Abstract
Endothelin (EDN, also known as ET) signaling has been suggested to be an important mediator of retinal ganglion cell (RGC) death in glaucoma. Antagonism of EDN receptors (EDNRA and EDNRB, also known as ET-A and ET-B) prevented RGC death in mouse models of chronic ocular hypertension, and intravitreal injection of EDN ligand was sufficient to drive RGC death. However, it remains unclear which cell types EDN ligands directly affect to elicit RGC death. Multiple cell types in the retina and optic nerve express EDNRA and EDNRB and thus could respond to EDN ligands in the context of glaucoma. Here, we systematically deleted Edn receptors from specific cell types to identify the critical EDN receptor mediating RGC death in vivo. Deletion of both Ednra and Ednrb from retinal neurons (including RGCs) and macroglia did not prevent RGC loss after exposure to EDN1 ligands, suggesting EDN1 ligands cause RGC death via an indirect mechanism involving a secondary cell type. Deletion of Ednra from the full body, and then specifically from vascular mural cells, prevented EDN1-induced vasoconstriction and RGC death. Together, these data suggest EDN ligands cause RGC death via a mechanism initiated by vascular mural cells. It is possible RGC death is a consequence of vascular mural cell-induced vasoconstriction and its pathological sequelae. These results highlight the potential importance of neurovascular dysfunction in glaucoma.
Similar content being viewed by others
Introduction
Glaucoma is a neurodegenerative condition affecting the output neurons of the retina—the retinal ganglion cells (RGCs). One of the most important risk factors for developing glaucomatous neurodegeneration is elevated intraocular pressure (IOP) [1]. To date, elevated IOP is the only clinically treatable component of glaucoma, and unfortunately, normalizing IOP does not prevent glaucoma progression or development in many patients [2]. Therefore, understanding the molecular signaling pathways that lead from ocular hypertensive injury to RGC death is critical for understanding the pathobiology of glaucoma. Recent evidence has suggested the importance of RGC-extrinsic signaling events (e.g., neuroinflammation, neurovascular dysfunction) in triggering glaucomatous RGC injury [3,4,5,6]. Molecular clustering analysis of ocular hypertensive DBA/2J retinas and optic nerves revealed several candidate mechanisms that are potentially critical in driving RGC injury in glaucoma, including activation of the endothelin system [5, 7].
The endothelin system is a family of three ligands (EDN1, 2, and 3, also known as ET-1, 2, and 3) and two G-protein coupled receptors (EDNRA and EDNRB, also known as ET-A and ET-B). The canonical role of the endothelin system is to regulate blood flow and vasoconstriction. Potent vasoconstriction occurs when EDN ligands bind to EDNRA [8,9,10,11], which is highly expressed by vascular mural cells [6, 12, 13], including smooth muscle cells [14,15,16,17] and pericytes [18, 19]. EDNRB is expressed by vascular endothelial cells [20,21,22], and is thought to mediate vasorelaxation in response to ligand binding. Many organ systems, including the central nervous system, use the endothelin system to maintain normal physiology [23, 24].
As with many signaling systems that have a physiological role, endothelin signaling has also been broadly implicated in the pathophysiology of numerous diseases, including retinal diseases and glaucoma [25, 26]. EDN ligands and receptors are known to be expressed by glaucoma-relevant cell types. EDN ligands have been shown to be expressed by retinal and optic nerve macroglia [6, 27] and myeloid-derived cells [5, 6], while both EDN receptors are expressed by retinal neurons (including RGCs) [12, 14, 25, 28, 29] and macroglia [26, 30,31,32,33]. Endothelin signaling has been hypothesized to play a role in human glaucoma. Levels of EDN ligand were found to be higher in the aqueous humor and plasma of glaucoma patients [34, 35]. Changes in blood flow have been documented in human [36,37,38,39] and animal models [3, 5] of glaucoma, and it is hypothesized that these changes could be important factors in the development and progression of glaucoma. Animal models of ocular hypertension have also indicated a potential role for endothelin signaling in glaucoma. Edn ligands and receptors were significantly upregulated in retinas and optic nerve heads of ocular hypertensive DBA/2J mice before the onset of glaucomatous neurodegeneration [3, 5, 6]. Similar patterns of endothelin system upregulation were found in models of acutely induced ocular hypertension [28] and after glaucoma-relevant optic nerve crush [40]. EDN ligands are sufficient to cause RGC death— intravitreal injection or transgenic overexpression of EDN ligands caused significant RGC loss and axonal degeneration [3, 5, 11, 12, 41,42,43,44]. Caspase 3 activation in RGCs and later RGC loss after EDN1 exposure was dependent upon JUN activation, similar to RGC death after glaucoma-relevant injuries including optic nerve crush [45] and ocular hypertension [46]. Importantly, pan-antagonism of EDN receptors with Bosentan or Macitentan conferred significant protection from glaucomatous neurodegeneration in DBA/2J ocular hypertensive mice [5, 6]. Thus, targeting endothelin signaling may have potential as a neuroprotective treatment for glaucoma.
Despite their apparent role in glaucoma pathology, it is unclear how EDN ligands act in the retina or optic nerve to ultimately drive RGC death. It is possible EDN ligands cause RGC death directly via RGC-expressed EDN receptors, as has been demonstrated in vitro [12]. But it is also possible EDN ligands bind to receptors expressed by astrocytes or vasculature, thereby triggering a neurotoxic response. Understanding the cell types important in EDN-induced RGC death will provide insight into early, critical pathways of glaucomatous neurodegeneration and can identify potential therapeutic targets for neuroprotective glaucoma treatments. The present work utilized cell-specific deletions of Ednra and/or Ednrb to investigate the mechanisms by which EDN ligands drive RGC death in vivo.
