Abstract
Osteoprotegerin (OPG), also known as tumor necrosis factor receptor superfamily member 11B (TNFRSF11B), is a member of the tumor necrosis factor (TNF) receptor superfamily. Characterized by its ability to bind to receptor activator of nuclear factor kappa B ligand (RANKL), OPG is critically involved in bone remodeling. Emerging evidence implies that OPG is far beyond a bone-specific modulator, and is involved in multiple physiological and pathological processes, such as immunoregulation, vascular function, and fibrosis. Notably, numerous preclinical and clinical studies have been conducted to assess the participation of OPG in tumorigenesis and cancer development. Mechanistic studies have demonstrated that OPG is involved in multiple hallmarks of cancer, including tumor survival, epithelial to mesenchymal transition (EMT), neo-angiogenesis, invasion, and metastasis. In this review, we systematically summarize the basis and advances of OPG from its molecular structure to translational applications. In addition to its role in bone homeostasis, the physiological and pathological impacts of OPG on human health and its function in cancer progression are reviewed, providing a comprehensive understanding of OPG. We aim to draw more attention to OPG in the field of cancer, and to propose it as a promising diagnostic or prognostic biomarker as well as potential therapeutic target for cancer.
Similar content being viewed by others
Facts
-
OPG is well-recognized for its participation in bone homeostasis and the skeletal metastasis of cancer.
-
Emerging evidence has revealed versatile roles of OPG in human physiological and pathological processes, such as vascular formation, immune modulation and fibrosis.
-
Except for the RANKL and TRAIL, the regulation of OPG functional activity and expression levels are modulated by various other ligands and signaling pathways.
-
OPG is widely expressed in numerous types of cancer cells. Advances in the field of cancer have unveiled the diagnostic, prognostic and therapeutic value of OPG in various cancers, especially bone metastasis.
-
The roles of OPG in cancer are far from clear, and more research is needed to completely elucidate the involvement of OPG in cancers.
Open Questions
-
The published results regarding the impact of OPG on some types of cancer are inconsistent and even contradictory. How can these discrepancies be explained?
-
RANKL and OPG are considered antagonistic effectors in most cases. However, independent researches studies have shown that they may fulfil concurrent functions in cancers. Do these two proteins have conflicting therapeutic applications in cancers? How can we better understand the relationship between these two cancer players?
-
The levels of OPG in different forms and compartments (mRNA, intracellular OPG protein and serum OPG protein) are often discordant. What is the molecular mechanism underlying this discrepancy? Which index is most important for cancer diagnosis or prognosis?
-
As a secreted protein, OPG is competent for acting as a signaling molecule between different cells. Is it more important to identify the main source of secreted OPG in a particular micro-environment than to focus on the expression pattern of OPG in cancer cells?
-
How can the antitumor effect of OPG be selectively improved to broaden its therapeutic prospects?
Introduction
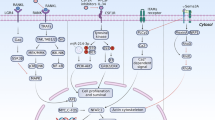
As a member of the TNF receptor superfamily, OPG is well recognized for its protective effect against excessive bone resorption [1]. Overall, OPG protein contains a signal peptide and seven functional domains. After proteolytic cleavage of the signal peptide and homodimerization, the mature form of OPG is secreted from the cytoplasm to the extracellular compartment [2] (Fig. 1).
By binding to RANKL, OPG suppresses the maturation and activation of pre-osteoclasts via antagonizing RANK/RANKL signaling (Fig. 2), thus inhibiting osteoclast function and promoting bone formation [3]. Therefore, attenuated OPG expression or an impaired RANKL/OPG ratio results in osteoporosis [4], rheumatoid arthritis [5], or periodontitis [6]. Another well-recognized endogenous ligand of OPG is TNF-related apoptosis-inducing ligand (TRAIL). OPG hinders the interaction between TRAIL and its death receptors (DRs), and thus blocks TRAIL-induced apoptosis [7] (Fig. 2). The crucial roles of RANKL and TRAIL signaling in the development of the immune system and various stages of tumor progression potentiate OPG as a key player in cancer formation and progression. In fact, as a secreted protein, the clinical significance of OPG has been studied in several tumors including breast cancer, prostate cancer (PCa), multiple myeloma (MM), and hepatocellular carcinoma (HCC). In these studies, OPG exhibits its capacity as a predictive index of bone metastasis, a diagnostic or prognostic marker of malignancies. Moreover, the therapeutic effect of OPG was investigated in established mouse models of MM [8, 9] and in tumor bone metastasis [10] by using exogenous bioactive OPG.
OPG is secreted in a homodimeric form. By binding with RANKL and TRAIL, OPG exerts its inhibitory effects on their downstream signaling pathways. Besides, OPG itself is regulated by many signaling pathway. Imbalanced signaling cross-talks between PI3K/AKT, p38/MAPK, wnt/β-catenin, TGF-β, mTOR and OPG contribute to the pathological cell behaviors. Other ligands, such as E2, vWF, FVIII-vWF and glycosaminoglycans (GAGs), bind with secreted OPG to impede its function. DR death receptor.
Molecular structure and expression of OPG
Independently identified by Simonet et al. [1] and Tsuda et al. [11] in 1997, OPG was previously named osteoclastogenesis inhibitory factor (OCIF), TNF receptor-related molecule-1 (TR-1) or follicular dendritic cell receptor-1 (FDCR-1). It was eventually named osteoprotegerin (OPG) in 2000 [12], due to its major function in bone homeostasis. OPG is encoded at chromosome 8q23-24, and is a secreted protein without a transmembrane region [3]. Biochemically, full length OPG protein, with a monomeric weight of 55–62 kDa, is 401 amino acids long and contains a signal peptide of 21 amino acids, which is then proteolytically cleaved to yield a 380 amino acid form (Fig. 1). There are four cysteine-rich N-terminal domains (domains 1–4), two death domain homologous regions (domains 5 and 6), and a C-terminal heparin-binding domain (domain 7) [2] (Fig. 1). OPG is secreted as a homodimer linked by disulfide bonds within 4 or 5 potential glycosylation sites, resulting in a mature secreted form of 110–120 kDa. In this process, the cysteine at position 400 is essential for homodimerization. The OPG dimer has the highest heparin-binding ability and the highest calcium-lowering ability [3].
