Abstract
The etiopathology of Parkinson’s disease has been associated with mitochondrial defects at genetic, laboratory, epidemiological, and clinical levels. These converging lines of evidence suggest that mitochondrial defects are systemic and causative factors in the pathophysiology of PD, rather than being mere correlates. Understanding mitochondrial biology in PD at a granular level is therefore crucial from both basic science and translational perspectives. In a recent study, we investigated mitochondrial alterations in fibroblasts obtained from PD patients assessing mitochondrial function in relation to clinical measures. Our findings demonstrated that the magnitude of mitochondrial alterations parallels disease severity. In this study, we extend these investigations to blood cells and dopamine neurons derived from induced pluripotent stem cells reprogrammed from PD patients. To overcome the inherent metabolic heterogeneity of blood cells, we focused our analyses on metabolically homogeneous, accessible, and expandable erythroblasts. Our results confirm the presence of mitochondrial anomalies in erythroblasts and induced dopamine neurons. Consistent with our previous findings in fibroblasts, we observed that mitochondrial alterations are reversible, as evidenced by enhanced mitochondrial respiration when PD erythroblasts were cultured in a galactose medium that restricts glycolysis. This observation indicates that suppression of mitochondrial respiration may constitute a protective, adaptive response in PD pathogenesis. Notably, this effect was not observed in induced dopamine neurons, suggesting their distinct bioenergetic behavior. In summary, we provide additional evidence for the involvement of mitochondria in the disease process by demonstrating mitochondrial abnormalities in additional cell types relevant to PD. These findings contribute to our understanding of PD pathophysiology and may have implications for the development of novel biomarkers and therapeutic strategies.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a high-prevalence chronic neurodegenerative disorder. The disease is mostly idiopathic and its complex etiopathogenesis progresses along multiple, different, and typically converging biological pathways that include protein quality control, oxido-reductive homestasis, and intracellular trafficking [1, 2]. Within the intricate pathobiology of PD, anomalies in mitochondrial function are central and have been reported in both genetic and idiopathic PD cases, and there is consequently solid and general consensus on the prominent role of mitochondrial dysfunction in PD etiopathogenesis (see e.g. [3,4,5,6]). Reduced activity of respiratory complex I have been implicated in PD by evidence gathered at the epidemiological level and in laboratory studies, as well as by clinical investigations that identified reduced peripheral complex I activity in patients’ platelets [7]. More specifically, PD has been associated with exposure to the complex I inhibitor rotenone in population studies [8], administration of this toxin in laboratory animals elicits a phenotype closely recapitulating that of the human disease [9], and genetic deletion of a complex I essential subunit in mice causes a phenotype that closely recapitulates that of human PD [10]. Moreover, several genes associated with familial forms of PD such as PARK2, PARK6, and PARK7 are involved in mitochondrial quality control [11]. These convergent data, obtained at both central and peripheral levels, suggest that mitochondrial defects are systemic and causative of PD pathophysiology, rather than being a simple correlate. Such a causative relationship constitutes the fundamental requirement for a valid surrogate endpoints [12] and therefore indicates that mitochondrial biology holds potential both in terms of biomarker development and as targets for disease-modifying therapies [3, 13]. Understanding mitochondrial biology in PD at a granular level is therefore of primary relevance from both the basic science and translational standpoints.
To gather better insights into the relationship between mitochondrial function and PD, we recently analyzed mitochondrial function in fibroblasts from skin biopsies from a relatively large and well-characterized cohort of idiopathic PD (iPD) patients (n = 47) and correlated these biochemical parameters with both dopaminergic and non-dopaminergic clinical measures [14]. To increase sensitivity toward mitochondrial defects, we used cell culturing conditions forcing energetic metabolism through oxidative phosphorylation (OXPHOS), which is not permissive for cells with severe mitochondrial defects and has been used as a diagnostic tool for mitochondrial diseases [15]. Using this simple, yet powerful stratagem we were able to show that alterations in mitochondrial function under these experimental setting correlate with clinical measures, and that the magnitude of mitochondrial alterations correlates with disease severity [14].
