Abstract
Sorafenib is an anti-tumor drug widely used in clinical treatment, which can inhibit tyrosine kinase receptor on cell surface and serine/threonine kinase in downstream Ras/MAPK cascade signaling pathway of cells. Tyrosine kinase phosphorylation plays an important role in inflammatory mechanism, such as TLR4 tyrosine phosphorylation, MAPK pathway protein activation, and activation of downstream NF-кB. However, the effects of sorafenib on LPS-induced inflammatory reaction and its specific mechanism have still remained unknown. We found that sorafenib inhibited the phosphorylation of tyrosine kinase Lyn induced by LPS, thereby reducing the phosphorylation level of p38 and JNK, inhibiting the activation of c-Jun and NF-κB, and then inhibiting the expression of inflammatory factors IL-6, IL-1β, and TNF-α. Furthermore, sorafenib also decreased the expression of TLR4 on the macrophage membrane to inhibit the expression of inflammatory factors latterly, which may be related to the inactivation of Lyn. These results provide a new perspective and direction for the clinical treatment of sepsis.
Similar content being viewed by others
Introduction
Sepsis, a syndrome of physiologic, pathologic, and biochemical abnormalities induced by infection, refers to the life-threatening organ dysfunction, which is a leading cause of mortality and critical illness worldwide [1,2,3]. It is estimated that more than 30 million people are hospitalized for sepsis every year in the world, and sepsis may cause as many as 5.3 million deaths every year [3]. Although sepsis is associated with high morbidity and mortality, there are few effective therapies to date [4, 5]. At present, antibiotics and symptomatic treatment of complications are the main clinical treatments for sepsis. Antibiotics can effectively kill bacteria, but the internal and external toxins, bacterial DNA and cell wall components released by dead bacteria could cause an excessive inflammatory reaction, and then lead to multiple organ failure and death [6]. Therefore, in the treatment of sepsis, it is particularly important to inhibit inflammation on the basis of antibiosis.
The recognition of bacterial lipopolysaccharide (LPS), RNA, or DNA by TLR receptor activates the pro-inflammatory cytokine profile in macrophages, thus destroying the dynamic balance regulation of the immune system [7]. Src family kinase (SFKs) is a non-receptor protein tyrosine kinase family composed of nine members: Src, Lyn, Fgr, Hck, Lck, Fyn, Blk, Yes, and Ylk. As a protein tyrosine kinase, SFK is involved in the regulation of many cell functions, such as proliferation, migration, differentiation, survival, immune response of B lymphocytes and T lymphocytes, and perception of microorganisms by pattern-recognition receptors [8, 9]. Lyn is the most abundant Src family kinase in B cells, which is closely related to the B-cell receptor complex. Besides, Lyn also exists in many hematopoietic cells [10,11,12]. What’s more, Lyn is a key regulator of the mucosal immune system, which plays an important role in mucus secretion of airway inflammation and airway remodeling, and can reduce excessive secretion of airway mucus and strictly regulate airway remodeling [13, 14]. In the case of Lyn knockout, the activation of the MAPK pathway was impaired and the activation of IKK-NF-κB pathway triggered by TLR4 was also inhibited after LPS stimulation [15,16,17].
Tyrosine kinase phosphorylation plays an important role in inflammatory mechanism, such as TLR4 tyrosine phosphorylation, activation of MAPK pathway protein and downstream NF-кB [18,19,20]. Sorafenib (BAY 43-9006) is a multikinase inhibitor with anti-tumor activity in various tumor models [21], which can inhibit the Raf/MEK/ERK signaling pathway [22], and inhibit receptor tyrosine kinases which promote angiogenesis, such as vascular endothelial growth factor receptor (VEGFR)1, VEGFR2, VEGFR 3, platelet-derived growth factor receptor -β(PDGFR-β), Flt3 and c-Kit, playing a key role in tumor cell proliferation and tumor angiogenesis [23,24,25]. Studies have shown that sorafenib can not only inhibit the proliferation and angiogenesis of tumor cells but also regulate the function of immune cells. In addition to inhibiting the phenotype and function of dendritic cells, sorafenib also inhibits caspase-1 overexpression through limiting nuclear transport of p65, which leads to the inactivation of NF-κB [26, 27]. However, the effect of sorafenib, a tyrosine kinase inhibitor, on LPS-induced inflammatory reaction and its specific mechanism are still remained unknown.
