Abstract
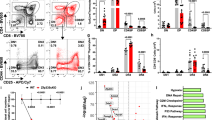
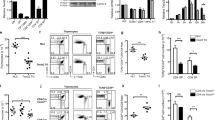
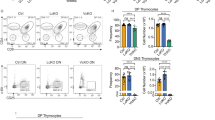
T cell development proceeds under the influence of a network of transcription factors (TFs). The precise role of Zeb1, a member of this network, remains unclear. Here, we report that Zeb1 expression is induced early during T cell development in CD4−CD8− double-negative (DN) stage 2 (DN2). Zeb1 expression was further increased in the CD4+CD8+ double-positive (DP) stage before decreasing in more mature T cell subsets. We performed an exhaustive characterization of T cells in Cellophane mice that bear Zeb1 hypomorphic mutations. The Zeb1 mutation profoundly affected all thymic subsets, especially DN2 and DP cells. Zeb1 promoted the survival and proliferation of both cell populations in a cell-intrinsic manner. In the periphery of Cellophane mice, the number of conventional T cells was near normal, but invariant NKT cells, NK1.1+ γδ T cells and Ly49+ CD8 T cells were virtually absent. This suggested that Zeb1 regulates the development of unconventional T cell types from DP progenitors. A transcriptomic analysis of WT and Cellophane DP cells revealed that Zeb1 regulated the expression of multiple genes involved in the cell cycle and TCR signaling, which possibly occurred in cooperation with Tcf1 and Heb. Indeed, Cellophane DP cells displayed stronger signaling than WT DP cells upon TCR engagement in terms of the calcium response, phosphorylation events, and expression of early genes. Thus, Zeb1 is a key regulator of the cell cycle and TCR signaling during thymic T cell development. We propose that thymocyte selection is perturbed in Zeb1-mutated mice in a way that does not allow the survival of unconventional T cell subsets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Shah, D. K. & Zúñiga-Pflücker, J. C. An overview of the intrathymic intricacies of T cell development. J. Immunol. 192, 4017–4023 (2014).
Rothenberg, E. V., Moore, J. E. & Yui, M. A. Launching the T-cell-lineage developmental programme. Nat. Rev. Immunol. 8, 9–21 (2008).
Kurd, N. & Robey, E. A. T-cell selection in the thymus: a spatial and temporal perspective. Immunol. Rev. 271, 114–126 (2016).
Hogquist, K. A. & Jameson, S. C. The self-obsession of T cells: how TCR signaling thresholds affect fate “decisions” and effector function. Nat. Immunol. 15, 815–823 (2014).
Gascoigne, N. R. J., Rybakin, V., Acuto, O. & Brzostek, J. TCR signal strength and T cell development. Annu. Rev. Cell Dev. Biol. 32, 327–348 (2016).
Moran, A. E. et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011).
Godfrey, D. I., Uldrich, A. P., McCluskey, J., Rossjohn, J. & Moody, D. B. The burgeoning family of unconventional T cells. Nat. Immunol. 16, 1114–1123 (2015).
Kronenberg, M. & Kinjo, Y. Innate-like recognition of microbes by invariant natural killer T cells. Curr. Opin. Immunol. 21, 6 (2009).
Tuttle, K. D. et al. TCR signal strength controls thymic differentiation of iNKT cell subsets. Nat. Commun. 9, 2650 (2018).
Zhao, M. et al. Altered thymic differentiation and modulation of arthritis by invariant NKT cells expressing mutant ZAP70. Nat. Commun. 9, 2627 (2018).
Malhotra, N. et al. SOX4 controls invariant NKT cell differentiation by tuning TCR signaling. J. Exp. Med 215, 2887–2900 (2018).
Ziętara, N. et al. Critical role for miR-181a/b-1 in agonist selection of invariant natural killer T cells. Proc. Natl Acad. Sci. USA 110, 7407–7412 (2013).
Henao-Mejia, J. et al. The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity 38, 984–997 (2013).
Wencker, M. et al. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat. Immunol. 15, 80–87 (2014).
Seo, W. & Taniuchi, I. Transcriptional regulation of early T-cell development in the thymus. Eur. J. Immunol. 46, 531–538 (2016).
Maillard, I., Fang, T. & Pear, W. S. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev. Immunol. 23, 945–974 (2005).
Murre, C. Helix-loop-helix proteins and lymphocyte development. Nat. Immunol. 6, 1079–1086 (2005).
Hosokawa, H. & Rothenberg, E. V. Cytokines, transcription factors, and the initiation of T-cell development. Cold Spring Harb. Perspect. Biol. 10, a028621 (2018).
