Abstract
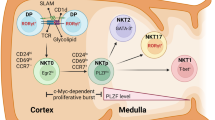
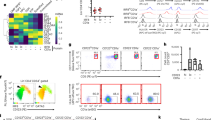
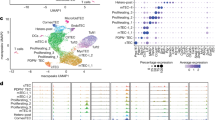
The vast majority of Foxp3+ regulatory T cells (Tregs) are generated in the thymus, and several factors, such as cytokines and unique thymic antigen-presenting cells, are known to contribute to the development of these thymus-derived Tregs (tTregs). Here, we report the existence of a specific subset of Foxp3+ Tregs within the thymus that is characterized by the expression of IL-1R2, which is a decoy receptor for the inflammatory cytokine IL-1. Detailed flow cytometric analysis of the thymocytes from Foxp3hCD2xRAG1GFP reporter mice revealed that the IL-1R2+ Tregs are mainly RAG1GFP– and CCR6+CCR7–, demonstrating that these Tregs are recirculating cells entering the thymus from the periphery and that they have an activated phenotype. In the spleen, the majority of IL-1R2+ Tregs express neuropilin-1 (Nrp-1) and Helios, suggesting a thymic origin for these Tregs. Interestingly, among all tissues studied, the highest frequency of IL-1R2+ Tregs was observed in the thymus, indicating preferential recruitment of this Treg subset by the thymus. Using fetal thymic organ cultures (FTOCs), we demonstrated that increased concentrations of exogenous IL-1β blocked intrathymic Treg development, resulting in a decreased frequency of CD25+Foxp3+ tTregs and an accumulation of CD25+Foxp3− Treg precursors. Interestingly, the addition of IL-1R2+ Tregs, but not IL-1R2− Tregs, to reaggregated thymic organ cultures (RTOCs) abrogated the IL-1β-mediated blockade, demonstrating that these recirculating IL-1R2+ Tregs can quench IL-1 signaling in the thymus and thereby maintain thymic Treg development even under inflammatory conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Josefowicz, S. Z., Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Klein, L., Robey, E. A. & Hsieh, C. S. Central CD4+ T cell tolerance: deletion versus regulatory T cell differentiation. Nat. Rev. Immunol. 19, 7–18 (2019).
Lio, C. W. & Hsieh, C. S. A two-step process for thymic regulatory T cell development. Immunity 28, 100–111 (2008).
Lio, C. W. & Hsieh, C. S. Becoming self-aware: the thymic education of regulatory T cells. Curr. Opin. Immunol. 23, 213–219 (2011).
Klein, L., Kyewski, B., Allen, P. M. & Hogquist, K. A. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat. Rev. Immunol. 14, 377–391 (2014).
Garg, G. et al. Unique properties of thymic antigen-presenting cells promote epigenetic imprinting of alloantigen-specific regulatory T cells. Oncotarget 8, 35542–35557 (2017).
Malek, T. R., Yu, A., Vincek, V., Scibelli, P. & Kong, L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity 17, 167–178 (2002).
Fontenot, J. D., Rasmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005).
Soper, D. M., Kasprowicz, D. J. & Ziegler, S. F. IL-2Rbeta links IL-2R signaling with Foxp3 expression. Eur. J. Immunol. 37, 1817–1826 (2007).
Chinen, T. et al. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 17, 1322–1333 (2016).
Liu, Y. et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol. 9, 632–640 (2008).
Konkel, J. E., Jin, W., Abbatiello, B., Grainger, J. R. & Chen, W. Thymocyte apoptosis drives the intrathymic generation of regulatory T cells. Proc. Natl Acad. Sci. USA 111, E465–E473 (2014).
Thiault, N. et al. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat. Immunol. 16, 628–634 (2015).
Cowan, J. E., McCarthy, N. I. & Anderson, G. CCR7 controls thymus recirculation, but not production and emigration, of Foxp3+ T cells. Cell Rep. 14, 1041–1048 (2016).
Toker, A. et al. Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus. J. Immunol. 190, 3180–3188 (2013).
McMahan, C. J. et al. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J. 10, 2821–2832 (1991).
Colotta, F. et al. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science 261, 472–475 (1993).
Garlanda, C., Riva, F., Bonavita, E. & Mantovani, A. Negative regulatory receptors of the IL-1 family. Semin. Immunol. 25, 408–415 (2013).
Peters, V. A., Joesting, J. J. & Freund, G. G. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav. Immun. 32, 1–8 (2013).
Kollewe, C., Neumann, D. & Martin, M. U. The first two N-terminal immunoglobulin-like domains of soluble human IL-1 receptor type II are sufficient to bind and neutralize IL-1beta. FEBS Lett. 487, 189–193 (2000).
Neumann, D., Kollewe, C., Martin, M. U. & Boraschi, D. The membrane form of the type II IL-1 receptor accounts for inhibitory function. J. Immunol. 165, 3350–3357 (2000).
Boraschi, D., Italiani, P., Weil, S. & Martin, M. U. The family of the interleukin-1 receptors. Immunol. Rev. 281, 197–232 (2018).
Kuwata, N., Igarashi, H., Ohmura, T., Aizawa, S. & Sakaguchi, N. Cutting edge: absence of expression of RAG1 in peritoneal B-1 cells detected by knocking into RAG1 locus with green fluorescent protein gene. J. Immunol. 163, 6355–6359 (1999).
Thornton, A. M. et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 184, 3433–3441 (2010).
Yadav, M. et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med 209, 1713–1722 (2012).
Weiss, J. M. et al. Neuropilin-1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ Treg cells. J. Exp. Med. 209, 1723–1742 (2012).