Different cells express and secrete OPG in bone marrow, including osteoblasts, B cells, megakaryocytes, platelets, vascular endothelial cells, and vascular smooth muscle cells [1]. In addition to skeletal expression, OPG expression is also detected in many tissues including the heart, kidney, liver and spleen [13].
Physiological function of OPG
Bone remodeling
The delicate balance between osteolytic and osteoblastic activity in bone depends mainly on two major cell types: mesenchymal stem cells (MSC) derived osteoblasts and myeloid lineage-derived osteoclasts [2, 14]. The RANKL/RANK/OPG axis is the utmost determinant of bone remodeling. Total bone mineral density is decreased in OPG−/− mice, resulting in severe trabecular and cortical porosity, thin parietal bone, and a high incidence of fractures [15]. The bone protective effect of OPG is also supported by a report of a 100,000-bp homozygous deletion of OPG in two young patients with Paget’s disease, an autosomal recessive disorder characterized by increased bone remodeling, osteopenia and frequent fractures [16]. The imbalance of OPG/RANKL leads to a series of bone diseases including osteoporosis, rheumatoid arthritis, and periodontitis, manifesting as excessive activation of osteoclast activity and an increased RANKL/OPG ratio [6, 17, 18]. Numerous literatures have detailed the functions of OPG in skeletal disease, but this topic is not the main focus of this review and can be referred to Kearns et al. [19].
Vascular biology
The function of OPG in vascular biology was first revealed by the observation that OPG-deficient mice suffered marked calcification of the aorta and renal arteries [15]. Di et al. reported that OPG regulated insulin-like growth factor 1 receptor (IGF1R) expression and activity could modulate vascular smooth muscle cell calcification in vitro [20]. Deletion of OPG in apolipoprotein E knockout mice accelerated calcified atherosclerosis, suggesting that OPG can prevent such process of atherosclerosis [16]. Gordin et al. reported that serum OPG was an independent indicator of cardiovascular complications in adults with type 1 diabetes; OPG might play a role in extraosseous calcification, resulting in atherosclerosis and subsequent vascular dysfunction [21]. Moreover, elevated serum OPG levels were detected in patients with carotid calcification [22]. Furthermore, serum OPG levels demonstrated a prognostic value independent of other known predictors of mortality and cardiovascular events in patients with heart failure after acute myocardial infarction [23].
Immunity modulator
OPG-dependent self-regulation of microfold cell differentiation was discovered by Kimura et al. [24], who demonstrated that OPG expressed by microfold cells suppressed the differentiation of adjacent follicle-associated epithelial cells into microfold cells, and thus created a weakened immune microenvironment [24]. The accumulation of type 1 transitional B cells and isotype class switch defects were observed in OPG−/− mice, suggesting the role of OPG in regulating B cell maturation and development [25]. In vitro experiments revealed that, OPG activated Akt/PI3K signaling pathway in endothelial cells (ECs), subsequently leading to the recruitment and adhesion of monocytes [26]. OPG mediated negative regulation of the transcription factor Spi-B during the development of medullary thymic epithelial cells weakened the proliferative ability of regulatory T cells and inhibited tumor development in mice [27], suggesting a regulatory role of OPG in antitumor immunity.
Fibrosis
Recently, OPG has been found to be involved in fibrosis, which is characterized by the excessive formation and accumulation of extracellular matrix proteins generated by myofibroblasts [28]. The administration of exogenous OPG to chondrosarcoma cells lowered the expression of both mRNA and protein of matrix metalloprotease-13 [29], which is considered an important anti-fibrotic factor. Adhyatmika et al. reported that OPG produced by liver tissue promoted fibrogenesis in a transforming growth factor-β (TGF-β) dependent manner, and this was restrained by treatment with the TGF-β receptor kinase inhibitor galunisertib (LY2157299) [30]. To develop noninvasive approaches to assess liver fibrosis in patients with chronic liver diseases, Bosselut et al. constructed a score-based blood test for liver fibrosis involving OPG, which showed an improved diagnostic accuracy compared to existing scoring systems [31]. Compared with healthy livers, fibrotic livers in humans and mice had increased OPG levels, resulting in an increased expression of genes associated with fibrogenesis through TGF-β1 [32].
Regulation of OPG expression
As one of the most important ligands of OPG, RANKL produced by osteoblasts binds to RANK on the surface of osteoclast precursors and recruits the cohesive protein TRAF6, resulting in the activation of NF-κB and translocation to the nucleus. This subsequently leads to the increased expression of c-Fos, which interacts with nuclear factor of activated T cells c1 (NFATc1) to trigger the transcription of osteoclast genes [16]. The downstream signaling pathway initiated by RANKL activation also includes PI3K, ERK, JNK, and p38/MAPK [33] (Fig. 2). Besides, a membrane receptor called leucine-rich repeat-containing G-protein-coupled receptor 4 (LRG4) of RANKL was recently identified. RANKL binds to the extracellular domain of LGR4 and negatively regulates osteoclastogenesis by activating Gαq/GS3K-β signaling and inhibiting the NFATc1 pathway [2]. OPG restrains this process by binding to extracellular RANKL (Fig. 2). Meanwhile, screening of OPG protein with TNF-related ligands resulted in the discovery that OPG binds to TRAIL, thus preventing its interaction with death receptors and blocking TRAIL-induced apoptosis in a human T lymphocyte cell line named Jurkat [7]. Reciprocally, OPG mRNA expression was significantly inhibited by TRAIL stimulation in normal human bone marrow-derived stromal cells [34] (Fig. 2).