Gheldof, A., Hulpiau, P., van Roy, F., De Craene, B. & Berx, G. Evolutionary functional analysis and molecular regulation of the ZEB transcription factors. Cell Mol. Life Sci. CMLS 69, 2527–2541 (2012).
Takagi, T., Moribe, H. & Kondoh, H. Higashi Y. DeltaEF1, a zinc finger and homeodomain transcription factor, is required for skeleton patterning in multiple lineages. Dev. Camb. Engl. 125, 21–31 (1998).
Caramel, J., Ligier, M. & Puisieux, A. Pleiotropic roles for ZEB1 in cancer. Cancer Res. 78, 30–35 (2018).
Conidi, A. et al. Few Smad proteins and many Smad-interacting proteins yield multiple functions and action modes in TGFβ/BMP signaling in vivo. Cytokine Growth Factor Rev. 22, 287–300 (2011).
Scott, C. L. & Omilusik, K. D. ZEBs: novel players in immune cell development and function. Trends Immunol. 40, 431–446 (2019).
van Helden, M. J. et al. Terminal NK cell maturation is controlled by concerted actions of T-bet and Zeb2 and is essential for melanoma rejection. J. Exp. Med. 212, 2015–2025 (2015).
Dominguez, C. X. et al. The transcription factors ZEB2 and T-bet cooperate to program cytotoxic T cell terminal differentiation in response to LCMV viral infection. J. Exp. Med. 212, 2041–2056 (2015).
Omilusik, K. D. et al. Transcriptional repressor ZEB2 promotes terminal differentiation of CD8+ effector and memory T cell populations during infection. J. Exp. Med. 212, 2027–2039 (2015).
Higashi, Y. et al. Impairment of T cell development in deltaEF1 mutant mice. J. Exp. Med. 185, 1467–1479 (1997).
Arnold, C. N. et al. A forward genetic screen reveals roles for Nfkbid, Zeb1, and Ruvbl2 in humoral immunity. Proc. Natl Acad. Sci. 109, 12286–12293 (2012).
Heng, T. S. P. & Painter, M. W., Immunological Genome Project Consortium. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Guan, T. et al. ZEB1, ZEB2, and the miR-200 family form a counterregulatory network to regulate CD8+ T cell fates. J. Exp. Med. 215, 1153–1168 (2018).
Jones, M. E. & Zhuang, Y. Stage-specific functions of E-proteins at the β-selection and T-cell receptor checkpoints during thymocyte development. Immunol. Res. 49, 202–215 (2011).
Emmanuel, A. O. et al. TCF-1 and HEB cooperate to establish the epigenetic and transcription profiles of CD4+CD8+ thymocytes. Nat. Immunol. 19, 1366–1378 (2018).
Rahim, M. M. A. et al. Ly49 receptors: innate and adaptive immune paradigms. Front. Immunol. 5, 145 (2014).
Grigoriadou, K., Boucontet, L. & Pereira, P. Most IL-4-producing gamma delta thymocytes of adult mice originate from fetal precursors. J. Immunol. 171, 2413–2420 (2003).
Gapin, L. iNKT cell autoreactivity: what is “self” and how is it recognized? Nat. Rev. Immunol. 10, 272–277 (2010).
Tuttle, K. D. & Gapin, L. Characterization of thymic development of natural killer T cell subsets by multiparameter flow cytometry. Methods Mol. Biol. Clifton NJ 1799, 121–133 (2018).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Szklarczyk, D. et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452 (2015).
Liston, A. et al. Impairment of organ-specific T cell negative selection by diabetes susceptibility genes: genomic analysis by mRNA profiling. Genome Biol. 8, R12 (2007).
Kastner, P. et al. Bcl11b represses a mature T-cell gene expression program in immature CD4(+)CD8(+) thymocytes. Eur. J. Immunol. 40, 2143–2154 (2010).
Yoshida, H. et al. The cis-regulatory atlas of the mouse immune system. Cell 176, 897–912.e20 (2019).
Bedel, R. et al. Effective functional maturation of invariant natural killer T cells is constrained by negative selection and T-cell antigen receptor affinity. Proc. Natl Acad. Sci. 111, E119–E128 (2014).
Hayes, S. M. & Love, P. E. Strength of signal: a fundamental mechanism for cell fate specification. Immunol. Rev. 209, 170–175 (2006).
Haks, M. C. et al. Attenuation of gammadeltaTCR signaling efficiently diverts thymocytes to the alphabeta lineage. Immunity 22, 595–606 (2005).