Results
Sorafenib inhibits pro-inflammatory cytokines induced by LPS in macrophages
We first determined the effects of sorafenib on LPS-induced inflammatory response. BMDM and RAW264.7 cells were pretreated with 10 μmol/L sorafenib for 30 min, and then 1 μg/mL LPS was added to stimulate the inflammatory response of macrophages for 2 or 4 h, respectively. The mRNA expression levels of pro-inflammatory cytokines including IL-6, IL-1β, and TNF-α were determined by real-time qPCR. As shown in Fig. 1A–C, the mRNA expression of IL-6, IL-1β, and TNF-α were significantly upregulated in LPS-treated BMDMs in a time-dependent manner compared with the control group, while the mRNA expression of IL-6, IL-1β, and TNF-α were weakened as expected after the pretreatment of sofafenib. The same results were observed in RAW264.7 cells (Fig. 1D–F). The aforementioned evidence suggested that sorafenib has inhibitory effects on LPS-induced inflammatory reaction in macrophages.
mRNA expression levels of IL-6, IL-1β, and TNF-α were measured by quantitative PCR in BMDMs which pretreated with 10 μmol/L sorafenib for 30 min prior to LPS (1 μg/mL) treatment for 2 and 4 h (A–C). The same method was verified on Raw264.7 D–F. All mRNA levels were normalized to the level of β-actin mRNA. The data are expressed as the means ± SEM of three independent experiments. ns, P > 0.05; *P < 0.05; **P < 0.01 compared with other groups.
Sorafenib alleviates LPS-induced inflammation in vivo
To establish an inflammatory mouse model, aged 6–8 weeks C57BL/6 mice were injected with 20 mg/kg LPS intraperitoneally. The mice injected with saline were used as controls. To explore the effect of sorafenib on LPS-induced inflammation in mice, 100 mg/kg sorafenib was pretreated through intragastric administration for 2 h before LPS injection. Compared with the LPS group, sorafenib pretreatment restrained the mRNA expression of inflammatory factors IL-6, IL-1β, and TNF-α in lung tissue (Fig. 2A–C), and the ELISA results also showed that soarfenib could decrease the expression of inflammatory factors in mouse serum at 12 h after LPS treatment (Fig. 2D–F). Meanwhile, H&E staining was carried out to observe the injured condition of lung tissue samples (Fig. 2G). Massive inflammatory cells such as macrophages, neutrophils, and lymphocytes infiltrate, perivascular edema, and severe alveolar space destruction were observed following LPS injection compared to the control group, which was proved that the inflammatory mouse model was set up correctly. However, pretreatment with sorafenib significantly reversed the severity of lung injury compared with the mice that received LPS treatment alone. These results indicated that sorafenib inhibits the production of inflammatory factors and alleviates LPS-induced inflammatory response in vivo.
In all, 6–8 W WT mice were + /− pretreated with 100 mg/kg sorafenib by intragastric administration for 2 h, and then LPS (20 mg/kg) were injected intraperitoneally for 12 h to establish the inflammatory model. The transcription levels of IL-6 A, IL-1β (B), and TNF-α (C) in lung tissue were detected by qRT-PCR and protein levels in serum were measured by ELISA D–F. H&E staining microscopy images (magnification ×20 and 40) of lung sections of mice in the control group and different treatment groups were shown in (G). All images are representative of at least three independent experiments. The graphs show means ± SEM, n = 3; *P < 0.05; **P < 0.01 compared with other groups.
Sorafenib inhibits phosphorylation of tyrosine kinase Lyn in LPS-treated macrophages
Sorafenib is a novel multiple kinase inhibitor, which targets at the Raf/MEK/ERK signaling pathway and tyrosine kinase receptor related to tumor progression and tumor angiogenesis [21,22,23]. The phosphorylation of tyrosine kinase plays an important role in regulating TLR4 signals, such as TLR4 tyrosine phosphorylation, p38MAPK, and NF-κB activation in response to LPS [18,19,20]. However, it is still unclear whether sorafenib can affect LPS-induced inflammatory response through acting on tyrosine kinase Lyn. To study the effect of sorafenib on LPS-induced tyrosine kinase activation, we assessed the level of phosphorylated Lyn (p-Lyn) in the whole-cell lysates by western blot. The results showed that sorafenib significantly inhibited the phosphorylation of Lyn induced by LPS at 10 min, 30 min, and 1 h (Fig. 3A). Interestingly, the time of LYN phosphorylation is short and fades with time, which indicates Lyn acts on the inflammation rapidly. Taken together, the results show that sorafenib inhibits LPS-induced inflammatory reaction, which may be related to the phosphorylation of tyrosine kinase Lyn.
BMDMs were pretreated with 10 μmol/L sorafenib for 30 min before incubation with 1 μg/mL LPS for 10 min, 30 min and 1 h. Lyn and the phosphorylation level of Lyn in the whole-cell lysates of BMDMs under the culture conditions as mentioned above were analyzed by western blot analysis. GAPDH used as a loading control A. Using gray analysis, the band density quantified in bar graph below as a relative expression compared with loading control B. All the images are representative of three independent experiments. The graphs show means ± SEM, n = 3; *P < 0.05; **P < 0.01 compared with other groups.