Ritvo, P. G. et al. Tfr cells lack IL-2Ralpha but express decoy IL-1R2 and IL-1Ra and suppress the IL-1-dependent activation of Tfh cells. Sci. Immunol. 2, eaan0368 (2017).
Richards, D. M. et al. Treg cell differentiation: from thymus to peripheral tissue. Prog. Mol. Biol. Transl. Sci. 136, 175–205 (2015).
Sharma, A. & Rudra, D. Emerging functions of regulatory T cells in tissue homeostasis. Front Immunol. 9, 883 (2018).
Delacher, M. et al. Genome-wide DNA-methylation landscape defines specialization of regulatory T cells in tissues. Nat. Immunol. 18, 1160–1172 (2017).
Delacher, M. et al. Rbpj expression in regulatory T cells is critical for restraining TH2 responses. Nat. Commun. 10, 1621 (2019).
Tai, X. et al. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity 38, 1116–1128 (2013).
Owen, D. L. et al. Thymic regulatory T cells arise via two distinct developmental programs. Nat. Immunol. 20, 195–205 (2019).
Shenderov, K. et al. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J. Immunol. 190, 5722–5730 (2013).
Su, S. B. et al. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J. Immunol. 175, 6303–6310 (2005).
Burchill, M. A., Yang, J., Vogtenhuber, C., Blazar, B. R. & Farrar, M. A. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 178, 280–290 (2007).
Vang, K. B. et al. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J. Immunol. 181, 3285–3290 (2008).
Tran, D. Q. et al. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood 113, 5125–5133 (2009).
Mercer, F., Kozhaya, L. & Unutmaz, D. Expression and function of TNF and IL-1 receptors on human regulatory T cells. PLoS ONE 5, e8639 (2010).
Tan, T. G., Mathis, D. & Benoist, C. Singular role for T-BET+CXCR3+ regulatory T cells in protection from autoimmune diabetes. Proc. Natl Acad. Sci. USA 113, 14103–14108 (2016).
De Simone, M. et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity 45, 1135–1147 (2016).
Plitas, G. et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity 45, 1122–1134 (2016).
Molgora, M. et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature 551, 110–114 (2017).
Guo, D. et al. Exosomes from heat-stressed tumour cells inhibit tumour growth by converting regulatory T cells to Th17 cells via IL-6. Immunology 154, 132–143 (2018).
Walters, S. N., Webster, K. E., Daley, S. & Grey, S. T. A role for intrathymic B cells in the generation of natural regulatory T cells. J. Immunol. 193, 170–176 (2014).
Yamano, T. et al. Thymic B cells are licensed to present self antigens for central T cell tolerance induction. Immunity 42, 1048–1061 (2015).
Ki, S. et al. Global transcriptional profiling reveals distinct functions of thymic stromal subsets and age-related changes during thymic involution. Cell Rep. 9, 402–415 (2014).
Mufazalov, I. A. et al. Generation of a novel T cell specific interleukin-1 receptor type 1 conditional knock out mouse reveals intrinsic defects in survival, expansion and cytokine production of CD4 T cells. PLoS ONE 11, e0161505 (2016).
Eriksson, U. et al. Activation of dendritic cells through the interleukin 1 receptor 1 is critical for the induction of autoimmune myocarditis. J. Exp. Med 197, 323–331 (2003).
Luft, T. et al. IL-1 beta enhances CD40 ligand-mediated cytokine secretion by human dendritic cells (DC): a mechanism for T cell-independent DC activation. J. Immunol. 168, 713–722 (2002).
Wesa, A. & Galy, A. Increased production of pro-inflammatory cytokines and enhanced T cell responses after activation of human dendritic cells with IL-1 and CD40 ligand. BMC Immunol. 3, 14 (2002).
Korn, T. et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl Acad. Sci. USA 105, 18460–18465 (2008).
Wei, J. et al. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc. Natl Acad. Sci. USA 104, 18169–18174 (2007).
Liu, B. et al. Severe influenza A(H1N1)pdm09 infection induces thymic atrophy through activating innate CD8+CD44hi T cells by upregulating IFN-gamma. Cell Death Dis. 5, e1440 (2014).
Miyao, T. et al. Plasticity of Foxp3+ T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 36, 262–275 (2012).
Kim, J. M., Rasmussen, J. P. & Rudensky, A. Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007).
Cossarizza, A. et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 49, 1457–1973 (2019).
Acknowledgements
We thank Marina Wuttke and Lothar Groebe for their technical and cell sorting support. This work was supported by the CRC 738 (to J.H.), CRC/TR 128 (to A.W.) and CRC/TRR 221 (to M.F.) of the German Research Foundation and by grants from the European Research Council (ERC-CoG, #648145 REGiREG) to M.F. Y.E. was supported by Ph.D scholarship program no. 57129429 of the German Academic Exchange Service (DAAD).
Author information
Authors and Affiliations
Contributions
E.N., Y.E., S.H., M.D., C.S. and I.A.M. performed the experiments and interpreted the data. A.W., C.F. and M.F. interpreted the data. E.N. and J.H. designed the research, interpreted the data, and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Nikolouli, E., Elfaki, Y., Herppich, S. et al. Recirculating IL-1R2+ Tregs fine-tune intrathymic Treg development under inflammatory conditions. Cell Mol Immunol 18, 182–193 (2021). https://doi.org/10.1038/s41423-019-0352-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0352-8
Keywords
This article is cited by
-
A distinct “repair” role of regulatory T cells in fracture healing
Frontiers of Medicine (2024)