Results
EDN ligand did not act through RGC- or macroglia-expressed EDN receptors to cause RGC death
Intravitreal delivery of EDN ligand was sufficient to drive RGC death [3, 5, 11, 12, 41, 42]. Studies have suggested EDN ligands cause RGC death directly. Primary RGCs in culture underwent cell death after EDN ligand exposure [12, 25], suggesting EDN ligands can bind to RGC-expressed EDN receptors and drive cell death. There is evidence to suggest RGCs express both EDNRB [6, 12, 13, 28, 29] and EDNRA [12, 28, 29], therefore, EDN ligands could bind to either or both RGC-expressed EDN receptors to directly cause RGC death. To investigate whether EDN ligands affect RGCs directly, Six3-cre was used to recombine homozygous floxed alleles of Ednra and/or Ednrb. Six3-cre is well known to recombine floxed alleles in retinal neurons, including in 80% of RGCs [45]. Of note, studies have reported Six3-cre-mediated recombination of floxed alleles in macroglia (astrocytes and Müller glia), but not in vascular cells [47,48,49]. Macroglia (Müller glia and astrocytes) are known to robustly express EDNRB [6], and some studies have shown EDNRA expression by astrocytes [31,32,33]. Therefore, EDN ligand could feasibly bind to EDN receptors expressed by macroglia to ultimately cause RGC death.
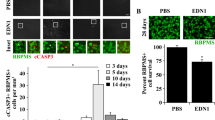
To determine whether neuronal and/or macroglial EDN receptors are required for EDN-induced RGC death, EDN1 was intravitreally injected into the eyes of WT (Six3-cre−Ednra+/+Ednrb+/+, Six3-cre+Ednrafl/flEdnrb+/+, and Six3-cre+Ednra+/+Ednrbfl/fl mice. PBS was injected into the contralateral eye as a vehicle-matched control. As expected,` Six3-cre-mediated deletion of Edn receptors did not interfere with EDN1-induced vasoconstriction (vascular smooth muscle-expressed EDNRA is known to induce vasoconstriction upon ligand binding [8,9,10,11]). Intravitreal EDN1 injection caused similar levels of vasoconstriction in WT, Six3-cre+Ednrafl/fl, Six3-cre+Ednrbfl/fl, and Six3-cre+Ednrafl/flEdnrbfl/fl retinas (Fig. 1A). Previous reports have shown EDN1 caused caspase 3 activation (cleavage; cCASP3) in RGCs 5 days post-intravitreal injection, which corresponded to RGC dropout by 28 days [44]. Genetic manipulations that prevented later RGC dropout also prevented early caspase 3 activation [44]—a pattern which is also observed after other glaucoma-relevant injuries [46, 50]. Therefore, the presence of cCASP3+ RGCs was used to assess RGC injury after EDN1 injection. As reported previously, intravitreal injection of PBS did not drive appreciable caspase 3 activation in RGCs, and intravitreal injection of EDN1 ligand drove significant caspase 3 activation in RGCs (Fig. 1B). Surprisingly, deletion of either Ednra or Ednrb from retinal neurons (including RGCs) and macroglia did not prevent EDN1-induced caspase 3 activation (cleavage) in RGCs (Fig. 1B). To address the possibility that both EDN receptors expressed by retinal neurons and/or macroglia are required to cause RGC death, EDN1 was injected into the eyes of Six3-cre+Ednrafl/flEdnrbfl/fl mice. Deletion of both Edn receptors from macroglia and retinal neurons did not prevent caspase 3 activation in RGCs in response to EDN1 (Fig. 1B). These data suggest EDN1 ligands did not directly affect neurons (including RGCs) or macroglia to drive RGC death. Rather, EDN1 ligands acted through either EDNRA or EDNRB expressed by a different cell type. These results necessitated the identification of the EDN receptor, regardless of the cell type expressing it, which ultimately drives EDN1-induced RGC death.
A Fluorescein angiography of retinal vasculature in naive and EDN1-injected eyes from WT, Six3-cre+Ednrafl/flEdnrb+/+, Six3-cre+Ednra+/+Ednrbfl/fl, and Six3-cre+Ednrafl/flEdnrbfl/fl animals. Deletion of either or both Edn receptors with Six3-cre did not prevent EDN1-induced vasoconstriction (n ≥ 3). B Retinal flat mounts and quantification of cleaved caspase 3+ (cCASP3+, red) RBPMS+ (green) cells from WT, Six3-cre+Ednra+/+Ednrbfl/fl, Six3-cre+Ednrafl/flEdnrb+/+, Six3-cre+Ednrafl/flEdnrbfl/fl 5 days post-EDN1 or PBS (vehicle control) injection. Each genotype group had significant increases in cCASP3+ RGCs compared to PBS controls. No significant difference in cCASP3+ RGCs was observed between genotype groups after EDN1. cCASP3+ RGCs/mm2 ± SEM: PBS: 0.8 ± 0.3, WT: 32.0 ± 7.2, Six3-cre+Ednrafl/flEdnrb+/+: 23.7 ± 8.6, Six3-cre+Ednra+/+Ednrbfl/fl: 32.3 ± 9.5, Six3-cre+Ednrafl/flEdnrbfl/fl: 19.8 ± 6.0 (n ≥ 7 per genotype, *P < 0.05, Kruskal–Wallis test). Scale bars, 50 μm.
Endothelin ligand acted through non-neuronal, non-macroglial EDNRA to elicit RGC death
Given EDN1 did not elicit RGC death via RGC- or macroglia-expressed EDN receptors, EDN1 must directly affect a different cell type through either EDNRA or EDNRB. Beyond retinal neurons and macroglia, Ednra is known to be expressed by vascular mural cells [6, 12,13,14,15,16,17,18,19], and Ednrb is known to be expressed by endothelial cells [20,21,22]. It is also possible Ednra and/or Ednrb are expressed at low levels by another cell type (e.g., myeloid cells) and are able to pathologically respond to EDN ligand exposure. To determine whether EDN acts through EDNRB or EDNRA to cause RGC death, EDN1-induced RGC death was assessed in mice with global deletions of Ednra or Ednrb (Cag-creERT2+Ednrafl/fl and Cag-creERT2+Ednrbfl/fl mice were treated with tamoxifen to produce full-body knockouts).
