Abstract
Are lipid droplets (LDs) necessary to maintain the viability of brain tumour cells as they move to new nutrient-poor environments? In turn, could cancers be targeted by attacking what you might think of as the cancer cells’ picnic basket? Lipid metabolism reprogramming, represented by increased lipid uptake, activation of de novo lipogenesis and increased lipid storage, is a newly identified hallmark of cancers. Recently, the presence of lipid droplets has been detected in several types of cancers, such as metastatic hepatocellular carcinoma, pancreatic and breast. LDs are storage organelles that provide a source of nutrients which may drive metastasis in different tumours. Currently, several roles of LDs have been posited in various tumours. This perspective aims to review and discuss the currently understood role of LDs in brain tumours.
Similar content being viewed by others
Introduction
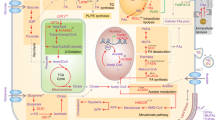
Lipid droplets (LDs) are dynamic organelles within the cytoplasm, rich in lipids, that play a role in storage and break down of neutral lipids. They are predominantly localised in adipose tissue [1]. LDs are spherical with a core composed of triacylglycerols (TAG) and cholesteryl esters serving as a reservoir for membrane formation and energy homeostasis (Fig. 1A) [2]. Interestingly, LDs can be identified in non-adipose tissues, however, their role and significance, both at homeostasis and in the pathology of various diseases, is still a matter of debate. In such non-adipose tissues, LDs protect against lipotoxicity, a type of cell death induced by the accumulation of excess lipids [2]. Furthermore, it has been reported that LDs in non-adipose tissues are a deposit of signalling precursors and vitamins, aiding protein turnover whilst protecting from oxidative stress [3]. Of interest, literature suggests that LDs tend to increase during cellular differentiation [4,5,6] and have been detected in various brain conditions such as Alzheimer’s and Parkinson’s disease [7]. Thus, highlighting the diverse potential functions of these dynamic organelles.
A Illustration depicting the structural composition of LDs. These droplets consist of a central hydrophobic core containing neutral lipids, primarily triacylglycerols (TAGs) and sterol esters. Surrounding this core is a monolayer of phospholipids, incorporating various proteins such as perilipin and lipid droplet-associated proteins (ADRP, TIP47). B Diagram outlining the fatty acid synthesis pathway responsible for generating triacylglycerols (TAG) and sterol esters stored within lipid droplets. TAG synthesis occurs on the endoplasmic reticulum membrane, involving sequential addition of fatty acids (FAs) in their activated acyl-CoA form to a glycerol-3-phosphate (G3P) backbone. This process yields lysophosphatidic acid (LPA), phosphatidic acid (PA), and diacylglycerol (DAG). Catalyzed by acyltransferase enzymes, including glycerol-3-phosphate acyltransferases (GPATs), acylglycerol-3-phosphate acyltransferases (AGPATs), and phosphatidic acid phosphatases (lipins), the final and pivotal step in the TAG synthesis cascade is carried out by diacylglycerol acyltransferase enzymes (DGATs). Figure created using BioRender.com.
As the most lipid-rich organ, the brain utilises lipid metabolism extensively for energy homeostasis, but it also plays a role in neuroinflammation and oxidative stress [8]. Therefore, we ask how much of what we know currently about the role of LDs in healthy adipose and non-adipose, such as stem cells, could be translated into a brain tumour.
Reprogramming of lipid metabolism is a recently recognized characteristic of cancer, where tumour cells increase both their production of new lipids and uptake of fatty acids [9]. This, therefore, increases intracellular lipid storage, reflected as an accumulation of LDs which can be catabolised to generate energy, thus, supporting growth, survival, and migration [10,11,12,13,14]. Recent publications have identified that glioblastoma tumours use LDs to grow under glucose-restricted conditions [1, 15]. Numerous potential roles of LDs have been identified in different cancers, such as enhancing cell proliferation, metastasis, chemotherapy resistance and crosstalk with the immune system [11,12,13,14]. Within the central nervous system (CNS), lipids utilised in the formation of LDs can be derived from de novo lipogenesis, lipoprotein particles, apoptotic cells, debris, myelin, neuron-released lipids and synaptic pruning, among other sources [16].
LDs formation occurs at the endoplasmic reticulum (ER) and involves a complex series of steps (Fig. 1B) [2]. Firstly, activated fatty acids (FAs) are esterified to diacylglycerol or sterol to form neutral lipids [2]. The formation of both compounds involves several enzyme-regulated steps (Fig. 1B). Most pertinent of these are, Acyl-CoA: cholesterol O-transferases (ACAT1/2), which catalyse the formation of sterol esters (SEs) and diacylglycerol acyltransferases (DGAT1/2), which catalyse the formation of triacylglycerols (TAGs) [2]. As the concentration of the neutral lipids increases, they form an oil lens by depositing between the inner and outer leaflets of the ER [2]. The oil lens expands, leading to LDs budding from the ER membrane [2]. This is facilitated by serpin, a class I protein which allows lipids to move into the LDs during budding [1, 2]. Class II binding proteins such as the perilipins-1-5 (PLIN1-5) protect LDs from hydrolysis by lipases such as adipose triglyceride lipase (ATGL) [1, 2].