Numerous signaling molecules or pathways have been demonstrated to regulate the expression of OPG (Fig. 2). Boyce et al. reported that β-catenin together with T-cell factor proteins regulated the expression of OPG in mouse osteoblasts [35]. TGF-β upregulated OPG and downregulated RANKL in osteoblasts through the canonical β-catenin dependent Wnt signaling pathway [28, 36]. However, the exogenous administration of TGF-β1 to oral squamous cell carcinoma cells upregulated RANKL and downregulated OPG [37]. Additionally, the activation of p38MAPK signaling increased the expression of OPG in HCC and mouse mesenchymal stem cells (MSCs) [38, 39]. However, Hao et al. demonstrated that the OPG expression was negatively regulated by p38MAPK in pre-osteoblast cells [40]. The female sex hormone estradiol was found to upregulate OPG and thus interfere with RANKL/RANK signal activation, which explains the tendency of elderly women to suffer from osteoporosis due to the decreased production of estradiol [41]. The deletion of Notch1 in osteoblast lineage cells promoted osteoclastogenesis by decreasing the OPG/RANKL expression ratio [42]. Qiang et al. reported that Wnt3a-regulated OPG and RANKL expression was disrupted by MM cell-derived DKK1, a soluble inhibitor of canonical Wnt signaling in osteoblasts [43].
In addition to RANKL and TRAIL, OPG was also found to bind to von Willebrand factor (vWF), and was subsequently stored within Weibel-Palade (WP) bodies, which fused with the outer membrane of endothelial cells and released their contents into the plasma when activated [44]. Likewise, the FVIII/vWF complex is capable of binding to OPG, which blocks the interaction of OPG with TRAIL, indicating the potential of the FVIII/vWF complex in tumorigenesis [26]. Heparan sulfate proteoglycans on the surface of cultured myeloma cell lines were found to bind to OPG, subsequently leading to its internalization and degradation [45].
OPG mediated hallmarks of cancer
OPG was found to prevent cell apoptosis and promote tumor survival. Lane et al. reported that OPG inhibited the TRAIL-mediated apoptosis of ovarian cancer cells in an αvβ3 and αvβ5 integrin-dependent manner [46]. In inflammatory breast cancer, OPG interacted with glucose-regulated protein/binding immunoglobulin protein (GRP78/BiP), the endoplasmic reticulum (ER) chaperone and master regulator of the unfolded protein response, to promote cell apoptosis and prolong the survival of inflammatory breast cancer cells [47]. By forming complexes with the lipid raft core protein Cav-1 and EGFR, RANK promoted gastric cancer cell migration, and this effect was mitigated by OPG [48]. Recombinant OPG (rOPG) restrained breast cancer stemness by suppressing the β-catenin pathway, thus inhibiting tumor growth, EMT, and metastasis in orthotopic breast cancer xenografts [49].
The role of OPG has also been elaborated in angiogenesis, a process essential for the maintenance, development, and progression of malignancies. Cross et al. reported that the expression of OPG in the endothelium of malignant colorectal, breast, and metastatic cancer tumors was higher than that in benign tumors or in normal tissues. Exogenous stimulation with OPG facilitated the formation of cord-like structures by endothelial cells in vitro [50]. In addition, αvβ3 was found indispensable for the resistance to apoptosis by administration of rOPG in human microvascular endothelial cells [51]. Benslimane et al. reported that OPG combined with fibroblast growth factor (FGF-2) promoted neovascularization in vivo, accompanied by the activation of proangiogenic pathways involving MAPK signaling pathway, as well as Akt or mTOR cascades in endothelial colony forming cells (ECFCs) [52]. Moreover, OPG-pretreated ECFCs secreted stromal cell-derived factor-1 (SDF-1), which promoted neovascularization in ischemic tissue [53].
Role of OPG in cancers
OPG in cancer bone metastasis
Bone is one of the most common sites of cancer metastasis, especially for prostate, breast, and lung cancer [54]. Given the crucial role of OPG in bone homeostasis, the involvement of OPG in cancer bone metastasis has been widely investigated. Among the bone markers tALP, NTX, BSP, P1NP, bALP, TRAP, and OPG, this last marker displayed the highest sensitivity and accuracy for identifying bone metastases of PCa [55]. In the reseach of benign prostatic hyperplasia, primary and metastatic PCa disease, elevated OPG mRNA expression was found in metastatic tumor tissues compared to local disease. A higher RANKL/OPG ratio was observed in tumors with bone metastases than in normal prostate tissues [56]. Using conditionally replicating adenoviruses (CRAds), Jung et al. demonstrated that overexpressing OPG suppressed osteoclastogenesis in vitro and inhibited the progression of advanced PCa bone metastases [57]. Corey et al. reported that increased expression of OPG in C4-2 PCa cells did not inhibit its proliferation directly, but suppressed the growth of skeletal metastatic PCa tumors [58]. By testing serum OPG and RANKL levels, Elfa et al. concluded that the sensitivity and specificity of serum OPG in detecting bone metastasis in breast cancer patients were 59% and 92%, respectively, while the corresponding values for the RANKL/OPG ratio were 73% and 72%, respectively [59]. In liver cancer, Huang et al. reported that the long noncoding RNA H19 promoted HCC bone metastasis by attenuating OPG expression to create a pro-metastatic bone microenvironment [39].
With the ability to inhibit osteoclast activity and prevent the vicious cycle of osteoclast-tumor cell interactions, bisphosphonates are commonly administered to clinical patients with bone metastases. Rachner et al. found that the TRAIL/OPG ratio was increased by zoledronic acid (ZA) in TRAIL-sensitive MDA-MB-231 cells, suggesting that the TRAIL/OPG cytokine system is among the bisphosphonate-responsive targets in breast cancer in vitro [60]. After 12 months of treatment with ZA, a decrease in the RANKL/OPG ratio was observed, indicating that ZA decreased osteoclast activity by modulating RANKL and OPG expression [61].