Sorafenib inhibits phosphorylation of MAPKp38, JNK, and Akt caused by LPS
To further investigate the effects of sorafenib on LPS-induced phosphorylation of MAPK pathway protein, the relative protein expression levels of MAPKp38, JNK, and ERK were analyzed by western blot. BMDM and RAW264.7 cells were pretreated with sorafenib at a concentration of 10 μmol/L for 30 min, and then stimulated with 1 μg/mL LPS for 1 h. Levels of both p-p38 and p-JNK increased significantly induced by LPS (Fig. 4A–D), with no increase in protein expression of total p38 or JNK. As expected, sorafenib inhibited phosphorylation of p38 and JNK. At the same time, DMSO of the same volume had no inhibitory effect on p-p38 and p-JNK, which excluded the effect of solvent. Surprisingly, sorafenib did not inhibit the phosphorylation of ERK, but enhanced its phosphorylation level a bit, which may be related to the inhibition of Akt phosphorylation by sorafenib. Collectively, these data indicate that the inhibition of Lyn will affect the activation of MAPK pathway proteins p38 and JNK in its downstream.
Immunoblots for p38, phospho (p)-p38, JNK, p-JNK, ERK, p-ERK, Akt, and p-Akt of whole-cell lysates of BMDMs after pretreatment with 10 μmol/L sorafenib followed by LPS (1 μg/mL) stimulation for 1 h A. DMSO was used as solvent control group. C was verified on Raw264.7 cells in the same way. The band density of each blot was quantified in bar graph as the ratio of phosphorylation (activation) to total protein B, D. All the images are representative of three independent experiments. The graphs show means ± SEM, n = 3; *P < 0.05; **P < 0.01 compared with other groups.
Sorafenib negatively regulates LPS-induced activation of NF-κB/AP-1
As mentioned above, inflammation response induced by LPS has been inhibited in sorafenib pretreatment. NF-κB and AP-1 signaling pathway is a key to modulate pro-inflammatory cytokine releases. Thus, this classic pathway was also investigated to prove whether NF-κB and AP-1 were involved in our study. As shown in Fig. 5A, LPS increased the phosphorylation level of c-Jun, which was most obvious at 30 min, while sorafenib could significantly inhibit the phosphorylation level of c-Jun stimulated by LPS at each time point. We also found the phosphorylation level of p65 increased most obviously after LPS stimulation for 10 min, decreased after 30 min, and almost completely degraded at 1 h. Sorafenib inhibited phosphorylation of p65 unapparently at 10 min, but most remarkable at 30 min. Using DMSO as the control group, we found that DMSO had no inhibitory effect on the phosphorylation level of c-Jun and p65, which ruled out the interference of solvent. In addition, we also used confocal microscopy to clarify the activation and localization of NF-κB. As shown in Fig. 5D, NF-κB with green fluorescence mainly distributed in the cytoplasm, but rarely distributed in the nucleus in the control group. After LPS stimulation, activated NF-κB with green fluorescence rapidly entered the nucleus, while sorafenib pretreatment decreased distribution of NF-κB in the nucleus. To sum up, sorafenib negative regulation of NF-κB and AP-1 activation serves as one of the major mechanisms that attenuates inflammation in response to LPS.
BMDMs were pretreated with 10 μmol/L sorafenib for 30 min before incubation with LPS for 10 min, 30 min, and 1 h. Then the phosphorylation level of NF-κB and AP-1 in the whole-cell lysates of BMDMs were detected by western blot analysis. GAPDH used as a loading control A. Using gray analysis, the band density quantified in bar graph below as a relative expression compared with loading control B, C. Fluorescent microscopy images of NF-κB in BMDMs treated with LPS (1 μg/mL) for 10 min with or without 10 μmol/L sorafenib pretreatment for 30 min D. All the images are representative of three independent experiments. The graphs show means ± SEM, n = 3; *P < 0.05; **P < 0.01 compared with other groups.
Sorafenib downregulates the expression of TLR4 on the membrane in LPS-induced macrophage
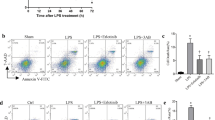
TLR4 is the ligand of LPS, which can be recognized and activated by LPS, and then dimerize the receptor on the cell membrane, initiating the protein-protein cascade reaction, leading to the production of inflammatory cytokines, thus initiating inflammation and immune response [28, 29]. In order to determine whether sorafenib negative regulation of LPS-induced inflammation is related to TLR4, the expression of TLR4 on the membrane were calculated using flow cytometry in vivo and vitro. As shown in Fig. 6A, B, LPS increased the expression of TLR4 on BMDMs membrane compared with the control group, and was positively correlated with stimulation time, while sorafenib pretreatment inhibited its expression. In the same way, 100 mg/kg sorafenib pretreatment by intragastric administration or not, WT (C57BL/6) mice were treated with LPS (20 mg/kg) for time points up to 12 h, after which macrophages were collected from abdominal lavage fluid. The higher expression of TLR4 on the membrane was observed following LPS treatment. Compared with the LPS group, the expression of TLR4 on the membrane of macrophages in the abdominal cavity decreased with sorafenib pretreatment. The results suggested that sorafenib inhibited the expression of TLR4 on the macrophage membrane induced by LPS.
