Abstract
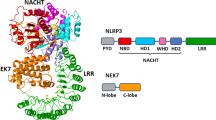
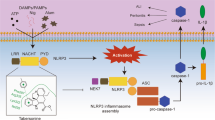
The NLRP3 inflammasome plays a crucial role in innate immune-mediated inflammation and contributes to the pathogenesis of multiple autoinflammatory, metabolic and neurodegenerative diseases, but medications targeting the NLRP3 inflammasome are not available for clinical use. RRx-001 is a well-tolerated anticancer agent currently being investigated in phase III clinical trials, but its effects on inflammatory diseases are not known. Here, we show that RRx-001 is a highly selective and potent NLRP3 inhibitor that has strong beneficial effects on NLRP3-driven inflammatory diseases. RRx-001 inhibits the activation of the canonical, noncanonical, and alternative NLRP3 inflammasomes but not the AIM2, NLRC4 or Pyrin inflammasomes. Mechanistically, RRx-001 covalently binds to cysteine 409 of NLRP3 via its bromoacetyl group and therefore blocks the NLRP3-NEK7 interaction, which is critical for the assembly and activation of the NLRP3 inflammasome. More importantly, RRx-001 treatment attenuates the symptoms of lipopolysaccharide (LPS)-induced systemic inflammation, dextran sulfate sodium (DSS)-induced colitis and experimental autoimmune encephalomyelitis (EAE) in mice. Thus, our study identifies RRx-001 as a new potential therapeutic agent for NLRP3-driven diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Martinon, F., Mayor, A. & Tschopp, J. The Inflammasomes: guardians of the Body. Annu. Rev. Immunol. 27, 229–265 (2009).
Davis, B. K., Wen, H. T. & Ting, J. P. Y. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29 29, 707–735 (2011).
Chen, G., Shaw, M. H., Kim, Y. G. & Nunez, G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev. Pathol.-Mech. 4, 365–398 (2009).
Broz, P. & Dixit, V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 (2016).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Masters, S. L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat. Immunol. 11, 897–904 (2010).
Wen, H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 (2011).
Unamuno, X. et al. NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling. Cell Mol. Immunol. 18, 1045–1057 (2021).
He, Y. et al. Immunopathobiology and therapeutic targets related to cytokines in liver diseases. Cell Mol. Immunol. 18, 18–37 (2021).
Heneka, M. T. et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Bauer, C. et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59, 1192–1199 (2010).
Zaki, M. H. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010).
Lamkanfi, M. & Dixit, V. M. Inflammasomes and their roles in health and disease. Annu Rev. Cell Dev. Biol. 28, 137–161 (2012).
He, Y. et al. 3,4-Methylenedioxy-beta-nitrostyrene Inhibits NLRP3 Inflammasome Activation by Blocking Assembly of the Inflammasome. J. Biol. Chem. 289, 1142–1150 (2014).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
Jiang, H. et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 214, 3219–3238 (2017).
Huang, Y. et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 10, e8689 (2018).
He, H. et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 9, 2550 (2018).
Marchetti, C. et al. OLT1177, a beta-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl Acad. Sci. USA 115, E1530–E1539 (2018).
Coll, R. C. et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 15, 556–559 (2019).
Mullard, A. NLRP3 inhibitors stoke anti-inflammatory ambitions. Nat. Rev. Drug Disco. 18, 405–407 (2019).
Kluck, V. et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2, e270–e280 (2020).
Oronsky, B. et al. Rockets, radiosensitizers, and RRx-001: an origin story part I. Disco. Med 21, 173–180 (2016).
Kim, M. M. et al. Whole brain radiotherapy and RRx-001: two partial responses in radioresistant melanoma brain metastases from a phase I/II clinical trial: a TITE-CRM phase I/II clinical trial. Transl. Oncol. 9, 108–113 (2016).
Carter, C. et al. Early results: “Rocket” a Phase II Study of Rrx-001, a novel triple epigenetic inhibitor, resensitization to irinotecan in colorectal cancer. Ann. Oncol. 26, ii3 (2015).
Morgensztern, D. et al. RRx-001 followed by platinum plus etoposide in patients with previously treated small-cell lung cancer. Br. J. Cancer 121, 211–217 (2019).
Oronsky, B. et al. REPLATINUM Phase III randomized study: RRx-001 + platinum doublet versus platinum doublet in third-line small cell lung cancer. Future Oncol. 15, 3427–3433 (2019).
Carter, C. A. et al. Partial response to platinum doublets in refractory EGFR-positive non-small cell lung cancer patients after RRx-001: evidence of episensitization. Case Rep. Oncol. 9, 62–67 (2016).
Oronsky, B. et al. RRx-001: a systemically non-toxic M2-to-M1 macrophage stimulating and prosensitizing agent in Phase II clinical trials. Expert Opin. Investig. Drugs 26, 109–119 (2017).
Oronsky, B. et al. RRx-001, a novel dinitroazetidine radiosensitizer. Invest N. Drugs 34, 371–377 (2016).
Scicinski, J. et al. Preclinical evaluation of the metabolism and disposition of RRx-001, a novel investigative anticancer agent. Drug Metab. Dispos. 40, 1810–1816 (2012).
Zhao, H. et al. Epigenetic effects of RRx-001: a possible unifying mechanism of anticancer activity. Oncotarget 6, 43172–43181 (2015).