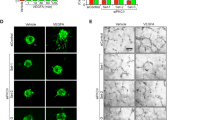
Cag-creERT2+TdTomato+ retinas and optic nerves were first evaluated to assess Cag-creERT2 recombination efficiency. Cag-creERT2+TdTomato+ retinas had TdTomato expression in nearly all DAPI+ cells in the retina, including retinal neurons, macroglia, and vascular cells (Fig. 2A). Furthermore, EDN ligand is known to induce vasoconstriction upon binding to vascular mural cell-expressed EDNRA [8,9,10,11]. Cag-creERT2-mediated deletion of Ednra completely prevented retinal vasoconstriction in response to intravitreal EDN1 (Fig. 2B). Therefore, EDN receptors were successfully deleted from the major cell types in the retina and optic nerve known to endogenously express each receptor. To determine whether EDN ligand binds to EDNRA or EDNRB to ultimately elicit RGC death, EDN1 was intravitreally injected into both Cag-creERT2+Ednrafl/fl and Cag-creERT2+Ednrbfl/fl mice. Global deletion of Ednra, but not Ednrb, prevented EDN1-induced caspase 3 activation in RGCs 5 days following injury (Fig. 3A). Furthermore, global Ednra deletion prevented RGC loss 28 days post-EDN1 (Fig. 3B). These data suggest, in contrast to previous reports [12, 25], EDN ligands act through non-neuronal/macroglial cell types expressing EDNRA to ultimately cause RGC death.
A Cag-creERT2+Tdtomato+ retinal and optic nerve head sections depicting cell-type-specific expression of Tdtomato. Cag-creERT2 robustly recombined floxed alleles in DAPI+ cells, including RBPMS+ RGCs, SOX2+ Müller glia, GFAP+ retinal astrocytes, CD31+ vascular cells, and SOX2+ GFAP+ optic nerve head (ONH) astrocytes (n = 3). Scale bars, 50 μm. B Fluorescein angiography of retinal vasculature in naive and EDN1-injected eyes from WT, Cag-creERT2+Ednrbfl/fl, and Cag-creERT2+Ednrafl/fl animals. Full body deletion of Ednra ablated EDN1-induced vasoconstriction (n ≥ 5).
A Retinal flat mounts from WT, Cag-creERT2+Ednrbfl/fl, and Cag-creERT2+Ednrafl/fl mice immunoassayed for cCASP3 and RBPMS 5 days post-EDN1. Cag-creERT2+Ednrbfl/fl retinas had similar numbers of cCASP3+ RGCs compared to WT controls, while Cag-creERT2+Ednrafl/fl mice had significantly reduced cCASP3+ RGCs compared to both WT and Cag-creERT2+Ednrbfl/fl retinas. cCASP3+ RGCs/mm2 ± SEM: WT: 24.2 ± 8.9 Cag-creERT2+Ednrbfl/fl: 18.0 ± 7.4, Cag-creERT2+Ednrafl/fl: 0.7 ± 0.1 (n ≥ 6, *P < 0.01, Kruskal-Wallis test). Scale bar, 50 μm. B Flat mounted WT and Cag-creERT2+Ednrafl/fl retinas immunoassayed for RBPMS 28 days post-EDN1 injection. Full body deletion of Ednra prevented EDN1-induced RGC loss. %RBPMS+ cell survival±SEM for WT and Cag-creERT2+Ednrafl/fl respectively: PBS: 100.0 ± 3.4, 100.0 ± 2.7; EDN1: 82.0 ± 2.7, 97.0 ± 3.8 (n ≥ 6, *P < 0.05, two-way ANOVA, Holm–Sidak post hoc). Scale bars, 50 μm.
Endothelin ligand caused RGC death via mural cell-expressed EDNRA
Full-body deletion of Ednra prevented both EDN1-induced vasoconstriction and RGC death. Beyond expression by RGCs and astrocytes, EDNRA is expressed by vascular mural cells. Upon ligand binding, vascular mural cell EDNRA elicits contraction (vasoconstriction) [8,9,10,11]. Therefore, the role of vascular mural cell EDNRA in RGC death in response to EDN ligand was investigated. To accomplish this, Ednrafl alleles were recombined from vascular mural cells (vascular smooth muscle cells and pericytes) with Myh11-creERT2 upon tamoxifen treatment [51, 52]. Myh11-creERT2+TdTomato+ retinas were first evaluated to assess cre recombination efficiency and specificity in retinal vascular cells (Fig. 4A–C). In vivo angiography and ex vivo immunofluorescence revealed TdTomato expression was specifically localized to retinal arteries (identified by distinct branching pattern compared to retinal veins [53]) and capillaries in superficial, intermediate, and deep layers of the retina. Importantly, EDN1-induced vasoconstriction was attenuated in Myh11-creERT2+Ednrafl/fl retinas (Fig. 4D). Therefore, Ednrafl alleles were specifically and efficiently recombined from retinal vascular mural cells. To determine whether EDN acts through vascular mural cell-expressed EDNRA to cause RGC death, EDN1 ligand was intravitreally injected into Myh11-creERT2+Ednrafl/fl mice. Mural cell deletion of Ednra prevented caspase 3 activation in RGCs 5 days after EDN1 (Fig. 5A), and prevented RGC loss after 28 days (Fig. 5B). Therefore, EDN1 ligand acted through EDNRA expressed by mural cells to ultimately drive RGC death.
A Myh11-creERT2+Tdtomato+ fluorescein angiography overlayed with TdTomato fluorescence demonstrating TdTomato localization to retinal arteries (n = 4). B Myh11-creERT2Tdtomato+ retinal flat mounts counterstained with CD31 to visualize retinal vasculature. Tdtomato was robustly and specifically expressed by arterial cells and capillaries, but not by RBPMS+ RGCs or any other observable cell type. C Tdtomato+ cells surrounding retinal capillaries were apparent in superficial, intermediate, and deep layers of the retina (n = 3). Scale bars, 50 μm. D Fluorescein angiography of retinal vasculature in naive and EDN1-injected eyes from WT and Myh11-creERT2+Ednrafl/fl animals. Mural cell-specific deletion of Ednra ablated EDN1-induced vasoconstriction (n ≥ 5).