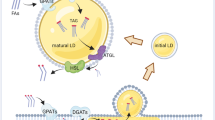
LDs catabolism is the process by which LDs are broken down to mobilize neutral lipid stores during periods of nutrient starvation or intense energy demand such as high rates of proliferation [2]. Two main breakdown pathways exist: lipophagy (Fig. 2A) and lipolysis (Fig. 2B) [2]. During lipolysis, lipases attach to the surface of lipid droplets and break down TAGs into FAs and glycerol (Fig. 2A). The breakdown of TAGs in lipolysis occurs through the step-by-step process involving adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and monoacylglycerol lipase (MAGL) (Fig. 2A) [14]. In the process of lipophagy, a type of autophagy, LDs are engulfed and merge with lysosomes, where they are broken down by lysosomal acid lipase (LAL) (Fig. 2B) [17].
LDs breakdown is orchestrated through two primary mechanisms: lipolysis and lipophagy. A Schematic illustration delineating TAG intracellular lipolysis. This process unfolds in a sequential cascade involving adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and monoacylglycerol lipase (MAGL), all situated on the LDs surface. ATGL initiates the process, catalyzing the conversion of triacylglycerols (TAGs) into diacylglycerol (DAG) and fatty acids (FAs). Subsequently, HSL further breaks down DAG into monoacylglycerol (MAG) and FAs. Finally, MAGL liberates FAs and glycerol. The lipolytic products, including DAG, MAG, FAs, and glycerol, serve as energy resources, membrane biosynthesis and others. B Lipophagy, an intricately regulated form of autophagy, engages the encapsulation of lipid droplets within autophagosomal membranes. These engulfed droplets are subsequently transported to lysosomes, where degradation occurs through acid lipolysis. Lysosomal TAG lipolysis is orchestrated by lysosomal acid lipase (LAL). Figure crafted using BioRender.com.
It has been recently documented that LDs play an important role in cancer [9,10,11,12,13,14,15]. Tumour cells are exposed to a dynamic environment where nutrient and oxygen levels fluctuate between the core and periphery of the tumour mass [9, 10]. As a direct result, cancer cells utilise multiple metabolic pathways to obtain energy and survive and proliferate [9, 10]. They maintain their lipid supply by increasing de novo lipogenesis and recycling structural lipids by autophagy or enzymatic remodelling [18]. This lipid supply is incorporated in LDs that, when required, are used to generate ATP, regulate autophagy, maintain redox balance and synthesise cell membranes [18, 19]. During tumour progression, this reduces the cellular stress of energy demand, allowing further development of the tumour [19].
We suggest that brain tumour cells accumulate LDs, which essentially act as a ‘picnic basket’, supplying an energy source for tumour progression during periods of environmental and cellular stress. Glioblastoma multiforme (GBM) is a prevalent and highly invasive brain tumour found in adults [15, 19]. In a study comparing fast-cycling (FC) and slow-cycling (SC) cells present in GBM tumours, it was found that SC cells displayed greater levels of lipid metabolites derived from LDs when exposed to low glucose conditions [15]. Thus, implicating a role for LDs as an energy reserve in SC cells [15]. SC cells in GBM also demonstrated a more invasive and migratory phenotype than FC cells [15]. Another study investigated the link between hyperactivation of mammalian target of rapamycin complex (mTORC)-1 and lipophagy in GBM cells [19]. mTORC1 integrates growth factor signals, nutrients and available energy to drive cell growth and catabolism [20]. It was shown that GBM cells sustain their energy production through lipophagy of LDs. Furthermore, use of the glycolysis inhibitor, 2-Deoxy-D-Glucose, correlated with hyperactivation of mTORC, linked with poor prognoses in GBM [19]. Yang K et al. (2021) discovered a physical interaction between choline kinase α2 (CKα2) and LDs [21]. AMP-activated protein kinase (AMPK) induces post-translational modifications at lysine247 through phosphorylation of CKα2 when GBM cells become starved of glucose, an action mediated by lysine acetyltransferase 5 [21]. This enables CKα2 to function as a protein kinase, phosphorylating PLIN2/3 on the surface of LDs, resulting in lipolysis [21]. Therefore, it appears that oxidation of FAs released from LDs fuels GBM, highlighting its role as a ‘picnic basket’ in brain tumour cells. Interestingly, there may also be a central role for LDs in stem cell fate determination [4]. Feng Yue et al., found that LDs accumulated in stem cells that are committed to differentiation rather than self-renewal. This suggests that LDs are linked with the differentiation mechanism of stem cells, although their role in stem cells fate remains ambiguous.
Based on the evidence discussed here and in published literature on different cancers, it can be concluded that one of the primary functions of LDs in brain tumours is to be the ‘picnic basket’. As a fully modulatory energy source, they are able to support energy-intense processes such as cell proliferation and in turn, drive aggressive phenotypes.