BMDMs isolated from WT mice were treated with LPS (1 μg/mL) in vitro for 6 h, 12 h, and 24 h with or without 10 μmol/L sorafenib pretreatment for 30 min followed by flow cytometry analysis of cell surface TLR4 intensity A. TLR4 cell surface expression in peritoneal macrophages (gated by 7AAD—, F4/80 + ) were measured by FACS analysis (C). The quantitative analysis bar graph of TLR4 expression on the cell membrane is shown in (B, D). All images are representative of three independent experiments. The graphs show means ± SEM, n = 3; *P < 0.05; **P < 0.01 compared with other groups.
Discussion
Sorafenib is a multiple kinase inhibitor (MKI) approved for the treatment of primary advanced renal cell carcinoma and advanced primary liver cancer, which is the standard systemic chemotherapy drug for hepatocellular carcinoma (HCC) [30]. Studies have also proved that sorafenib could enhance survival and improve the prognosis in breast cancer, multiple myeloma, and thyroid cancer, etc. [31,32,33]. Although clinical studies demonstrated some benefits of sorafenib on the time to progression (TTP) and overall survival (OS), its efficacy against HCC remains moderate [34]. The alternative effective treatment regimens or combined treatment is also being explored, which is expected to be a reliable way to treat HCC and ameliorate sorafenib resistance. Uncontrollable inflammation is an important factor to change the microenvironment, which is closely related to the occurrence, development, invasion, and metastasis of tumors. Sorafenib is an effective drug for the treatment of cancer, so what role it plays in the inflammatory mechanism is of great interest to us.
Sorafenib can inhibit tyrosine kinase receptor on the cell surface and serine/threonine kinase in downstream Ras/MAPK cascade signaling pathway of cells. Since these kinases are involved in tumor cell proliferation and tumor angiogenesis, sorafenib is widely used in clinical anti-tumor treatment [35]. Sorafenib is also an effective inhibitor of TNF-dependent necrotic apoptosis through down-regulating the activities of RIPK1 and RIPK3 kinases [36]. Here, we found that sorafenib could significantly inhibit the inflammatory response induced by LPS both in vitro and vivo.
In order to further explore the mechanism of sorafenib-inhibiting LPS-induced inflammatory response, we first observed the tyrosine kinase inhibitory properties of sorafenib. Lyn belongs to a non-receptor tyrosine kinase-cytoplasmic tyrosine kinase. It has been reported that inhibition of tyrosine kinase significantly reduced the production of inflammatory factors such as TNF-α and IL-6 [15, 37, 38]. However, other studies have the opposite conclusion. They found that activation of SFK could inhibit inflammation induced by LPS, or silencing of Lyn upregulated LPS-induced cytokine production [19, 39]. We observed that LPS increased phosphorylation of Lyn, while sorafenib pretreatment could inhibit the activation of Lyn. These results showed that sorafenib inhibited not only the activation of receptor tyrosine kinase, but also the activation of non-receptor tyrosine kinase indicating that sorafenib inhibits LPS-induced inflammatory reaction, which may be partially due to its role in the activation of Lyn.
Mitogen-activated protein kinase (MAPK) transports extracellular signals such as hormones, growth factors, cytokines, and environmental stresses, and triggers various physiological functions, including cell proliferation, differentiation, development, and apoptosis. The three major subfamilies of MAPK include extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 protein [16, 40, 41]. Previous studies have shown that Lyn knockout affected the activation of MAPK pathway proteins [15]. We proved that sorafenib inhibited the phosphorylation of p38MAPK and JNK protein, which may be caused by the lower phosphorylation level of lyn. However, sorafenib did not inhibit the phosphorylation of ERK, but enhanced its phosphorylation level, which was consistent with the previously reported results [26, 42]. This may be explained as the fact that weakened phosphorylation of Akt promotes active phosphorylation of Raf-1 and subsequent phosphorylation of ERK. As we know, the ubiquitous Raf serine/threonine kinase is the key molecule in the Raf/ MEK/ ERK signaling pathway. Once Raf is activated, MEK will be phosphorylated and activated in Raf subtype, and then phosphorylate and activate ERK [43]. AKT is an inhibitor of Raf-1, which inhibits Raf-1 by phosphorylating Ser259 to create a binding site for the negative regulator of Raf [44]. It, therefore, appeared that sorafenib inhibited the phosphorylation of Akt, decreased the inhibitory effect on Raf, and enhanced the activation of ERK. Further investigation will be required to define the contribution of the different phosphorylation events in p38, JNK, and ERK mediate by sorafenib.