OPG in breast cancer
A nested case control study in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort revealed that higher serum OPG levels might be a novel risk factor for ER-negative breast cancer [62]. In addition, in the EPIC cohort, Sarink et al. found that high concentrations of serum OPG were associated with elevated breast cancer specific and overall mortality, especially in those with ER-positive breast cancer [63]. In the Tromsø study, serum OPG was correlated with a reduced risk of breast cancer in women <60 years after adjustment. However, a worse prognosis was associated with patients with OPG levels in the upper quartile [64]. RANKL and OPG levels were reported to be upregulated in patients with breast cancer, and OPG was associated with the burden of bone metastases according to Mountzios et al. [65]. While BRAC1/2 mutation is a risk factor for breast cancer, lower serum OPG levels were correlated with the germline mutations of BRAC1/2 known to facilitate breast cancer risk [66]. Musculoskeletal toxicity is the major adverse effect of aromatase inhibitor therapy, which significantly affects the quality of life of patients. Lintermans et al. found that the SNP rs2073618 in OPG was associated with an elevated risk of musculoskeletal symptoms and pain after aromatase inhibitor therapy [67].
OPG in prostate cancer
Based on a strong negative correlation between endogenous OPG levels and TRAIL-induced apoptosis in vitro, Holen et al. concluded that OPG produced by PCa might be a considerable survival factor in hormone-resistant PCa cells [68]. In addition to predicting bone metastasis, OPG was found by multivariate regression analysis to be an independent prognostic factor for PCa related death [55]. Conditioned medium from PCa cells stimulated osteoclast formation, and this effect could be weakened by pre-exposure of osteoblasts to NF-κB inhibitor parthenolide (PTN), which reduced RANKL/OPG ratio [69]. By analyzing the correlation of p53 and the OPG/RANKL axis in PCa, Velletri et al. demonstrated that the loss of p53 was associated with high OPG levels both in vivo and in vitro, and that this predicted poor prognosis [70].
OPG in multiple myeloma
As one of the major features of MM, osteolytic lesions are caused by the excessive activation of osteoclasts and inhibition of osteoblasts [71]. Therefore, the imbalance in OPG/RANKL signaling has attracted much attention. Goranova-Marinova et al. found that serum OPG, RANKL, and RANKL/OPG ratios were significantly higher in MM patients than in healthy controls [72]. Inactivation of osteoclasts and trabecular bone loss in the vertebrae and tibiae was observed in mice following systemic administration of OPG expressing MSCs, suggesting the protective effect of OPG in MM [73].
OPG/RANKL is also used as a marker of bone remodeling. After the administration of two doses of lenalidomide, serum RANKL level, as well as the RANKL/OPG ratio, was significantly reduced, whereas serum OPG was elevated [71]. In a study of 104 patients who received at least 1 cycle of bortezomib, serum RANKL declined significantly, but serum OPG levels remained unchanged, causing a drop in the RANKL/OPG ratio [74]. The therapeutic effect of ZA was evaluated by measuring bone turnover markers including OPG. Interestingly, OPG levels in gingival crevicular fluid (GCF) were higher after 3 months of ZA therapy in patients with 5 or more bone metastases [75]. After analyzing the genotypes of 3774 MM patients of European ancestry, rs4407910 at 8q24.12 (odds ratio = 1.38, P = 4.09 × 10−9) and rs74676832 at 19q13.43 (odds ratio = 1.97, P = 9.33 × 10−7) showed the strongest association with MM bone disease (MBD). These data further support and highlight the role of OPG in the development of MM [76].
OPG in hepatocellular carcinoma
By analyzing 40 pairs of tumor tissues and corresponding adjacent normal tissues utilizing real-time PCR, Jiang et al. found that RANK and OPG levels were elevated in tumor tissue [77]. The prognostic value of serum OPG in HCC was reported by Zhang et al., who revealed that high serum OPG levels were correlated with worse prognosis and OPG was an independent risk factor for liver cancer [78]. A prediction model for early stage hepatitis B-related liver cancer containing five proteins including OPG was established by Cheng et al. [79], demonstrating the potential diagnostic value of OPG.
Gao et al. observed lower mRNA expression of OPG in highly metastatic HCC cell lines (MHCC97H, HCCLM3, and Sk-Hep1) compared to lines with low metastatic potential (HuH7 and HepG2) [80]. Long non-coding RNA H19 was found to promote HCC bone metastasis by inhibiting OPG expression [39]. These results implied that OPG may be an anti-metastatic factor in HCC. Interestingly, MHCC97-L cells expressed higher levels of OPG protein under hypoxic conditions, despite lower levels of OPG mRNA [80]. This inconsistency between the protein and mRNA expression levels may be attributed to the special properties of OPG as a secreted protein.
Clinical relevance of OPG in other cancers
The mRNA and protein expression of OPG was found to be upregulated in pancreatic cancer compared to normal pancreatic tissues, and both univariate and multivariate analyses demonstrated that the elevated levels of OPG were adversely correlated with the overall survival of cancer patients [81]. Aversa et al. showed that OPG was one of 12 biomarkers that were positively correlated with esophageal squamous cell carcinoma risk [82]. For oral squamous cell carcinoma, the elevated OPG expression was shown to be associated with bone invasion and shorter long-term cancer-specific survival [83] (Table 1).
Translational endeavors of OPG for cancer treatment
As early as 2001, the therapeutic potential of OPG was reported. Vanderkerken et al. found that the tumor burden of the recombinant OPG treatment group was reduced and the tumor onset time was delayed in MM murine model [8]. A recombinant OPG construct was also reported to inhibit bone resorption at the molecular level, which manifested as a decrease in the bone resorption marker urinary N-telopeptide of collagen (NTX) in patients with MM [9]. A mouse model of osteosarcoma expressing truncated OPG showed lower tumor incidence and longer survival [84]. However, OPG failed to inhibit pulmonary metastasis [84], illustrating the relationship of OPG with the bone microenvironment on OPG. What’s more, OPG-Fc treatment of mice with established bone metastases positively impacted prognosis [85]. After the administration of OPG-Fc to 12-week-old BALB/c mice, ovariectomy-induced osteoclast lesions and growth of disseminated tumor cells were prevented. This finding might suggest that bone metastases can be prevented by early administration of anti-resorptive therapy, including OPG, in a low-estrogen environment [86]. The capability of rAAV-OPG therapy to inhibit tumor growth and protect bone integrity was elucidated in a mouse model of bone metastasis [87]. In addition, OPG-Fc inhibited the occurrence and progression of bone metastases in mice bearing skeletal non-small cell lung cancer tumors [10]. Treatment with rOPG-Fc prevented bone damage by patient-derived B cell acute lymphoblastic leukemia (B-ALL) xenografts, even under conditions of a heavy tumor load [88].