A Flat mounted retinas immunoassayed for cCASP3 and RBPMS 5 days post-EDN1 injection. Ednra deletion from vascular mural cells significantly reduced numbers of cCASP3+ RGCs after EDN1 injury. cCASP3+ RGCs/mm2: WT: 23.6 ± 7.6, Myh11-creERT2+Ednrafl/fl: 6.0 ± 4.4 (n ≥ 9, *P = 0.009, Mann–Whitney test). B Flat mounted WT and Myh11-creERT2+Ednrafl/fl retinas immunoassayed for RBPMS 28 days post-EDN1 injection. Mural cell deletion of Ednra prevented EDN-induced RGC loss. %RBPMS+ cell survival ± SEM for WT and Myh11-creERT2+Ednrafl/fl respectively: PBS: 100.0 ± 2.3, 100.0 ± 1.4; EDN1: 85.0 ± 4.3, 101.4 ± 1.3 (n ≥ 8, *P ≤ 0.001, two-way ANOVA, Holm–Sidak post hoc). Scale bars, 50 μm.
Discussion
Glaucoma is a multifactorial, heterogeneous neurodegenerative condition. Often In glaucoma, an increase in IOP leads to RGC injury and subsequent death. Several hypotheses have been postulated as to how ocular hypertension leads to RGC injury in glaucoma. Recent work has provided strong evidence for the role of endothelin signaling in causing RGC injury and subsequent death in models of chronic ocular hypertension [5, 6]. EDN ligands were upregulated in human [34, 54] and animal models [3, 5, 25, 40] of glaucoma, and intravitreal injection of EDN ligand was sufficient to drive caspase 3 activation in RGCs, which corresponded with later RGC death [44]. Similar to models of glaucoma-relevant axonal injury [45] and ocular hypertension [46], deletion of Jun from RGCs prevented caspase 3 activation in RGCs and prevented later RGC loss after intravitreal EDN1 injection [44]. Pan-antagonism of EDN receptors significantly slowed RGC loss in the DBA/2J model of ocular hypertension [5, 6], suggesting a causal role for the endothelin system in glaucoma pathogenesis. However, it was unknown which cell type EDN ligands directly affect in order to ultimately drive RGC death, and through which receptor (EDNRA or EDNRB) this occurs.
Previous in vitro studies have suggested EDN ligands can cause primary RGC death, suggesting EDN ligands are directly neurotoxic to RGCs (acting through RGC-expressed EDN receptors) [12]. Here, we demonstrate EDN1 ligands did not cause RGC death directly and did not cause RGC death by affecting other retinal neurons or macroglia in vivo (Fig. 1). Also, in contrast with previous studies suggesting EDN ligands act through EDNRB to drive RGC death in vitro, after EDN injection in vivo, and in a model of chronic ocular hypertension [12, 25], EDNRB was not required for EDN1-induced RGC death. Rather, EDNRA was the receptor that was necessary for EDN1-induced RGC death (Fig. 3). Given the canonical role of EDNRA is to mediate vasoconstriction [8,9,10,11], we investigated the importance of mural cell-expressed EDNRA in EDN-induced RGC death. We demonstrated EDN1-induced RGC death was driven by vascular mural cell (smooth muscle and pericyte)-expressed EDNRA (Fig. 5). These data do not preclude the possibility that vascular mural cells respond to EDN1 by eliciting neurotoxic paracrine or endocrine signaling. However, it is likely EDN1-induced RGC death is a result of EDNRA-mediated vasoconstriction and its sequalae.
Because Edn ligands were upregulated in DBA/2J glaucoma [3, 5, 7], pan-antagonism of EDN signaling lessened RGC loss after ocular hypertensive insults [5, 6], and EDN1-induced RGC death was driven by vascular mural cell-expressed EDNRA (Fig. 5), it is possible that chronic vascular pathology or vasoconstriction is an important mediator of RGC death in glaucoma. Vascular involvement is consistent with several observations in human and animal models of glaucoma. Reduced ocular and retinal blood flow have been documented in human [36,37,38,39] and animal models [3, 5] of glaucoma, and it is hypothesized that these changes could be important factors in the development and progression of glaucoma. Hypoxic glia and RGCs were present after acute [55, 56] and chronic [57] ocular hypertension in rodents, suggesting the potential importance of hypoxia in driving glaucoma-relevant pathology.
If vascular EDNRA-induced vasoconstriction causes RGC death in response to EDN ligand, it will be important to investigate the pathological cellular events that lead from vasoconstriction to RGC death. While EDN1 injection was shown to cause regional RGC and glial hypoxia [44] (similar to glaucomatous ocular hypertension [55, 57]), oxygen deprivation itself is unlikely to cause this RGC death. Oxygen deprivation severe enough to cause RGC death is also known to cause loss of amacrine neurons [58,59,60,61,62]. Previous work has demonstrated that, similar to ocular hypertension [63], EDN1 injection was not sufficient to drive the death of amacrine cells [44]. Therefore, if vasoconstriction is important in EDN-induced RGC death, it most likely drives secondary neurotoxic pathological events.
Chronic low-level hypoxia mediated by endothelin signaling may lead to compromise of the blood-brain barrier after EDN1 exposure and in glaucoma. In vitro, hypoxic conditions were sufficient to degrade endothelial cell tight junctions and cause barrier permeability [64,65,66]. Chronic mild hypoxia [67] and transgenic overexpression or injection of EDN ligand [43, 68, 69] led to loss of blood–brain barrier integrity and vascular leakage in vivo. Breakdown of the blood-brain barrier and subsequent infiltration of peripheral immune cells has been suggested to drive neurodegeneration in glaucoma—prevention of immune cell infiltration with radiation therapy protected from glaucoma in DBA/2J mice [3]. Similarly, deletion of Cd11b (also known as Itgam—a cell adhesion protein critical for tissue infiltration of monocytes) was shown to lessen monocyte infiltration into the optic nerve head and protect from glaucomatous neurodegeneration in DBA/2J mice [70]. Consistent with these results, deletion of Glycam (a proteoglycan ligand for L-selectin known to prevent transendothelial migration of leukocytes) promoted monocyte infiltration into the optic nerve head and weakened the protection afforded to DBA/2J retinas by radiation treatment [4]. Given the importance of vascular compromise in glaucoma, it is possible that EDNRA-induced vasoconstriction and its sequelae damages the blood-brain barrier and plays a role in neurodegeneration in response to EDN1 ligand and in glaucoma.