Moreover, it is not without reason to posit that these organelles may play a key role in supporting or facilitating metastatic phenotypes within tumours. The complex role of LDs in cancer and other non-adipocyte cells remains largely uncharacterised. We believe that the different processes involving lipid mobilization from these dynamic organelles can be developed as a new strategy for the treatment of brain tumours.
References
Cruz ALS, Barreto EA, Fazolini NPB, Viola JPB, Bozza PT. Lipid droplets: platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020;11:105.
Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019;20:137–55.
Welte MA, Gould AP. Lipid droplet functions beyond energy storage. Biochimica et Biophysica Acta Mol Cell Biol Lipids. 2017;1862:1260–72.
Yue F, Oprescu SN, Qiu J, Gu L, Zhang L, Chen J, et al. Lipid droplet dynamics regulate adult muscle stem cell fate. Cell Rep. 2022;38:110267.
Sanjabi B, Dashty M, Özcan B, Akbarkhanzadeh V, Rahimi M, Vinciguerra M, et al. Lipid droplets hypertrophy: a crucial determining factor in insulin regulation by adipocytes. Sci Rep. 2015;5:8816.
Rugolo F, Bazan NG, Calandria J, Jun B, Raschellà G, Melino G, et al. The expression of ELOVL4, repressed by MYCN, defines neuroblastoma patients with good outcome. Oncogene. 2021;40:5741–51.
Farmer BC, Walsh AE, Kluemper JC, Johnson LA. Lipid droplets in neurodegenerative disorders. Front Neurosci. 2020;14:742.
Bruce KD, Zsombok A, Eckel RH. Lipid processing in the brain: a key regulator of systemic metabolism. Front Endocrinol (Lausanne). 2017;8:60.
Snaebjornsson MT, Janaki-Raman S, Schulze A. Greasing the wheels of the cancer machine: the role of lipid metabolism in cancer. Cell Metab. 2020;31:62–76.
Koizume S, Miyagi Y. Lipid droplets: a key cellular organelle associated with cancer cell survival under normoxia and hypoxia. Int J Mol Sci. 2016;17:1430.
Wang YY, Attané C, Milhas D, Dirat B, Dauvillier S, Guerard A, et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI insight. 2017;2:e87489.
Tirinato L, Liberale C, Di Franco S, Candeloro P, Benfante A, La Rocca R, et al. Lipid droplets: a new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem cells (Dayt, Ohio). 2015;33:35–44.
Qi W, Fitchev PS, Cornwell ML, Greenberg J, Cabe M, Weber CR, et al. FOXO3 growth inhibition of colonic cells is dependent on intraepithelial lipid droplet density. J Biol Chem. 2013;288:16274–81.
Zaytseva YY, Harris JW, Mitov MI, Kim JT, Butterfield DA, Lee EY, et al. Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. Oncotarget. 2015;6:18891–904.
Hoang‐Minh LB, Siebzehnrubl FA, Yang C, Suzuki‐Hatano S, Dajac K, Loche T, et al. Infiltrative and drug‐resistant slow‐cycling cells support metabolic heterogeneity in glioblastoma. EMBO J. 2018;37:c98772.
Ralhan I, Chang C-L, Lippincott-Schwartz J, Ioannou MS. Lipid droplets in the nervous system. J cell Biol. 2021;220:1.
Petan T, Jarc E, Jusović M. Lipid droplets in cancer: guardians of fat in a stressful world. Molecules (Basel, Switz). 2018;23:1941.
Xu G, Jiang Y, Xiao Y, Liu XD, Yue F, Li W, et al. Fast clearance of lipid droplets through MAP1S-activated autophagy suppresses clear cell renal cell carcinomas and promotes patient survival. Oncotarget. 2016;7:6255–65.
Wang C, Haas MA, Yeo SK, Paul R, Yang F, Vallabhapurapu S, et al. Autophagy mediated lipid catabolism facilitates glioma progression to overcome bioenergetic crisis. Br J cancer. 2021;124:1711–23.
Zou Z, Tao T, Li H, Zhu X. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci 2020;10:31.
Yang K, Rich JN. A delicate initiation: Lipolysis of lipid droplets fuels glioblastoma. Mol cell. 2021;81:2686–7.
Acknowledgements
The authors thank Dr Sergey Smirnov, Professor Laurence Hurst, Professor David Tosh, and Professor Stephen Ward from the University of Bath, for advice during the preparation of the manuscript.
Funding
This work was funded by a summer scholarship from the British Pharmacological Society to T. B. Also, it was funded by a grant from the Little Princes Trust (LPT2023A22) and a startup grant from the University of Bath to M.V.N-C.
Author information
Authors and Affiliations
Contributions
TB, KC, and MVN-C wrote the paper. KC prepared the figures. CC corrected the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bingi, T., Cotton, K., Comer, C. et al. Are lipid droplets the picnic basket of brain tumours?. Cell Death Discov. 10, 31 (2024). https://doi.org/10.1038/s41420-024-01797-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-024-01797-8