In addition to binding to RANKL to inhibit bone resorption, OPG also combines with TRAIL to exert an anti-apoptotic effect, thus limiting the clinical applications of OPG in cancer patients. Special genetically engineered MSCs overexpressing OPG that selectively bind to RANKL, but not to TRAIL were designed by Qiang et al. As expected, the osteoclast activity induced by tumor cells expressing mutant OPG was significantly suppressed, suggesting the potential therapeutic value of this technology [89]. This provided clues for us to develop OPG-related therapeutics with fewer side effects and greater efficacy.
Due to the antagonistic relationship between OPG and RANKL, inhibiting RANKL may somewhat mimic the effect of OPG. The clinical development of OPG-Fc could be replaced by a human monoclonal antibody called denosumab (AMG 162), which specifically inhibits RANKL [90]. Based on data from considerable preclinical studies, many clinical trials have been conducted for denosumab and certain phase III clinical trials achieved exhilarant outcomes in patients with early stage breast cancer or newly diagnosed MM [90,91,92,93,94].
Conclusions
The roles and molecular mechanisms of OPG in bone biology and pathology have been intensively investigated in the past decades. Beyond its involvement in bone diseases, comprehensive understanding of OPG in other benign diseases and in cancer progression has been achieved. OPG is a multifaceted player that can regulate vascular biology, fibrosis, immunity, EMT, and the apoptosis of cancer cells (Fig. 3). As a secreted protein, OPG exerts bioactive functions by modulating various signal transduction pathways in cells, as well as remodeling the local microenvironment. At the same time, the presence of secreted OPG in human blood and the unique expression pattern provide a potential non-invasive approach to the diagnosis and prognosis of cancer patients. The function of OPG is contextual, given it influences numerous signaling, which exerts contradictory functions for cancer progression. Thus, it is essential to modify the endogenous OPG. Engineered OPG should display selective targeting effects to avoid synchronous side effects.
References
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997;89:309–19.
Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front Immunol. 2014;5:511.
Holen I, Shipman CM. Role of osteoprotegerin (OPG) in cancer. Clin Sci (Lond). 2006;110:279–91.
Chen CY, Rao SS, Tan YJ, Luo MJ, Hu XK, Yin H, et al. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. 2019;7:18.
Takayanagi H, Oda H, Yamamoto S, Kawaguchi H, Tanaka S, Nishikawa T, et al. A new mechanism of bone destruction in rheumatoid arthritis: synovial fibroblasts induce osteoclastogenesis. Biochem Biophys Res Commun. 1997;240:279–86.
Renn TY, Huang YK, Feng SW, Wang HW, Lee WF, Lin CT, et al. Prophylactic supplement with melatonin successfully suppresses the pathogenesis of periodontitis through normalizing RANKL/OPG ratio and depressing the TLR4/MyD88 signaling pathway. J Pineal Res. 2018;64. https://doi.org/10.1111/jpi.12464.
Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–7.
Vanderkerken K, De Leenheer E, Shipman C, Asosingh K, Willems A, Van Camp B, et al. Recombinant osteoprotegerin decreases tumor burden and increases survival in a murine model of multiple myeloma. Cancer Res. 2003;63:287–9.
Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP, et al. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97:887–92.
Miller RE, Jones JC, Tometsko M, Blake ML, Dougall WC. RANKL inhibition blocks osteolytic lesions and reduces skeletal tumor burden in models of non-small-cell lung cancer bone metastases. J Thorac Oncol. 2014;9:345–54.
Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, et al. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun. 1997;234:137–42.
American Society for B, Mineral Research President’s Committee on N. Proposed standard nomenclature for new tumor necrosis factor family members involved in the regulation of bone resorption. The American Society for Bone and Mineral Research President’s Committee on Nomenclature. J Bone Min Res. 2000;15:2293–6.
Dawson S, Lawrie A. From bones to blood pressure, developing novel biologic approaches targeting the osteoprotegein pathway for pulmonary vascular disease. Pharm Ther. 2017;169:78–82.
Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–25.
Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–8.
Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl 1): S1.
Scholtysek C, Katzenbeisser J, Fu H, Uderhardt S, Ipseiz N, Stoll C, et al. PPARbeta/delta governs Wnt signaling and bone turnover. Nat Med. 2013;19:608–13.
van Tuyl LH, Voskuyl AE, Boers M, Geusens P, Landewe RB, Dijkmans BA, et al. Baseline RANKL:OPG ratio and markers of bone and cartilage degradation predict annual radiological progression over 11 years in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1623–8.
Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–92.
Di Bartolo BA, Schoppet M, Mattar MZ, Rachner TD, Shanahan CM, Kavurma MM, et al. Calcium and osteoprotegerin regulate IGF1R expression to inhibit vascular calcification. Cardiovasc Res. 2011;91:537–45.
Gordin D, Soro-Paavonen A, Thomas MC, Harjutsalo V, Saraheimo M, Bjerre M, et al. Osteoprotegerin is an independent predictor of vascular events in Finnish adults with type 1 diabetes. Diabetes Care. 2013;36:1827–33.
Rochette L, Meloux A, Rigal E, Zeller M, Cottin Y, Vergely C. et al. The role of osteoprotegerin in the crosstalk between vessels and bone: its potential utility as a marker of cardiometabolic diseases. Pharm Ther. 2018;182:115–32.