It is also possible that EDN-induced regional mild hypoxia can affect immune cells in the retina or optic nerve. Astrocytes took on a reactive phenotype in response to hypoxia in vitro [71, 72] and in vivo [73, 74], which could potentially lead to a neurotoxic gliotic response. Furthermore, astrocytes aid in maintaining blood-brain barrier integrity. Chronic hypoxic conditions led to a loss of astrocyte-endothelial cell contacts [65]. Astrocytes are also known to upregulate and secrete VEGF upon hypoxic insult [75]. Astrocyte-specific VEGF was critical for pathological neovascularization after retinal hypoxic injury in vivo [76]. VEGF was required for hypoxia-induced blood-brain barrier breakdown [66], and astrocyte-specific VEGF was shown to cause blood–brain barrier breakdown in vitro [77]. Together, these data suggest hypoxia can cause changes in retinal astrocytes, which can in turn drive neurotoxic signaling and/or contribute to breakdown of the blood–brain barrier. The mechanisms by which EDN-EDNRA signaling drive RGC death must be elucidated, and the importance of these events in driving glaucomatous neurodegeneration upon chronic ocular hypertension merits future investigation.
Materials and methods
Mice
All mice used were 1.5–6 months of age. Mice were fed chow and water ad libitum and housed on a 12-hour light-to-dark cycle. All experiments were conducted in adherence to the Association for Research in Vision and Ophthalmology’s statement on the use of animals in ophthalmic and vision research and were approved by the University of Rochester’s University Committee on Animal Resources. C57BL/6N-Atm1BrdEdnratm1a(EUCOMM)Hmgu/JMmucd knockout-first mice with promoter-driven alleles were obtained through UC Davis KOMP Repository. These mice were crossed with flippase recombinase transgenic mice (Flptg, URMC genomics research core) to generate offspring with Ednrafl alleles. Ednrbtm1.1Nat/J alleles were obtained from the Jackson Laboratory (Ednrbfl; Stock #011080 [47]). Mice with Ednrafl and Ednrbfl alleles were bred to Tg(Six3-cre)69Frty/GcoJ transgenic mice (Six3-cre+; Jackson Laboratory, Stock# 019755) [48] to generate mice with a conditional deletion of Ednra and/or Ednrb from retinal neurons and macroglia. Mice with Ednrafl or Ednrbfl alleles were also bred to Tg(CAG-cre/Esr1*)5Amc/J transgenic mice (Cag-creERT2+; JAX Stock #004682) [78] to produce offspring with full-body deletions of Ednra or Ednrb upon tamoxifen treatment. Mice with Ednrafl alleles were bred to Tg(Myh11-cre/ERT2)1Soff/J transgenic mice (Myh11-creERT2+; Jackson Laboratory, Stock# 019079) [79] to generate mice with a conditional deletion of Ednra from vascular mural cells upon tamoxifen treatment. Mice transgenic for Cag-creERT2 or Myh11-creERT2 recombinase were bred to Gt(ROSA)26Sortm75.1(CAG-tdTomato*)Hze/J conditional reporter mice (TdTomato+; JAX stock 025106) to generate offspring as TdTomato reporters of cre expression.
Statistical analysis and experimental rigor
Power calculations were performed before experiments were conducted to determine the appropriate sample size. Data were analyzed using GraphPad Prism9 software. Data from experiments designed to test differences between two groups were subjected to an F test to compare variance and a Shapiro-Wilk test to test normality to ensure appropriate statistical tests were utilized. For non-normally distributed data designed to test differences between two groups, a Mann–Whitney test was utilized. Data from experiments designed to test differences among more than two groups across one condition were subjected to a Brown–Forsythe test to compare variance and a Shapiro–Wilk test to test normality to ensure an appropriate statistical test was utilized. Data from experiments designed to detect differences among multiple groups and across one condition were analyzed using a Kruskal–Wallis test with Dunn’s post hoc test. Data from experiments designed to detect differences among multiple groups and across two conditions were analyzed using a two-way analysis of variance followed by Holm–Sidak’s post hoc test. For these statistical tests, every possible comparison was made when relevant, and multiplicity adjusted P values are reported. In all cases, data met the assumptions of the statistical test used. P values < 0.05 were considered statistically significant. Throughout the manuscript, results are reported as mean ± standard error of the mean (SEM).
Roughly equal numbers of male and female mice were used for each experimental group, except for Myh11-creERT2Ednrafl mice (the Myh11-creERT2 transgene is inserted into the Y chromosome, thus, all animals used for this line of experiments were male). Phenotypically wild-type (WT) controls included tamoxifen treated and untreated cre+ and cre− animals. Littermate controls were used wherever possible. Animals were randomly assigned to experimental groups. Before experiments were performed, it was established that animals with pre-existing abnormal eye phenotypes (e.g., displaced pupil, cataracts) would be excluded from the study. All procedures were conducted by an observer masked to genotype and condition.
Tamoxifen treatment and animal procedures
At 6 weeks of age or older, animals were intraperitoneally injected with 125 mg/kg tamoxifen (Sigma, T5648) dissolved in corn oil at a concentration of 20 mg/mL once per day for five consecutive days. Experiments were conducted no earlier than 7 days after the last tamoxifen dose to allow for recombination of floxed alleles and degeneration of endogenous protein. Intravitreal injections and fluorescein angiography were performed as previously described [44]. EDN1 (Sigma, E7764) was dissolved in sterile PBS at a concentration of 500 μM. As previously performed, 2 μL of 500 μM EDN1 dissolved in sterile PBS was intravitreally injected into one eye. Sterile PBS was injected into the contralateral eye as a volume-matched vehicle control.
Tissue processing, immunofluorescence, and cell quantification
Tissue processing, immunostaining, and cell quantification were performed as previously described [44] using the following primary antibodies: rabbit anti-cCASP3 (R&D Systems, AF835, 1:1000), rabbit anti-RBPMS (GeneTex, GTX118619, 1:250), guinea pig anti-RBPMS (PhosphoSolutions, 1832-RBPMS, 1:250), goat anti-SOX2 (Santa Cruz, sc-17320, 1:200), goat anti-CD31 (R&D Systems, AF3628, 1:1000), and chicken anti-GFAP (Abcam, ab4674, 1:500).