A few urgent standing questions, however, remain open. Evidence of peripheral mitochondrial dysfunction in PD, for instance, is discrepant and some studies failed to detect respiratory alterations in peripheral blood mononuclear cells (e.g. [16]). It is unclear whether this inconsistency is due to a selective characteristic that limits mitochondrial defects to certain subsets/subtypes of PD, and/or if it rather depends upon the specific cell type that has been analyzed because of intrinsic cell-dependent differences in bioenergetics. Correlation between mitochondrial respiration parameters and clinical measures is of potential translational relevance and should be validated on a larger scale; it is however unknown whether correlation is detectable also in other peripheral cells than fibroblasts. Reconfirming the findings in cells different than fibroblasts would clarify these important aspects. Addressing these issues in blood cells would be highly desirable given they are easily accessible with non-invasive methodologies and would therefore greatly enhance the value of mitochondria in biomarker research. Bioenergetic studies in blood cells, however, pose critical issues because of the sharp differences in the metabolic layout of different cell types and even subtypes [17]. While purification via cell sorting methodologies may potentially circumvent this problem, it also comes with limitations regarding the amount of biological material available for the assays [16]. A further fundamental question, which is essential to define in greater detail the role of mitochondrial dysfunction in PD, is whether peripheral mitochondrial anomalies parallel comparable alterations in neurons.
This study describes the bioenergetic characterization of immature erythroid cells. i.e. erythroblasts and iPSCs-derived dopaminergic neurons (iDAN) obtained from the very same and well-characterized cohort analyzed in our previous study, and from the same patient. Moreover, it explores the correlation between clinical severity and mitochondrial alterations at the transcription level in the large and well-characterized PPMI cohort. The results reveal mitochondrial anomalies in erythroblasts and in iDAN. In peripheral cells, the observed reduction in mitochondrial respiration in PD peripheral cells is reversible because it can be potentiated in galactose culturing conditions. This effect, however, is not observed in iDAN, which therefore displays a distinctive bioenergetic behavior. In summary, our study substantiates the relevance of mitochondrial biology in PD.
Methods
Human subjects
PD patients were recruited from the outpatient clinic for Movement Disorders of the Department of Neurology of the Leiden University Medical Center (Leiden, the Netherlands) and nearby university and regional hospitals. All participants fulfilled the U.K. Parkinson’s Disease Society Brain Bank criteria for idiopathic PD. The study was approved by the medical ethics committee of the Leiden University Medical Center (P12.194/NV/ib), and written informed consent was obtained from all PD patients.
Fibroblasts were isolated at Leiden University Medical Center from skin biopsies derived from the ventral side of the upper leg and cultured under highly standardized conditions as previously described in [14]. Peripheral whole blood was collected from PD patients at Leiden University Medical Center and PBMCs were isolated at the Department of Molecular Genetics at the Erasmus Medical Center in Rotterdam. Control iPSC were obtained from the Eramsus MC iPS Core facility.
Peripheral whole blood from 24 age-matched healthy controls (age >55 years) was obtained from Sanquin Rotterdam (NVT0585.00 Mantel, NVT0585.01 Annex).
Bioinformatic analysis was performed using the Parkinson’s Progression Markers Initiative (PPMI) database.
Cell culture
To generate erythroblasts, PD patients’ peripheral blood mononuclear cells (PBMC) were extracted from 10 ml of freshly extracted blood with the use of Lympholyte-H (Cedarline) and Leucosep polypropylene tubes (227290, Greiner) according to manufacturer’s indications. Briefly, blood was diluted in PBS at a 1:2 ratio and loaded on a 15 mL Lympholyte – Leucosep tube. Blood was centrifuged at 800 g for 25 min with no brakes at 4 C. Upon removal of the plasma, the PBMC enriched cell fraction was collected, washed several times with sterile PBS and upon PBMCs were cultured in StemSpan SFEM medium (Stemcell Technologies) containing 2 mM Ultraglutamine (Lonza), 1% Nonessential aminoacids (NEAA), 1% penicillin/streptomycin, 50 ng/ml Stem Cell Factor, 2 U/ml Erythropoietin, 1 uM Dexamethasone (Sigma), 10 ng/ml Interleukin-3 (R&D Systems), 10 ng/ml Interleukin-6 (R&D Systems), 40 ng/ml IGF-1 (R&D Systems) and 50 ug/ml Ascorbic Acid (Merck) for 6–9 days refreshing half of the medium every other day starting from day 2. Erythroblasts were isolated when reaching 60–70% of the total cell population by gradient centrifugation at 1000 × g for 20 minutes at room temperature over Percoll (GE Healthcare). Isolated erythroblasts were frozen in FBS containing 10% DMSO at −80 °C. Metabolic analysis was performed within 2 days after thawing.