Ueland T, Jemtland R, Godang K, Kjekshus J, Hognestad A, Omland T, et al. Prognostic value of osteoprotegerin in heart failure after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1970–6.
Kimura S, Nakamura Y, Kobayashi N, Shiroguchi K, Kawakami E, Mutoh M, et al. Osteoprotegerin-dependent M cell self-regulation balances gut infection and immunity. Nat Commun. 2020;11:234.
Yun TJ, Tallquist MD, Aicher A, Rafferty KL, Marshall AJ, Moon JJ, et al. Osteoprotegerin, a crucial regulator of bone metabolism, also regulates B cell development and function. J Immunol. 2001;166:1482–91.
Baud’huin M, Duplomb L, Teletchea S, Lamoureux F, Ruiz-Velasco C, Maillasson M, et al. Osteoprotegerin: multiple partners for multiple functions. Cytokine Growth Factor Rev. 2013;24:401–9.
Akiyama N, Shinzawa M, Miyauchi M, Yanai H, Tateishi R, Shimo Y, et al. Limitation of immune tolerance-inducing thymic epithelial cell development by Spi-B-mediated negative feedback regulation. J Exp Med. 2014;211:2425–38.
Habibie H, Adhyatmika A, Schaafsma D, Melgert BN. The role of osteoprotegerin (OPG) in fibrosis: its potential as a biomarker and/or biological target for the treatment of fibrotic diseases. Pharm Ther. 2021;228:107941.
Meng H, Bai X, Yu H, Wang Z, Guo A. Osteoprotegerin promotes cell growth by regulating matrix metalloprotease-13 in chondrocytes. J Biomater Tissue Eng. 2017;7:257–60.
Adhyatmika A, Putri KSS, Gore E, Mangnus KA, Reker-Smit C, Schuppan D, et al. Osteoprotegerin Expression in Liver is Induced by IL13 through TGFβ. Cell Physiol Biochem. 2022;56:28–38.
Bosselut N, Taibi L, Guechot J, Zarski JP, Sturm N, Gelineau MC, et al. Including osteoprotegerin and collagen IV in a score-based blood test for liver fibrosis increases diagnostic accuracy. Clin Chim Acta. 2013;415:63–8.
Adhyatmika A, Beljaars L, Putri KSS, Habibie H, Boorsma CE, Reker-Smit C, et al. Osteoprotegerin is more than a possible serum marker in liver fibrosis: a study into its function in human and murine liver. Pharmaceutics. 2020;12:471.
Raje NS, Bhatta S, Terpos E. Role of the RANK/RANKL pathway in multiple myeloma. Clin Cancer Res. 2019;25:12–20.
Sundaram K, Sambandam Y, Balasubramanian S, Pillai B, Voelkel-Johnson C, Ries WL, et al. STAT-6 mediates TRAIL induced RANK ligand expression in stromal/preosteoblast cells. Bone. 2015;71:137–44.
Glass DA 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–64.
Duan P, Bonewald LF. The role of the wnt/beta-catenin signaling pathway in formation and maintenance of bone and teeth. Int J Biochem Cell Biol. 2016;77:23–9.
Quan J, Elhousiny M, Johnson NW, Gao J. Transforming growth factor-beta1 treatment of oral cancer induces epithelial-mesenchymal transition and promotes bone invasion via enhanced activity of osteoclasts. Clin Exp Metastasis. 2013;30:659–70.
Jiao K, Niu LN, Li QH, Chen FM, Zhao W, Li JJ, et al. Biphasic silica/apatite co-mineralized collagen scaffolds stimulate osteogenesis and inhibit RANKL-mediated osteoclastogenesis. Acta Biomater. 2015;19:23–32.
Huang Z, Chu L, Liang J, Tan X, Wang Y, Wen J, et al. H19 Promotes HCC bone metastasis through reducing osteoprotegerin expression in a protein phosphatase 1 catalytic subunit alpha/p38 mitogen-activated protein kinase-dependent manner and sponging microRNA 200b-3p. Hepatology. 2021;74:214–32.
Hao Y, Gao R, Lu B, Ran Y, Yang Z, Liu J, et al. Ghrelin protects against depleted uranium-induced bone damage by increasing osteoprotegerin/RANKL ratio. Toxicol Appl Pharm. 2018;343:62–70.
Nagy V, Penninger JM. The RANKL-RANK story. Gerontology. 2015;61:534–42.
Bai S, Kopan R, Zou W, Hilton MJ, Ong CT, Long F, et al. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem. 2008;283:6509–18.
Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J, et al. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008;112:196–207.
O’Regan N, Moxon C, Gegenbauer K, O’Sullivan JM, Chion A, Smith OP, et al. Marked elevation in plasma osteoprotegerin constitutes an early and consistent feature of cerebral malaria. Thromb Haemost. 2016;115:773–80.
Standal T, Seidel C, Hjertner O, Plesner T, Sanderson RD, Waage A, et al. Osteoprotegerin is bound, internalized, and degraded by multiple myeloma cells. Blood. 2002;100:3002–7.
Lane D, Matte I, Rancourt C, Piche A. Osteoprotegerin (OPG) protects ovarian cancer cells from TRAIL-induced apoptosis but does not contribute to malignant ascites-mediated attenuation of TRAIL-induced apoptosis. J Ovarian Res. 2012;5:34.
Alsterda A, Asha K, Powrozek O, Repak M, Goswami S, Dunn AM, et al. Salubrinal exposes anticancer properties in inflammatory breast cancer cells by manipulating the endoplasmic reticulum stress pathway. Front Oncol. 2021;11:654940.
Wan X, Song Y, Fang H, Xu L, Che X, Wang S, et al. RANKL/RANK promotes the migration of gastric cancer cells by interacting with EGFR. Clin Transl Med. 2020;9:3.