The innate immune response triggered by LPS is mediated by TLR4, which activates transcription factors NF-κB and AP-1 and their nuclear translocation [45]. NF-κB is one of the essential transcription factors in the classical inflammatory pathway, which triggers transcription of inflammatory factors in the recognition of pathogen-related molecular patterns (PAMPs) by pattern-recognition receptors (PRRs) [17, 46]. In an inactive state, NF-κB complex binds to the inhibitory IκB protein in the cytoplasm, which limits its translocation to the nucleus. Activation and phosphorylation of IκB complex promotes the degradation and release of NF-κB, and then the released NF-κB subunits (mainly p65 and p50) are transported to the nucleus to induce the production and release of inflammatory cytokines such as IL-6, IL-1β, and TNF-α [47,48,49]. AP-1 is one of the other major transcription factors mediated by TLR. Stimulated by LPS, phosphorylation of MAPK proteins lead to the activation and nuclear translocation of AP-1 subunits, such as c-fos, c-jun, ETS domain protein (ELK-2), and activated transcription factor 2 (ATF-2), which combine with DNA reaction elements to promote the release of pro-inflammatory cytokines [50,51,52]. It was recently proposed that inhibition of src family kinases decreases the phosphorylation of c-Jun, inhibits the activation of IKK-NF-κB signaling pathway, and leads to the formation of AP-1 complex and the decrease of p65 nucleus entry under LPS stimulation, reducing the production and release of inflammatory factors lastly [15, 38, 53]. In addition, phosphorylation of MAPK especially JNK can activate c-Jun [54, 55]. We confirmed that pretreatment of sorafenib inhibited activation of c-Jun and p65 induced by LPS, which may be related to the inhibition of Lyn and MAPK phosphorylation.
The TLR4 signal pathway depends on the cascade reaction of serine–threonine phosphorylation and polyubiquitination, and the activation of TLR4 also triggers off several protein tyrosine kinases, including Syk kinase and SFKs [19, 56]. We discovered that sorafenib can inhibit the expression of TLR4 on macrophages in vitro and in vivo experiments. These data suggest that sorafenib inhibit inflammation response is related to its inhibition of TLR4 expression, and its mechanism may be connected with the inactivation of tyrosine kinase Lyn, which needs to be further studied in the future.
Our major findings in this paper suggest that sorafenib inhibit the phosphorylation of tyrosine kinase Lyn induced by LPS, thereby reducing the phosphorylation level of MAPKp38 and JNK, inhibiting the activation of c-Jun and NF-κB, and then inhibiting the expression of inflammatory factors IL-6, IL-1β, and TNF-α. Furthermore, sorafenib also decreased the expression of TLR4 on the macrophage membrane, which may be related to the inactivation of Lyn. The mechanism diagram is shown in Fig. 7. These results further clarified the effect of sorafenib on LPS-induced inflammation and its mechanism, which provided a new perspective and direction for the clinical treatment of sepsis.
In the inflammatory reaction induced by LPS, sorafenib inhibits the activation of tyrosine kinase Lyn, and then reduce the phosphorylation of its downstream proteins MAPKp38 and JNK. Sorafenib decreases the activation of c-jun and p65, and then reduces the nucleation of AP-1 and NF-κB, and then reduces the production and release of inflammatory factors such as IL-6, IL-1β, and TNF-α. In addition, sorafenib can also inhibit LPS-induced inflammatory response by inhibiting the expression of TLR4 on the macrophage membrane.
However, there are many limitations in our study. Sorafenib is generally used as an anti-tumor drug in clinic. We have not conducted clinical experiments to explore the efficacy of sorafenib in sepsis patients, whether it is superior to other antibiotics, and so on. These are the topics that we are going to carry out in the later stage. In terms of mechanism, it is necessary to further clarify the specific mechanism of sorafenib inhibiting the expression of TLR4 on the membrane, whether it is related to inhibiting the transport of TLR4 from endoplasmic reticulum to cell membrane or decreasing the transcription and translation of TLR4. In addition, it is interesting that sorafenib enhances the phosphorylation of ERK induced by LPS and it may also be the direction of further exploration in the future. Furthermore, inflammation is closely related to oxidative stress, and sorafenib has been proved to be a ferroptosis inducer, so how the effect of ferroptosis induced by sorafenib is related to its inhibition of LPS-induced inflammatory response will be a new field worthy of further study.