Data availability
The datasets used in the current study are available from the corresponding author on reasonable request.
References
Blumberg D, Skaat A, Liebmann JM. Emerging risk factors for glaucoma onset and progression. Prog Brain Res. 2015;221:81–101.
Werner EB, Drance SM. Progression of glaucomatous field defects despite successful filtration. Can J Ophthalmol. 1977;12:275–80.
Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, et al. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Investig. 2012;122:1246–61.
Williams PA, Braine CE, Foxworth NE, Cochran KE, John SWM. GlyCAM1 negatively regulates monocyte entry into the optic nerve head and contributes to radiation-based protection in glaucoma. J Neuroinflammation. 2017;14:93.
Howell GR, Macalinao DG, Sousa GL, Walden M, Soto I, Kneeland SC, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Investig. 2011;121:1429–44.
Howell GR, MacNicoll KH, Braine CE, Soto I, Macalinao DG, Sousa GL, et al. Combinatorial targeting of early pathways profoundly inhibits neurodegeneration in a mouse model of glaucoma. Neurobiol Dis. 2014;71:44–52.
Howell GR, Walton DO, King BL, Libby RT, John SW. Datgan, a reusable software system for facile interrogation and visualization of complex transcription profiling data. BMC Genomics. 2011;12:429.
Aliancy J, Stamer WD, Wirostko B. A review of nitric oxide for the treatment of glaucomatous disease. Ophthalmol Ther. 2017;6:221–32.
Prasanna G, Krishnamoorthy R, Yorio T. Endothelin, astrocytes and glaucoma. Exp Eye Res. 2011;93:170–7.
Iglarz M, Silvestre JS, Duriez M, Henrion D, Levy BI. Chronic blockade of endothelin receptors improves ischemia-induced angiogenesis in rat hindlimbs through activation of vascular endothelial growth factor-no pathway. Arterioscler Thromb Vasc Biol. 2001;21:1598–603.
Sasaoka M, Taniguchi T, Shimazawa M, Ishida N, Shimazaki A, Hara H. Intravitreal injection of endothelin-1 caused optic nerve damage following to ocular hypoperfusion in rabbits. Exp Eye Res. 2006;83:629–37.
Krishnamoorthy RR, Rao VR, Dauphin R, Prasanna G, Johnson C, Yorio T. Role of the ETB receptor in retinal ganglion cell death in glaucoma. Can J Physiol Pharmacol. 2008;86:380–93.
Torbidoni V, Iribarne M, Suburo AM. Endothelin receptors: do they have a role in retinal degeneration? Adv Exp Med Biol. 2008;613:399–405.
MacCumber MW, D’Anna SA. Endothelin receptor-binding subtypes in the human retina and choroid. Arch Ophthalmol. 1994;112:1231–5.
Kallakuri S, Kreipke CW, Schafer PC, Schafer SM, Rafols JA. Brain cellular localization of endothelin receptors A and B in a rodent model of diffuse traumatic brain injury. Neuroscience. 2010;168:820–30.
Donato AJ, Lesniewski LA, Stuart D, Walker AE, Henson G, Sorensen L, et al. Smooth muscle specific disruption of the endothelin-A receptor in mice reduces arterial pressure, and vascular reactivity and affects vascular development. Life Sci. 2014;118:238–43.
Wirth A, Wang S, Takefuji M, Tang C, Althoff TF, Schweda F, et al. Age-dependent blood pressure elevation is due to increased vascular smooth muscle tone mediated by G-protein signalling. Cardiovas Res. 2016;109:131–40.
Chow LH, Subramanian S, Nuovo GJ, Miller F, Nord EP. Endothelin receptor mRNA expression in renal medulla identified by in situ RT-PCR. Am J Physiol. 1995;269(Pt 2):F449–57.
Kitazawa T, Sato T, Nishiyama K, Asai R, Arima Y, Uchijima Y, et al. Identification and developmental analysis of endothelin receptor type-A expressing cells in the mouse kidney. Gene Expr Patterns. 2011;11:371–7.
Bagnall AJ, Kelland NF, Gulliver-Sloan F, Davenport AP, Gray GA, Yanagisawa M, et al. Deletion of endothelial cell endothelin B receptors does not affect blood pressure or sensitivity to salt. Hypertension. 2006;48:286–93.
Ghoneim MA, Yamamoto T, Hirose S, Nagasawa T, Hagiwara H. Endothelium localization of ETB receptor revealed by immunohistochemistry. J Cardiovasc Pharmacol. 1993;22(Suppl 8):S111–2.
Eguchi S, Hirata Y, Marumo F. Endothelin subtype B receptors are coupled to adenylate cyclase via inhibitory G protein in cultured bovine endothelial cells. J Cardiovasc Pharmacol. 1993;22(Suppl 8):S161–3.
MacCumber MW, Ross CA, Snyder SH. Endothelin in brain: receptors, mitogenesis, and biosynthesis in glial cells. Proc Natl Acad Sci USA. 1990;87:2359–63.
Maguire JJ, Davenport AP. Endothelin@25 - new agonists, antagonists, inhibitors and emerging research frontiers: IUPHAR review 12. Br J Pharmacol. 2014;171:5555–72.
Minton AZ, Phatak NR, Stankowska DL, He S, Ma HY, Mueller BH, et al. Endothelin B receptors contribute to retinal ganglion cell loss in a rat model of glaucoma. PLoS ONE. 2012;7:e43199.
Rogers SD, Demaster E, Catton M, Ghilardi JR, Levin LA, Maggio JE, et al. Expression of endothelin-B receptors by glia in vivo is increased after CNS injury in rats, rabbits, and humans. Exp Neurol. 1997;145:180–95.
Patel C, Narayanan SP, Zhang W, Xu Z, Sukumari-Ramesh S, Dhandapani KM, et al. Activation of the endothelin system mediates pathological angiogenesis during ischemic retinopathy. Am J Pathol. 2014;184:3040–51.
