.
Barbaro NR, Kirabo A, Harrison DG. A new role of mister (MR) T in hypertension: mineralocorticoid receptor, immune system, and hypertension. Circ Res. 2017;120:1527–9.
Chang Y, Wei W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin Exp Immunol. 2015;179:137–45.
Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, et al. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension. 2010;55:99–108.
Zhang JD, Patel MB, Griffiths R, Dolber PC, Ruiz P, Sparks MA, et al. Type 1 angiotensin receptors on macrophages ameliorate IL-1 receptor-mediated kidney fibrosis. J Clin Investig. 2014;124:2198–203.
Zhang JD, Patel MB, Song YS, Griffiths R, Burchette J, Ruiz P, et al. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ Res. 2012;110:1604–17.
Marthey L, Cadiot G, Seksik P, Pouderoux P, Lacroute J, Skinazi F, et al. Olmesartan-associated enteropathy: results of a national survey. Aliment Pharmacol Ther. 2014;40:1103–9.
Rubio-Tapia A, Herman ML, Ludvigsson JF, Kelly DG, Mangan TF, Wu TT, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc. 2012;87:732–8.
Acknowledgements
The work of UW is supported by the German Research Foundation and the ADMIRE network. The authors thank Jonathan Jantsch for critical reading and discussion of the paper.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hengel, F.E., Benitah, JP. & Wenzel, U.O. Mosaic theory revised: inflammation and salt play central roles in arterial hypertension. Cell Mol Immunol 19, 561–576 (2022). https://doi.org/10.1038/s41423-022-00851-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-022-00851-8
Keywords
This article is cited by
-
Salt Behind the Scenes of Systemic Lupus Erythematosus and Rheumatoid Arthritis
Current Nutrition Reports (2023)