Alraouji NN, Hendrayani SF, Ghebeh H, Al-Mohanna FH, Aboussekhra A, Osteoprotegerin OPG, et al. mediates the anti-carcinogenic effects of normal breast fibroblasts and targets cancer stem cells through inhibition of the beta-catenin pathway. Cancer Lett. 2021;520:374–84.
Cross SS, Yang Z, Brown NJ, Balasubramanian SP, Evans CA, Woodward JK, et al. Osteoprotegerin (OPG)-a potential new role in the regulation of endothelial cell phenotype and tumour angiogenesis? Int J Cancer. 2006;118:1901–8.
Pritzker LB, Scatena M, Giachelli CM. The role of osteoprotegerin and tumor necrosis factor-related apoptosis-inducing ligand in human microvascular endothelial cell survival. Mol Biol Cell. 2004;15:2834–41.
Benslimane-Ahmim Z, Heymann D, Dizier B, Lokajczyk A, Brion R, Laurendeau I, et al. Osteoprotegerin, a new actor in vasculogenesis, stimulates endothelial colony-forming cells properties. J Thromb Haemost. 2011;9:834–43.
Benslimane-Ahmim Z, Pereira J, Lokajczyk A, Dizier B, Galy-Fauroux I, Fischer A-M, et al. Osteoprotegerin regulates cancer cell migration through SDF-1/CXCR4 axis and promotes tumour development by increasing neovascularization. Cancer Lett. 2017;395:11–9.
Wang H, Zhang W, Bado I, Zhang XH. Bone tropism in cancer metastases. Cold Spring Harb Perspect Med. 2020;10. https://doi.org/10.1101/cshperspect.a036848.
Jung K, Lein M, Stephan C, Von Hosslin K, Semjonow A, Sinha P, et al. Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. Int J Cancer. 2004;111:783–91.
Christoph F, Konig F, Lebentrau S, Jandrig B, Krause H, Strenziok R, et al. RANKL/RANK/OPG cytokine receptor system: mRNA expression pattern in BPH, primary and metastatic prostate cancer disease. World J Urol. 2018;36:187–92.
Cody JJ, Rivera AA, Lyons GR, Yang SW, Wang M, Ashley JW, et al. Expression of osteoprotegerin from a replicating adenovirus inhibits the progression of prostate cancer bone metastases in a murine model. Lab Invest. 2013;93:268–78.
Corey E, Brown LG, Kiefer JA, Quinn JE, Pitts TE, Blair JM, et al. Osteoprotegerin in prostate cancer bone metastasis. Cancer Res. 2005;65:1710–8.
Elfar GA, Ebrahim MA, Elsherbiny NM, Eissa LA. Validity of osteoprotegerin and receptor activator of NF-kappaB ligand for the detection of bone metastasis in breast cancer. Oncol Res. 2017;25:641–50.
Rachner TD, Singh SK, Schoppet M, Benad P, Bornhauser M, Ellenrieder V, et al. Zoledronic acid induces apoptosis and changes the TRAIL/OPG ratio in breast cancer cells. Cancer Lett. 2010;287:109–16.
Mercatali L, Ricci M, Scarpi E, Serra P, Fabbri F, Ricci R, et al. RANK/RANK-L/OPG in patients with bone metastases treated with anticancer agents and zoledronic acid: a prospective study. Int J Mol Sci. 2013;14:10683–93.
Fortner RT, Sarink D, Schock H, Johnson T, Tjonneland A, Olsen A, et al. Osteoprotegerin and breast cancer risk by hormone receptor subtype: a nested case-control study in the EPIC cohort. BMC Med. 2017;15:26.
Sarink D, Schock H, Johnson T, Chang-Claude J, Overvad K, Olsen A, et al. Receptor activator of nuclear factor kB ligand, osteoprotegerin, and risk of death following a breast cancer diagnosis: results from the EPIC cohort. BMC Cancer. 2018;18:1010.
Vik A, Brodin EE, Mathiesen EB, Brox J, Jorgensen L, Njolstad I, et al. Serum osteoprotegerin and future risk of cancer and cancer-related mortality in the general population: the Tromso study. Eur J Epidemiol. 2015;30:219–30.
Mountzios G, Dimopoulos MA, Bamias A, Papadopoulos G, Kastritis E, Syrigos K, et al. Abnormal bone remodeling process is due to an imbalance in the receptor activator of nuclear factor-kappaB ligand (RANKL)/osteoprotegerin (OPG) axis in patients with solid tumors metastatic to the skeleton. Acta Oncol. 2007;46:221–9.
Widschwendter M, Burnell M, Fraser L, Rosenthal AN, Philpott S, Reisel D, et al. Osteoprotegerin (OPG), the endogenous inhibitor of receptor activator of NF-kappaB ligand (RANKL), is dysregulated in BRCA mutation carriers. EBioMedicine 2015;2:1331–9.
Lintermans A, Van Asten K, Jongen L, Van Brussel T, Laenen A, Verhaeghe J, et al. Genetic variant in the osteoprotegerin gene is associated with aromatase inhibitor-related musculoskeletal toxicity in breast cancer patients. Eur J Cancer. 2016;56:31–6.
Holen I, Croucher PI, Hamdy FC, Eaton CL. Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res. 2002;62:1619–23.
Marino S, Bishop RT, Carrasco G, Logan JG, Li B, Idris AI. Pharmacological inhibition of NFkappaB reduces prostate cancer related osteoclastogenesis in vitro and osteolysis ex vivo. Calcif Tissue Int. 2019;105:193–204.
Velletri T, Huang Y, Wang Y, Li Q, Hu M, Xie N, et al. Loss of p53 in mesenchymal stem cells promotes alteration of bone remodeling through negative regulation of osteoprotegerin. Cell Death Differ. 2021;28:156–69.
Breitkreutz I, Raab MS, Vallet S, Hideshima T, Raje N, Mitsiades C, et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia. 2008;22:1925–32.
Goranova-Marinova V, Goranov S, Pavlov P, Tzvetkova T. Serum levels of OPG, RANKL and RANKL/OPG ratio in newly-diagnosed patients with multiple myeloma. Clinical correlations. Haematologica. 2007;92:1000–1.