Materials and methods
Cells culture and treatment
BMDM isolation and culture refer to the methods of previous study [57]. Bone marrow from male mice (6–8 weeks) was flushed out and cultured in DMEM containing 10% FBS complemented with 50 mg/ml penicillin/streptomycin and 10 ng/ml M-CSF (PeproTech, Lot#0817245). After 5–7 days of culture, BMDM differentiated and matured, and then changed into fresh medium (DMEM containing 10% FBS) for dosing treatment. Subcultured Raw264.7 murine macrophage was obtained from the ATCC (Bethesda, MD, USA). Cells were also cultured in DMEM containing 10% FBS(Gibco, USA), streptomycin (100 μg/mL, Life Technologies), and penicillin (100 units/ml, Life Technologies) and incubated overnight at 37 °C and 5% CO2. Sorafenib was prepared in dimethyl sulfoxide (DMSO) and diluted to the desired final concentration (0, 5, 10 μmol/L) in a culture medium for 30 min before LPS (1 μg/mL) treatment. The final concentration of DMSO did not exceed 0.1%.
Animals
Fifty C57BL/6 male (wild-type; WT) mice (6–8 wk old), weight range 20–22 g, were purchased from Beijing Huafukang Biotechnology (License No. SCXK (Jing) 2019). All the animal experimental protocols were reviewed and conformed to the committees of Guangdong Medical University. For animal studies, the mice were divided into the following four groups: (1) control group: mice received intraperitoneal (i.p.) injections of saline for 12 h; (2) sorafenib group: 100 mg/kg sorafenib(dissolved in 0.5% CMC-Na) for intragastric administration 2 h before saline i.p. 12 h; (3) LPS group: mice were treated with LPS (20 mg/kg, i.p.) for 12 h; (4) LPS + sorafenib group: mice were pretreated with 100 mg/kg sorafenib orally 2 h before LPS (20 mg/kg, i.p.) treatment 12 h.
Antibodies and reagents
Lipopolysaccharides (LPS) were purchased from Solarbio (Lot:L8880). Sorafenib (BAY 43-9006, CAS No.284461-73-0), and CMC-Na (Sodium carboxymethyl cellulose, CAS No. 9004-32-4) were purchased from Selleck. Abs specific for Phospho-Lyn (70926S), Lyn (4576S), Phospho-p38 (4631S), p38 (8690S), Phospho-Erk1/2 (9101S), Erk1/2 (9102S), Phospho-JNK (9255S), JNK (9252S), Phospho-Akt (4060S), Akt (4691S), Phospho-p65 (3033S), p65 (8642S), and Phospho-c-Jun (4691S) were obtained from Cell Signaling Technology. GAPDH (Cat No. 60004-1-Ig) was purchased from Proteintech.
Flow cytometry analysis
Macrophages collected from peritoneal lavage were labeled with F4/80 at 4 °C for 15 min. For measuring cell surface expression of TLR4, BMDMs and/or PLF cells were stained with PE-conjugated anti-mouse TLR4-PE (12-9041-80, 1:200; eBioscience) for 30 min. Cells were washed three times with PBS, after suspension in PBS, and then cells were analyzed by flow cytometry (BD Biosciences). Data were analyzed using FlowJo software (Tree Star).
RNA extraction and quantitative real-time PCR
Total cellular RNA was extracted using TRIzol reagent (Invitrogen). Real-time RT-PCR was done using the iTaq™ Universal SYBR® Green Supermix (172-5121, Bio-Rad, Hercules, CA, USA) in a Bio-Rad iQ5 real-time PCR machine (Bio-Rad). Reverse transcription was done using iScript Reverse Transcription Supermix (170-8841, Bio-Rad, Hercules, CA, USA). Amplification was performed with cycling conditions of 95 °C for 15 s then 60 °C for 30 s for 40 cycles. After the amplification protocol was over, the PCR product was subjected to melt-curve analysis using Bio-Rad iQ5 software (Bio-Rad). The real-time PCR primers were synthesized as follows:
β-actin (forward: CATGTACGTTGCTATCCAGGC;
reverse: CTCCTTAATGTCACGCACGAT).
IL-6 (forward: TCTATACCACTTCACAAGTCGGA;
reverse: GAATTGCCATTGCACAACTCTTT).
TNF-α (forward: AAGCCTGTAGCC CACGTCGTA;
reverse: GGCACCACTAGTTGGTTGTCTTTG);
IL-1β (forward: GCAACTGTTCCTGAACTCAACT;
reverse: ATCTTTTGGGGTCCGTCAACT).
Western blotting
Total proteins were extracted from the BMDM or Raw264.7 cells with the lysis buffer supplemented with 1 mM PMSF, and a protease inhibitor cocktail. Protein concentration determined by the bicinchoninic acid (BCA) protein assay (Beyotime). Proteins were subjected to SDS/PAGE, transferred onto polyvinylidene difluoride (PVDF) membranes, and blotted with 5% skimmed milk. The membranes were incubated overnight with primary antibodies against GAPDH, Phospho-Lyn, Total-Lyn, Phospho-p38, Total p38, Phospho-Erk1/2, Total-Erk1/2, Phospho-JNK, Total-JNK, Phospho-Akt, Total-Akt, Phospho-p65, p65, and Phospho-c-Jun, respectively. Horseradish peroxidase-conjugated anti-rabbit IgG was used as the secondary antibody.