Alonzo, E. S. et al. Development of promyelocytic zinc finger and ThPOK-expressing innate gamma delta T cells is controlled by strength of TCR signaling and Id3. J. Immunol. 184, 1268–1279 (2010).
Ilangumaran, S. et al. Loss of GIMAP5 (GTPase of immunity-associated nucleotide binding protein 5) impairs calcium signaling in rat T lymphocytes. Mol. Immunol. 46, 1256–1259 (2009).
Howie, D. et al. MS4A4B is a GITR-associated membrane adapter, expressed by regulatory T cells, which modulates T cell activation. J. Immunol. 183, 4197–4204 (2009).
Carlson, C. M. et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 442, 299–302 (2006).
Gubbels Bupp, M. R. et al. T cells require Foxo1 to populate the peripheral lymphoid organs. Eur. J. Immunol. 39, 2991–2999 (2009).
Li, L. & Bhatia, R. Molecular pathways: stem cell quiescence. Clin. Cancer Res J. Am. Assoc. Cancer Res. 17, 4936–4941 (2011).
Hedrick, S. M., Michelini, R. H., Doedens, A. L., Goldrath, A. W. & Stone, E. L. FOXO transcription factors throughout T cell biology. Nat. Publ. Group 12, 649–662. (2012).
Shi, L. Z. et al. Gfi1-Foxo1 axis controls the fidelity of effector gene expression and developmental maturation of thymocytes. Proc. Natl Acad. Sci. USA 114, E67–E74. (2017).
D’Cruz, L. M., Knell, J., Fujimoto, J. K. & Goldrath, A. W. An essential role for the transcription factor HEB in thymocyte survival, Tcra rearrangement and the development of natural killer T cells. Nat. Immunol. 11, 240–249 (2010).
Brabletz, T. et al. Negative regulation of CD4 expression in T cells by the transcriptional repressor ZEB. Int. Immunol. 11, 1701–1708 (1999).
Postigo, A. A., Ward, E., Skeath, J. B. & Dean, D. C. zfh-1, the Drosophila homologue of ZEB, is a transcriptional repressor that regulates somatic myogenesis. Mol. Cell Biol. 19, 7255–7263 (1999).
Grégoire, J. M. & Roméo, P. H. T-cell expression of the human GATA-3 gene is regulated by a non-lineage-specific silencer. J. Biol. Chem. 274, 6567–6578 (1999).
Wang, L. et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat. Genet. 47, 1426–1434 (2015).
Mishra, A. et al. Mechanism, consequences, and therapeutic targeting of abnormal IL15 signaling in cutaneous T-cell lymphoma. Cancer Discov. 6, 986–1005 (2016).
Doisne, J.-M. et al. iNKT cell development is orchestrated by different branches of TGF- signaling. J. Exp. Med. 206, 1365–1378 (2009).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
Acknowledgements
The authors acknowledge the contribution of the SFR Biosciences facilities (UMS3444/CNRS, ENSL, UCBL, and US8/INSERM), particularly the Plateau de Biologie Expérimentale de la Souris and the flow cytometry facility. We thank Bruce Beutler for sharing the Cellophane mutant mice. We also thank Andrew Griffiths and Kiyoto Kurima for discussions regarding Twirler mutant mice and Fotini Gounari and Christophe Benoist for providing RNA-seq/ChIP-seq data on T cell development. The TW lab is supported by the Agence Nationale de la Recherche (ANR GAMBLER to TW and ANR JC BaNK to AM) and the Institut National du Cancer and receives institutional grants from the Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), Université Claude Bernard Lyon and ENS de Lyon, and the Joint Research Institute for Science and Society (JORISS). JZ is the recipient of a fellowship from the China Scholarship Council (CSC). RS and YGH were funded by an FRM grant (AJE20161236686) to YGH.
Author information
Authors and Affiliations
Contributions
JZ, AB, MW, DL, DEC, ALM, AR, and AM performed the experiments. RS and QM performed the in silico analyses. JC, LG, and YGH provided reagents and conceptual insight and helped write the paper. TW wrote the paper and supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, J., Wencker, M., Marliac, Q. et al. Zeb1 represses TCR signaling, promotes the proliferation of T cell progenitors and is essential for NK1.1+ T cell development. Cell Mol Immunol 18, 2140–2152 (2021). https://doi.org/10.1038/s41423-020-0459-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-020-0459-y
Keywords
This article is cited by
-
The role and application of transcriptional repressors in cancer treatment
Archives of Pharmacal Research (2023)
-
Untangling the genetic link between type 1 and type 2 diabetes using functional genomics
Scientific Reports (2021)