McGrady NR, Minton AZ, Stankowska DL, He S, Jefferies HB, Krishnamoorthy RR. Upregulation of the endothelin A (ET(A)) receptor and its association with neurodegeneration in a rodent model of glaucoma. BMC Neurosci. 2017;18:27.
Williams PA, Harder JM, Foxworth NE, Cochran KE, Philip VM, Porciatti V, et al. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 2017;355:756–60.
Rattner A, Nathans J. The genomic response to retinal disease and injury: evidence for endothelin signaling from photoreceptors to glia. J Neurosci. 2005;25:4540–9.
Hammond TR, McEllin B, Morton PD, Raymond M, Dupree J, Gallo V. Endothelin-B receptor activation in astrocytes regulates the rate of oligodendrocyte regeneration during remyelination. Cell Rep. 2015;13:2090–7.
Hasselblatt M, Kamrowski-Kruck H, Jensen N, Schilling L, Kratzin H, Sirén AL, et al. ETA and ETB receptor antagonists synergistically increase extracellular endothelin-1 levels in primary rat astrocyte cultures. Brain Res. 1998;785:253–61.
Ehrenreich H, Costa T, Clouse KA, Pluta RM, Ogino Y, Coligan JE, et al. Thrombin is a regulator of astrocytic endothelin-1. Brain Res. 1993;600:201–7.
Tezel G, Kass MA, Kolker AE, Becker B, Wax MB. Plasma and aqueous humor endothelin levels in primary open-angle glaucoma. J Glaucoma. 1997;6:83–9.
Koukoula SC, Katsanos A, Tentes IK, Labiris G, Kozobolis VP. Retrobulbar hemodynamics and aqueous humor levels of endothelin-1 in exfoliation syndrome and exfoliation glaucoma. Clin Ophthalmol. 2018;12:1199–204.
Wang S, Xu L, Wang Y, Wang Y, Jonas JB. Retinal vessel diameter in normal and glaucomatous eyes: the Beijing eye study. Clin Exp Ophthalmol. 2007;35:800–7.
Jonas JB, Naumann GO. Parapapillary retinal vessel diameter in normal and glaucoma eyes. II. Correlations. Investig Ophthalmol Vis Sci. 1989;30:1604–11.
Mitchell P, Leung H, Wang JJ, Rochtchina E, Lee AJ, Wong TY, et al. Retinal vessel diameter and open-angle glaucoma: the Blue Mountains Eye Study. Ophthalmology. 2005;112:245–50.
Ramm L, Jentsch S, Peters S, Sauer L, Augsten R, Hammer M. Dependence of diameters and oxygen saturation of retinal vessels on visual field damage and age in primary open-angle glaucoma. Acta Ophthalmol. 2015;94:276–81.
Tonari M, Kurimoto T, Horie T, Sugiyama T, Ikeda T, Oku H. Blocking endothelin-B receptors rescues retinal ganglion cells from optic nerve injury through suppression of neuroinflammation. Investig Ophthalmol Vis Sci. 2012;53:3490–500.
Blanco R, Martínez-Navarrete G, Valiente-Soriano FJ, Avilés-Trigueros M, Pérez-Rico C, Serrano-Puebla A, et al. The S1P1 receptor-selective agonist CYM-5442 protects retinal ganglion cells in endothelin-1 induced retinal ganglion cell loss. Exp Eye Res. 2017;164:37–45.
Lau J, Dang M, Hockmann K, Ball AK. Effects of acute delivery of endothelin-1 on retinal ganglion cell loss in the rat. Exp Eye Res. 2006;82:132–45.
Mi X-S, Zhang X, Feng Q, Lo ACY, Chung SK, So K-F. Progressive retinal degeneration in transgenic mice with overexpression of endothelin-1 in vascular endothelial cells. Investig Ophthalmol Vis Sci. 2012;53:4842–51.
Marola OJ, Syc-Mazurek SB, Howell GR, Libby RT. Endothelin 1-induced retinal ganglion cell death is largely mediated by JUN activation. Cell Death Dis. 2020;11:811.
Syc-Mazurek SB, Fernandes KA, Wilson MP, Shrager P, Libby RT. Together JUN and DDIT3 (CHOP) control retinal ganglion cell death after axonal injury. Mol Neurodegener. 2017;12:71.
Syc-Mazurek SB, Fernandes KA, Libby RT. JUN is important for ocular hypertension-induced retinal ganglion cell degeneration. Cell Death Dis. 2017;8:e2945.
Rattner A, Yu H, Williams J, Smallwood PM, Nathans J. Endothelin-2 signaling in the neural retina promotes the endothelial tip cell state and inhibits angiogenesis. Proc Natl Acad Sci USA. 2013;110:E3830–9.
Furuta Y, Lagutin O, Hogan BL, Oliver GC. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis. 2000;26:130–2.
Rattner A, Williams J, Nathans J. Roles of HIFs and VEGF in angiogenesis in the retina and brain. J Clin Investig. 2019;129:3807–20.
Fernandes KA, Harder JM, John SW, Shrager P, Libby RT. DLK-dependent signaling is important for somal but not axonal degeneration of retinal ganglion cells following axonal injury. Neurobiol Dis. 2014;69:108–16.
Murgai M, Ju W, Eason M, Kline J, Beury DW, Kaczanowska S, et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat Med. 2017;23:1176–90.
Valdez CN, Arboleda-Velasquez JF, Amarnani DS, Kim LA, D’Amore PA. Retinal microangiopathy in a mouse model of inducible mural cell loss. Am J Pathol. 2014;184:2618–26.
Crist AM, Young C, Meadows SM. Characterization of arteriovenous identity in the developing neonate mouse retina. Gene Expr Patterns. 2017;23-24:22–31.
Shoshani YZ, Harris A, Shoja MM, Rusia D, Siesky B, Arieli Y, et al. Endothelin and its suspected role in the pathogenesis and possible treatment of glaucoma. Curr Eye Res. 2012;37:1–11.
Jassim AH, Inman DM. Evidence of hypoxic glial cells in a model of ocular hypertension. Investig Ophthalmol Vis Sci. 2019;60:1–15.