Rabin N, Kyriakou C, Coulton L, Gallagher OM, Buckle C, Benjamin R, et al. A new xenograft model of myeloma bone disease demonstrating the efficacy of human mesenchymal stem cells expressing osteoprotegerin by lentiviral gene transfer. Leukemia. 2007;21:2181–91.
Eom KS, Kim SJ, Lee JJ, Suh C, Kim JS, Yoon SS, et al. Changes in osteoblastic activity in patient who received bortezomib as second line treatment for plasma cell myeloma: a prospective multicenter study. Biomed Res Int. 2014;2014:245247.
Inanc M, Kaynar L, Enhos S, Pala C, Karaca H, Berk V, et al. Nuclear factor-kappa B ligand and osteoprotegerin levels in serum and gingival crevicular fluid in patients with bone metastases treated with zoledronic acid. Med Oncol. 2014;31:837.
Johnson DC, Weinhold N, Mitchell J, Chen B, Stephens OW, Forsti A, et al. Genetic factors influencing the risk of multiple myeloma bone disease. Leukemia 2016;30:883–8.
Jiang R, Xia Y, Li J, Deng L, Zhao L, Shi J, et al. High expression levels of IKKalpha and IKKbeta are necessary for the malignant properties of liver cancer. Int J Cancer. 2010;126:1263–74.
Zhang C, Lin J, Ni X, Li H, Zheng L, Zhao Z, et al. Prognostic value of serum osteoprotegerin level in patients with hepatocellular carcinoma following surgical resection. Front Oncol. 2021;11:731989.
Cheng K, Shi J, Liu Z, Jia Y, Qin Q, Zhang H, et al. A panel of five plasma proteins for the early diagnosis of hepatitis B virus-related hepatocellular carcinoma in individuals at risk. EBioMedicine. 2020;52:102638.
Gao YB, Xiang ZL, Zhou LY, Wu ZF, Fan J, Zeng HY, et al. Enhanced production of CTGF and IL-11 from highly metastatic hepatoma cells under hypoxic conditions: an implication of hepatocellular carcinoma metastasis to bone. J Cancer Res Clin Oncol. 2013;139:669–79.
Shi W, Qiu W, Wang W, Zhou X, Zhong X, Tian G, et al. Osteoprotegerin is up-regulated in pancreatic cancers and correlates with cancer-associated new-onset diabetes. Biosci Trends. 2014;8:322–6.
Aversa J, Song M, Shimazu T, Inoue M, Charvat H, Yamaji T, et al. Prediagnostic circulating inflammation biomarkers and esophageal squamous cell carcinoma: a case-cohort study in Japan. Int J Cancer. 2020;147:686–91.
Russmueller G, Moser D, Wurger T, Wrba F, Christopoulos P, Kostakis G, et al. Upregulation of osteoprotegerin expression correlates with bone invasion and predicts poor clinical outcome in oral cancer. Oral Oncol. 2015;51:247–53.
Lamoureux F, Richard P, Wittrant Y, Battaglia S, Pilet P, Trichet V, et al. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res. 2007;67:7308–18.
Canon JR, Roudier M, Bryant R, Morony S, Stolina M, Kostenuik PJ, et al. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis. 2008;25:119–29.
Ottewell PD, Wang N, Brown HK, Fowles CA, Croucher PI, Eaton CL, et al. OPG-Fc inhibits ovariectomy-induced growth of disseminated breast cancer cells in bone. Int J Cancer. 2015;137:968–77.
Chanda D, Isayeva T, Kumar S, Siegal GP, Szafran AA, Zinn KR, et al. Systemic osteoprotegerin gene therapy restores tumor-induced bone loss in a therapeutic model of breast cancer bone metastasis. Mol Ther. 2008;16:871–8.
Rajakumar SA, Papp E, Lee KK, Grandal I, Merico D, Liu CC, et al. B cell acute lymphoblastic leukemia cells mediate RANK-RANKL-dependent bone destruction. Sci Transl Med. 2020;12:eaba5942.
Higgs JT, Lee JH, Wang H, Ramani VC, Chanda D, Hardy CY, et al. Mesenchymal stem cells expressing osteoprotegerin variants inhibit osteolysis in a murine model of multiple myeloma. Blood Adv. 2017;1:2375–85.
Kostenuik PJ, Nguyen HQ, McCabe J, Warmington KS, Kurahara C, Sun N, et al. Denosumab, a fully human monoclonal antibody to RANKL, Inhibits bone resorption and increases BMD in knock-in mice that express chimeric (Murine/Human) RANKL. J Bone Miner Res. 2009;24:182–95.
Santini D, Galluzzo S, Zoccoli A, Pantano F, Fratto ME, Vincenzi B, et al. New molecular targets in bone metastases. Cancer Treat Rev 2010;36(Suppl 3):S6–S10.
Gnant M, Pfeiler G, Steger GG, Egle D, Greil R, Fitzal F, et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:339–51.
Coleman R, Finkelstein DM, Barrios C, Martin M, Iwata H, Hegg R, et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21:60–72.
Raje N, Terpos E, Willenbacher W, Shimizu K, García-Sanz R, Durie B, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19:370–81.
Beuselinck B, Jean-Baptiste J, Couchy G, Job S, De Reynies A, Wolter P, et al. RANK/OPG ratio of expression in primary clear-cell renal cell carcinoma is associated with bone metastasis and prognosis in patients treated with anti-VEGFR-TKIs. Br J Cancer. 2015;113:1313–22.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81372327, 81572427 and 81874189).
Author information
Authors and Affiliations
Contributions
ZH and YW designed this study. YW and YL drafted the manuscript. ZH, BX and XC revised the manuscript. BX obtained funding. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Liu, Y., Huang, Z. et al. The roles of osteoprotegerin in cancer, far beyond a bone player. Cell Death Discov. 8, 252 (2022). https://doi.org/10.1038/s41420-022-01042-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-022-01042-0