Generation of iPSCs
PD patients’ fibroblasts used in this study were prepared and isolated at Leiden University Medical Center from skin biopsies derived from the ventral side of the upper leg and cultured under highly standardized conditions as previously described in [14]. The study was approved by the medical ethics committee of the Leiden University Medical Center, and written informed consent was obtained from all PD patients.
Fibroblasts were reprogrammed to pluripotent stem cells using the CytoTune-iPS 2.0 Sendai Reprogramming Kit (A16517, Thermo Fisher) according to the manufacturer’s protocol.
Generation of small molecule neural precursor cells (smNPCs)
Human iPSC lines were generated as previously described [18]. Briefly, to generate embryoid bodies with neuroepithelial outgrowths (EBs), iPSC colonies were dissociated with 2 mg/mL collagenase IV and transferred to non-adherent plates in hESC medium.
(Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Thermo Fisher Scientific), 20% knockout serum (Thermo Fisher Scientific), 1% minimum essential medium/non-essential amino acid (NEAA, Sigma-Aldrich, St Louis, MO, USA), 7 nl ml−1 β-mercaptoethanol (Sigma-Aldrich), 1% L-glutamine (Thermo Fisher Scientific) and 1% penicillin/streptomycin (P/S, Thermo Fisher Scientific) supplemented with 10 µM SB-431542 (Ascent Scientific), 1 µM dorsomorphin (Tocris), 3 µM CHIR 99021 (Axon Medchem) and 0.5 µM Purmorphamine (Alexis) on a shaker in an incubator at 37 °C/5% CO2. On the second day, medium was replaced with N2B27 medium [DMEM-F12/neurobasal 50:50 (Thermo Fisher Scientific), 1% P/S, 1:100 B27 supplement lacking vitamin A (Thermo Fisher Scientific) and 1:200 N2 supplement (Thermo Fisher Scientific)] containing 10 µM SB-431542, 1 µM dorsomorphin, 3 µM CHIR99021 and 0,5 µM Purmorphamine. On day 4, N2B27 medium was replaced and supplemented with 3 µM CHIR99021, 0.5 µM Purmorphamine, and 150 µM Ascorbic Acid (Sigma).
On day 6, EBs were slightly triturated and plated on Matrigel-coated (Matrigel - 354277, Corning) plates at a density 10–15 EB per well containing smNPC expansion medium (N2B27 medium containing 3 µM CHIR 99021, 200 µM Ascorbic Acid, 0.5 µM Purmorphamine) and expanded for 5 passages before final differentiation. The medium was refreshed every other day.
Differentiation of smNPC into midbrain dopaminergic neurons (iDANs)
smNPC were dissociated with Accutase at RT, diluted, and seeded on Poly-D-lysine – Matrigel-coated cover glasses in a 12-well plate at the concentration of 5 × 104 cells per well in the Patterning medium [N2B27 medium containing 1 ng/mL GDNF (Peprotech), 2 ng/ml BDNF (Pepotech), 200 µM Ascorbic Acid and 0.5 µM Smoothened Agonist (SAG Pepotech)]. The medium was refreshed every 2 days.
At day 8, the medium wash switched to the Maturation medium containing N2B27 medium, 2 ng/ml GDNF, 2 ng/ml BDNF, 1 ng/mL TGF-b3 (Peprotech), 200 µM ascorbic Acid and 5 ng/ml of ActivinA for the first feeding and 2 ng/ml ActivinA for the following feedings. Medium change occurred every third day.
Flow cytometry
Erythroblasts and iPSCs were washed and resuspended in FC buffer (HBSS w/o calcium and magnesium + 0.5% BSA) and incubated with PE Mouse Anti-Human CD44 antibody (1:25, BD, 550989), FITC Mouse Anti-Human CD71 antibody (1:50, BD, 555536), or 7-AAD (Thermo Fisher, A1310) for 30 min at 4 °C. Mitochondria were stained with Mitotracker Green FM (100 nm, Cell Signaling, 9074) and active mitochondria with TMRM (100 nm, Thermo Fisher, T668) for 30 min at 37 °C. Cells were detected by flow cytometry using a LSRFortessa Cell Analyzer (BD, USA). Flowjo software (BD, USA) was used for data analysis.