Chidlow G, Wood JPM, Casson RJ. Investigations into hypoxia and oxidative stress at the optic nerve head in a rat model of glaucoma. Front Neurosci. 2017;11:478.
Jassim AH, Fan Y, Pappenhagen N, Nsiah NY, Inman DM. Oxidative stress and hypoxia modify mitochondrial homeostasis during glaucoma. Antioxid Redox Signal. 2021;35:1341–57.
Spix NJ, Liu L-L, Zhang Z, Hohlbein JP, Prigge CL, Chintala S, et al. Vulnerability of dopaminergic amacrine cells to chronic ischemia in a mouse model of oxygen-induced retinopathy. Investig Ophthalmol Vis Sci. 2016;57:3047–57.
Rojo Arias JE, Economopoulou M, Juárez López DA, Kurzbach A, Au Yeung KH, Englmaier V, et al. VEGF-Trap is a potent modulator of vasoregenerative responses and protects dopaminergic amacrine network integrity in degenerative ischemic neovascular retinopathy. J Neurochem. 2019;153:390–412.
Joachim SC, Renner M, Reinhard J, Theiss C, May C, Lohmann S, et al. Protective effects on the retina after ranibizumab treatment in an ischemia model. PLoS ONE. 2017;12:e0182407.
Palmhof M, Frank V, Rappard P, Kortenhorn E, Demuth J, Biert N, et al. From ganglion cell to photoreceptor layer: timeline of deterioration in a rat ischemia/reperfusion model. Front Cell Neurosci. 2019;13:174.
He M, Pan H, Chang RC, So KF, Brecha NC, Pu M. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS ONE. 2014;9:e84800.
Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171:313–25.
Baumann J, Tsao C-C, Huang S-F, Gassmann M, Ogunshola OO. Astrocyte-specific hypoxia-inducible factor 1 (HIF-1) does not disrupt the endothelial barrier during hypoxia in vitro. Fluids Barriers CNS. 2021;18:13.
Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J Cereb Blood Flow Metab. 2011;31:693–705.
Al Ahmad A, Gassmann M, Ogunshola OO. Maintaining blood-brain barrier integrity: pericytes perform better than astrocytes during prolonged oxygen deprivation. J Cell Physiol. 2009;218:612–22.
Halder SK, Milner R. A critical role for microglia in maintaining vascular integrity in the hypoxic spinal cord. Proc Natl Acad Sci USA. 2019;116:26029–37.
Narushima I, Kita T, Kubo K, Yonetani Y, Momochi C, Yoshikawa I, et al. Contribution of endothelin-1 to disruption of blood-brain barrier permeability in dogs. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:639–45.
Alrashdi SF, Deliyanti D, Talia DM, Wilkinson-Berka JL. Endothelin-2 injures the blood-retinal barrier and macroglial muller cells: interactions with angiotensin II, aldosterone, and NADPH oxidase. Am J Pathol. 2018;188:805–17.
Williams PA, Braine CE, Kizhatil K, Foxworth NE, Tolman NG, Harder JM, et al. Inhibition of monocyte-like cell extravasation protects from neurodegeneration in DBA/2J glaucoma. Mol Neurodegener. 2019;14:6.
Fang D, Li Z, Zhong-ming Q, Mei WX, Ho YW, Yuan XW, et al. Expression of bystin in reactive astrocytes induced by ischemia/reperfusion and chemical hypoxia in vitro. Biochim. Biophys Acta Mol Basis Dis. 2008;1782:658–63.
Badawi Y, Ramamoorthy P, Shi H. Hypoxia-inducible factor 1 protects hypoxic astrocytes against glutamate toxicity. ASN Neuro. 2012;4:231–41.
He M, Shi X, Yang M, Yang T, Li T, Chen J. Mesenchymal stem cells-derived IL-6 activates AMPK/mTOR signaling to inhibit the proliferation of reactive astrocytes induced by hypoxic-ischemic brain damage. Exp Neurol. 2019;311:15–32.
Angelova PR, Kasymov V, Christie I, Sheikhbahaei S, Turovsky E, Marina N, et al. Functional oxygen sensitivity of astrocytes. J Neurosci. 2015;35:10460–73.
Schmid-Brunclik N, Bürgi-Taboada C, Antoniou X, Gassmann M, Ogunshola OO. Astrocyte responses to injury: VEGF simultaneously modulates cell death and proliferation. Am J Physiol Regul Integr Comp Physiol. 2008;295:R864–73.
Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, Dorrell MI, et al. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58:1177–85.
Li Y-N, Pan R, Qin X-J, Yang W-L, Qi Z, Liu W, et al. Ischemic neurons activate astrocytes to disrupt endothelial barrier via increasing VEGF expression. J Neurochem. 2014;129:120–9.
Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–18.
Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–8.
Acknowledgements
The authors would like to acknowledge Alyssa Parker for her excellent technical support. This work was supported by EY027701 (RTL, GRH), EY030739 (OJM), and Research to Prevent Blindness, an unrestricted grant to the Department of Ophthalmology at the University of Rochester Medical Center. GRH is the Diana Davis Spencer Foundation Chair for Glaucoma Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding
This work was supported by EY027701 (RTL, GRH), EY030739 (OJM), and Research to Prevent Blindness, an unrestricted grant to the Department of Ophthalmology at the University of Rochester Medical Center. GRH is the Diana Davis Spencer Foundation Chair for Glaucoma Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
OJM designed and conducted experiments, analyzed and interpreted data, and drafted the manuscript. GRH and RTL conceived of and designed experiments and aided in drafting and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marola, O.J., Howell, G.R. & Libby, R.T. Vascular derived endothelin receptor A controls endothelin-induced retinal ganglion cell death. Cell Death Discov. 8, 207 (2022). https://doi.org/10.1038/s41420-022-00985-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-022-00985-8
This article is cited by
-
A ginger extract improves ocular blood flow in rats with endothelin-induced retinal blood flow dysfunction
Scientific Reports (2023)
-
Functions of retinal astrocytes and Müller cells in mammalian myopia
BMC Ophthalmology (2022)