Oronsky, B., Scicinski, J., Cabrales, P. & Minchinton, A. RRx-001, an epigenetic-based radio- and chemosensitizer, has vascular normalizing effects on SCCVII and U87 tumors. Clin. Epigenetics 8, 53 (2016).
Cabrales, P. RRx-001 acts as a dual small molecule checkpoint inhibitor by downregulating CD47 on cancer cells and SIRP-alpha on monocytes/macrophages. Transl. Oncol. 12, 626–632 (2019).
Oronsky, B., Scribner, C., Aggarwal, R. & Cabrales, P. RRx-001 protects normal tissues but not tumors via Nrf2 induction and Bcl-2 inhibition. J. Cancer Res Clin. Oncol. 145, 2045–2050 (2019).
Reid, T. et al. Safety and activity of RRx-001 in patients with advanced cancer: a first-in-human, open-label, dose-escalation phase 1 study. Lancet Oncol. 16, 1133–1142 (2015).
Gross, C. J. et al. K+ efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity 45, 761–773 (2016).
Yu, W. et al. One-carbon metabolism supports S-adenosylmethionine and histone methylation to drive inflammatory macrophages. Mol. Cell 75, 1147–1160 e1145 (2019).
Oronsky, B. et al. RRx-001, a novel clinical-stage chemosensitizer, radiosensitizer, and immunosensitizer, inhibits glucose 6-phosphate dehydrogenase in human tumor cells. Disco. Med 21, 251–265 (2016).
Petrilli, V. et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 (2007).
Munoz-Planillo, R. et al. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Daniels, M. J. et al. Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat. Commun. 7, 12504 (2016).
Tang, T. et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat. Commun. 8, 202 (2017).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Xie, J. H., Li, Y. Y. & Jin, J. The essential functions of mitochondrial dynamics in immune cells. Cell Mol. Immunol. 17, 712–721 (2020).
Lu, A. et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193–1206 (2014).
Dick, M. S., Sborgi, L., Ruhl, S., Hiller, S. & Broz, P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat. Commun. 7, 11929 (2016).
He, Y., Zeng, M. Y., Yang, D., Motro, B. & Nunez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357 (2016).
Shi, H. X. et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 17, 250–258 (2016).
Schmid-Burgk, J. L. et al. A genome-wide CRISPR (clustered regularly interspaced short palindromic repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J. Biol. Chem. 291, 103–109 (2016).
Haq, T. et al. Mechanistic basis of Nek7 activation through Nek9 binding and induced dimerization. Nat. Commun. 6, 8771 (2015).
Lomenick, B. et al. Target identification using drug affinity responsive target stability (DARTS). Proc. Natl Acad. Sci. USA 106, 21984–21989 (2009).
Scicinski, J. et al. NO to cancer: the complex and multifaceted role of nitric oxide and the epigenetic nitric oxide donor, RRx-001. Redox Biol. 6, 1–8 (2015).
He, Y., Franchi, L. & Nunez, G. TLR agonists stimulate Nlrp3-dependent IL-1beta production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J. Immunol. 190, 334–339 (2013).
Gris, D. et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J. Immunol. 185, 974–981 (2010).
Inoue, M., Williams, K. L., Gunn, M. D. & Shinohara, M. L. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 109, 10480–10485 (2012).
Sharif, H. et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570, 338–343 (2019).
Acknowledgements
We thank Dr. Feng Shao (National Institute of Biological Sciences, Beijing, China) for providing the TcdB toxin. This research was supported by the National Key Research and Development Program of China (grant numbers 2019YFA0508503 and 2020YFA0509101), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant number XDB29030102), the National Natural Science Foundation of China (grant numbers 82003765, 81821001, 31770991, and 91742202), the Fundamental Research Funds for the Central Universities and the University Synergy Innovation Program of Anhui Province (GXXT-2019-026), the Natural Science Foundation of Anhui Province (1908085QC99) .
Author information
Authors and Affiliations
Contributions
Y.C., H.H. and B.L performed the experiments of this work; X.D., W.J. and R.Z. designed the research. Y.C., W.J. and R.Z. wrote the manuscript. W.J. and R.Z. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
R.Z., W.J. and Y.C. are named as inventors on China National Intellectual Property Administration Application Serial No. 202011472140.0 related to RRx-001.
Rights and permissions
About this article
Cite this article
Chen, Y., He, H., Lin, B. et al. RRx-001 ameliorates inflammatory diseases by acting as a potent covalent NLRP3 inhibitor. Cell Mol Immunol 18, 1425–1436 (2021). https://doi.org/10.1038/s41423-021-00683-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-021-00683-y
Keywords
This article is cited by
-
HECTD3 inhibits NLRP3 inflammasome assembly and activation by blocking NLRP3-NEK7 interaction
Cell Death & Disease (2024)
-
GSNOR negatively regulates the NLRP3 inflammasome via S-nitrosation of MAPK14
Cellular & Molecular Immunology (2024)
-
The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases
Nature Reviews Cardiology (2024)
-
Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets
Nature Reviews Drug Discovery (2024)
-
New Insights on NLRP3 Inflammasome: Mechanisms of Activation, Inhibition, and Epigenetic Regulation
Journal of Neuroimmune Pharmacology (2024)