Bioenergetics assays
Oxygen consumption rates (OCR) and extracellular acidification rate (ECAR) were measured using a XF-24 Extracellular Flux Analyzer (Agilent Technologies), as previously described [14]. Erythroblasts were seeded at a density of 2 × 105 cells/well on Cell-Tak (Corning, 354240) coated Seahorse plates in unbuffered XF DMEM medium (Agilent Technologies) supplemented with 1 mM sodium pyruvate, 2 mM glutamine and 10 mM glucose or galactose. Immediately after seeding, cells were centrifuged at 200 g for 1 minute to attach evenly to the bottom of the well and the plate was equilibrated for 30 minutes at 37 °C in the absence of CO2. iPSCs derived from fibroblasts of PD patients and healthy controls were seeded at a density of 8 × 103 cells/well on Seahorse plates and differentiated to dopaminergic neurons over a period of 3 weeks according to the described methodology. On an experimental day, the medium was changed to an unbuffered XF DMEM medium supplemented with 1 mM sodium pyruvate, 2 mM glutamine and 10 mM glucose or galactose. Cells were incubated for 1 h at 37 °C in the absence of CO2, before the Seahorse assay. For each assay, medium and reagent acidity were adjusted to pH 7.4 on the day of the assay, according to the manufacturer’s procedure. Optimal cell densities were determined experimentally to ensure a proportional response to FCCP (oxidative phosphorylation uncoupler).
After 3 measurements to detect the oxygen consumption ratio baseline, cells were then challenged with sequential injections of mitochondrial toxins: 1 μM oligomycin (Adenosine triphosphate – ATP - synthase inhibitor), 1 μM FCCP, and 1 μM antimycin (complex III inhibitor). A minimum number of 5 replicates were performed for each cell line; data represent the mean of the different replicates. Basal respiration (measured as the average OCR rates at the baseline), maximal mitochondrial respiration (maximal respiration), reserve capacity (difference between maximal respiration and basal respiration), and respiration dedicated to ATP production (difference between basal respiration and oligomycin-dependent respiration) were used to investigate mitochondrial bioenergetics. Basal glycolysis, measured as extracellular acidification rate (ECAR) maximal glycolysis and reserve glycolytic capacity (difference between maximal glycolysis and basal glycolysis) were taken into account to investigate glycolytic properties.
Immunofluorescence
Reprogrammed iPSCs cells cultured in an 8-chamber slide were fixed with 4% PFA for 15 min at room temperature. After incubation in ice-cold methanol for 10 min cells were permeabilized in 0.1% Triton in PBS for 10 min and blocked using 1% BSA in PBS/0.05% Tween-20 for 30 min. Next, cells were incubated with primary antibodies diluted in blocking buffer overnight at 4 °C - Mouse Anti-Human TRA1-81 (1:75, Abcam, AB16289#20), Rabbit Anti-Human OCT4 (1:250, Abcam, AB19857#8), Goat Anti-Human NCAM (1:100, R&D, AF2408), Goat-Anti Human SOX17 (1:100, R&D, AF1924) or Mouse Anti-Human Beta-Tubulin (1:1000, Merck, T8660) primary Chicken Anti-Human MAP2 (1:2000, Abcam, AB5392), Mouse Anti-Human TH (1:200, Millipore, MAB318). After washing with PBS cells were incubated with respective secondary Goat Anti-Mouse Alexa Fluor 546 (1:500, Invitrogen, A21045), Goat Anti-Rabbit Alexa Fluor 488 (1:500, Invitrogen, A11008#8a), Donkey Anti-Goat Alexa Fluor 488 (1:500, Invitrogen, A11055) or Goat Anti-Mouse Dylight 594 (1:500, Jackson, 115-515-166#7) antibodies diluted in blocking buffer for 1 h at room temperature. Nuclei were stained with Hoechst 33342 (1:1000 in PBS, Thermo Fisher) for 10 min. Cells were next washed with PBS, mounted with ProLong Diamond Antifade Mountant (P36965, Thermo Fisher), and imaged using a Leica Stellaris5 confocal microscope.