Enzyme-linked immunosorbent assay (ELISA)
IL-6, IL-1β, and TNF-α concentrations in mouse serum were measured using murine cytokine-specific Quantikine ELISA kits (eBioscience) according to the manufacturer’s instructions.
Statistical analysis
Data were analyzed in SPSS19.0 (Carlsbad, CA). For multi-group comparisons, One-way ANOVA was performed. All graphs represent mean ± s.e.m. P < 0.05 was considered statistically significant. Graphs and figures were made with GraphPad Prism 5 (GraphPad software, Carlsbad, CA).
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). J Am Med Assoc. 2016;315:801–10.
Vincent JL, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380–6.
Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–72.
Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. J Am Med Assoc. 2017;318:1241–9.
Rowan KM, Angus DC, Bailey M, Barnato AE, Bellomo R, Canter RR, et al. Early, goal-directed therapy for septic shock—a patient-level meta-analysis. N. Engl J Med. 2017;376:2223–34.
Kohanski MA, Tharakan A, Lane AP, Ramanathan M Jr. Bactericidal antibiotics promote reactive oxygen species formation and inflammation in human sinonasal epithelial cells. Int Forum Allergy Rhinol. 2016;6:191–200.
Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33:449–58.
Senis YA, Mazharian A, Mori J. Src family kinases: at the forefront of platelet activation. Blood. 2014;124:2013–24.
Wu Y, Span LM, Nygren P, Zhu H, Moore DT, Cheng H, et al. The tyrosine kinase c-Src specifically binds to the active integrin αIIbβ3 to initiate outside-in signaling in platelets. J Biol Chem. 2015;290:15825–34.
Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M, et al. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol. 2003;4:274–9.
Lowell CA. Src-family kinases: rheostats of immune cell signaling. Mol Immunol. 2004;41:631–43.
Hibbs ML, Dunn AR. Lyn, a src-like tyrosine kinase. Int J Biochem Cell Biol. 1997;29:397–400.
Li G, Fox J 3rd, Liu Z, Liu J, Gao GF, Jin Y, et al. Lyn mitigates mouse airway remodeling by downregulating the TGF-β3 isoform in house dust mite models. J Immunol. 2013;191:5359–70.
Wang X, Li Y, Luo D, Wang X, Zhang Y, Liu Z, et al. Lyn regulates mucus secretion and MUC5AC via the STAT6 signaling pathway during allergic airway inflammation. Sci Rep. 2017;7:42675.
Avila M, Martinez-Juarez A, Ibarra-Sanchez A, Gonzalez-Espinosa C. Lyn kinase controls TLR4-dependent IKK and MAPK activation modulating the activity of TRAF-6/TAK-1 protein complex in mast cells. Innate Immun. 2012;18:648–60.
Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–6.
Cui J, Zhu L, Xia X, Wang HY, Legras X, Hong J, et al. NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell. 2010;141:483–96.
Henricson BE, Carboni JM, Burkhardt AL, Vogel SN. LPS and Taxol activate Lyn kinase autophosphorylation in Lps(n), but not in Lpsd), macrophages. Mol Med. 1995;1:428–35.
Mitchell J, Kim SJ, Seelmann A, Veit B, Shepard B, Im E, et al. Src family kinase tyrosine phosphorylates Toll-like receptor 4 to dissociate MyD88 and Mal/Tirap, suppressing LPS-induced inflammatory responses. Biochem Pharm. 2018;147:119–27.
Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, et al. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem. 2007;282:16042–53.
Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40.
Wilhelm S, Chien DS. BAY 43-9006: preclinical data. Curr Pharm Des. 2002;8:2255–7.
Hasskarl J. Sorafenib. Recent Results Cancer Res. 2010;184:61–70.
Miyanaga N, Akaza H. [Sorafenib(Nexavar)]. Gan Kagaku Ryoho. 2009;36:1029–33.
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109.
Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610–20.
Li J, Zhou Y, Liu Y, Dai B, Zhang YH, Zhang PF, et al. Sorafenib inhibits caspase-1 expression through suppressing TLR4/stat3/SUMO1 pathway in hepatocellular carcinoma. Cancer Biol Ther. 2018;19:1057–64.
Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol. 2002;270:109–20.
Roy A, Srivastava M, Saqib U, Liu D, Faisal SM, Sugathan S, et al. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int Immunopharmacol. 2016;40:79–89.
Brunetti O, Gnoni A, Licchetta A, Longo V, Calabrese A, Argentiero A, et al. Predictive and prognostic factors in HCC patients treated with sorafenib. Medicina. 2019;55:707.
Pitoia F, Jerkovich F. Selective use of sorafenib in the treatment of thyroid cancer. Drug Des Devel Ther. 2016;10:1119–31.
Bronte G, Andreis D, Bravaccini S, Maltoni R, Cecconetto L, Schirone A, et al. Sorafenib for the treatment of breast cancer. Expert Opin Pharmacother. 2017;18:621–30.
Gentile M, Martino M, Recchia AG, Vigna E, Morabito L, Morabito F. Sorafenib for the treatment of multiple myeloma. Expert Opin Investig Drugs. 2016;25:743–9.
Yang J, Eresen A, Scotti A, Cai K, Zhang Z. Combination of NK-based immunotherapy and sorafenib against hepatocellular carcinoma. Am J Cancer Res. 2021;11:337–49.
Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597–612.
Martens S, Jeong M, Tonnus W, Feldmann F, Hofmans S, Goossens V, et al. Sorafenib tosylate inhibits directly necrosome complex formation and protects in mouse models of inflammation and tissue injury. Cell Death Dis. 2017;8:e2904.
Stefanová I, Corcoran ML, Horak EM, Wahl LM, Bolen JB, Horak ID. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem. 1993;268:20725–8.
Smolinska MJ, Page TH, Urbaniak AM, Mutch BE, Horwood NJ. Hck tyrosine kinase regulates TLR4-induced TNF and IL-6 production via AP-1. J Immunol. 2011;187:6043–51.
Borzęcka-Solarz K, Dembińska J, Hromada-Judycka A, Traczyk G, Ciesielska A, Ziemlińska E, et al. Association of Lyn kinase with membrane rafts determines its negative influence on LPS-induced signaling. Mol Biol Cell. 2017;28:1147–59.
Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805.
Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–15.
Edwards JP, Emens LA. The multikinase inhibitor sorafenib reverses the suppression of IL-12 and enhancement of IL-10 by PGE(2) in murine macrophages. Int Immunopharmacol. 2010;10:1220–8.
Gollob JA, Wilhelm S, Carter C, Kelley SL. Role of Raf kinase in cancer: therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin Oncol. 2006;33:392–406.
Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science. 1999;286:1741–4.
Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–50.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76.
Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–51.
Landström M. The TAK1-TRAF6 signalling pathway. Int J Biochem Cell Biol. 2010;42:585–9.
Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95.
Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94.
Patel H, Shaw SG, Shi-Wen X, Abraham D, Baker DM, Tsui JC. Toll-like receptors in ischaemia and its potential role in the pathophysiology of muscle damage in critical limb ischaemia. Cardiol Res Pr. 2012;2012:121237.
Napolitani G, Bortoletto N, Racioppi L, Lanzavecchia A, D’Oro U. Activation of src-family tyrosine kinases by LPS regulates cytokine production in dendritic cells by controlling AP-1 formation. Eur J Immunol. 2003;33:2832–41.
Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. Philos Trans R Soc Lond B Biol Sci. 1996;351:127–34.
Subedi L, Lee JH, Yumnam S, Ji E, Kim SY. Anti-inflammatory effect of sulforaphane on LPS-activated microglia potentially through JNK/AP-1/NF-κB inhibition and Nrf2/HO-1 activation. Cells. 2019;8:194.
Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32.
Assouvie A, Daley-Bauer LP, Rousselet G. Growing murine bone marrow-derived macrophages. Methods Mol Biol. 2018;1784:29–33.
Acknowledgements
We would like to thank the Experimental platform provided by the Clinical Research Center of Affiliated Hospital of Guangdong Medical University, and all the subjects who participated in the study.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81873951 and 82072208) and the National Key R&D Program of China (grant numbers 2021YFC2701700).
Author information
Authors and Affiliations
Contributions
JT and XT conceived and designed the experiments. XL and MX performed the experiments with the help of JS and YL. QP, SL, and MZ analyzed the data and drafted the manuscript. All authors helped to interpret the results and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study was carried out in accordance with the recommendations of the Animal Ethics Committee of the Guangdong Medical University. The protocol was approved by the Animal Ethics Committee of the Guangdong Medical University (Zhanjiang, China).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Xu, M., Shen, J. et al. Sorafenib inhibits LPS-induced inflammation by regulating Lyn-MAPK-NF-kB/AP-1 pathway and TLR4 expression. Cell Death Discov. 8, 281 (2022). https://doi.org/10.1038/s41420-022-01073-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-022-01073-7
This article is cited by
-
Review of research progress on intestinal microbiota based on metabolism and inflammation for depression
Archives of Microbiology (2024)
-
Diagnostic model based on key autophagy-related genes in intervertebral disc degeneration
BMC Musculoskeletal Disorders (2023)
-
Novel VEGFR2 inhibitors with thiazoloquinoxaline scaffold targeting hepatocellular carcinoma with lower cardiotoxic impact
Scientific Reports (2023)
-
Heat-Killed Latilactobacillus sakei CNSC001WB and Lactobacillus pentosus WB693 Have an Anti-inflammatory Effect on LPS-Stimulated RAW 264.7 Cells
Probiotics and Antimicrobial Proteins (2023)