Bioinformatic analysis of the PPMI dataset
Blood transcriptome data from the Parkinson Progressive Markers Initiative (PPMI) cohort (PPMI website: https://ida.loni.usc.edu/pages/access/geneticData.jsp#441) were obtained. The libraries were prepared using the NEB/Kapa (NEBKAP) based library prep, with second-strand synthesis. RNA sequencing was performed at Hudson Alpha’s Genomic Services Lab on an Illumina NovaSeq6000, generating 100 million 125 bp paired reads per sample. The Salmon files were imported into R using Tximport. To identify differentially expressed genes between PD groups and controls, the DESeq2 package was used. Normalized counts were subjected to Rlog transformation to improve distances/clustering for the principal component analysis (PCA). The cohort of subjects was divided into subgroups based on the delta-UPDRS-III (MDS-Unified Parkinson’s Disease Rating Scale, UPDRS-III at last visit - UPDRS-III at first visit) of PD subjects: those with a delta-UPDRS-III less than 0 (defined as mild) and those with a delta-UPDRS-III greater than 0 (defined as severe), as well as controls (CTRL). A threshold of significance at FDR < 0.05 was applied.
Gene Set Enrichment Analysis (GSEA) was conducted on an unfiltered, ranked list of genes. The analysis involved various terms from the Kyoto Encyclopedia of Genes and Genomes (KEGG), Reactome Pathway Databases, Hallmark Gene Set Collection, and WikiPathways (GSEA website: http://www.gsea-msigdb.org/gsea/msigdb/collections.jsp). Pathway information was obtained from the Kyoto Encyclopedia of Genes and Genomes (KEGG) available at the Molecular Signatures Database (http://www.broadinstitute.org/gsea/msigdb/index.jsp) or from the Hallmark Gene Set Collection (http://www.gsea-msigdb.org/gsea/msigdb/collections.jsp). Gene set enrichment with FDR < 0.1 was considered significant. Genes in each PD group were ranked based on the level of differential expression using a signal-to-noise metric and a weighted enrichment statistic.
Transcriptomic analysis was performed using R Studio version 4.2.3. The experiments were conducted with a minimum of three independent biological replicates. GraphPad Prism version 9 (GraphPad Software, La Jolla California USA) was used for all statistical analyses and graphical representations. P values were denoted as *P < 0.05, **P < 0.01, ***P < 0.001, and were considered significant. In the absence of indications, comparisons should be considered non-significant. Comparisons between two groups were analyzed using unpaired two-tailed Student’s t-tests, and comparisons between more than two groups were analyzed using either one-way ANOVA followed by Dunnett’s (comparison of PD means vs. healthy subjects) or Tukey’s (comparison of all the means) posthoc test for multiple comparisons.
Results
Mitochondiral bioinformatic analysis
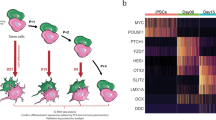
We took advantage of the PPMI cohort to study the expression of mitochondrial genes in peripheral mononuclear blood cells (PBMCs) from 393 iPD patients and healthy controls examined at two different time points, i.e. at the intake visit (i.e. visit 1), shortly after diagnosis, and in a follow-up visit, after 36 months (i.e. visit 8). Of the 340 of the 393 iPD patients and 159 of the 189 healthy controls that were part of visit 1 were also examined at visit 8. Given the intrinsic variability in PD progression, we divided the 340 patients examined at both visit 1 and 8 in two groups based on motor symptoms progression (i.e. UPDRS part III): mild cases included patients who did not show significant deterioration of motor symptoms (i.e. deltaUPDRS-III = UPDRS-IIIvisit8- UPDRS-IIIvisit1 ≤ 0), severe cases, encompassed patients with aggravation of motor symptoms who (i.e. deltaUPDRS-III = UPDRS-IIIvisit8- UPDRS-IIIvisit1 > 0) (Fig. 1A).
A iPD in the PPMI cohort was stratified based on the evolution of the UPDRS-III score between visit 1 and visit 8. B GSEA indicates that multiple mitochondrial pathways are downregulated in iPD, at visit 1. Deregulation of mitochondrial pathways was not detected at visit 8 when pathways related to reactive oxygen species were instead upregulated. C At visit 1, patients who will develop more severe symptoms display deregulation in a higher number of mitochondrial pathways.
Over-representation analysis (ORA) based on significantly differentially expressed genes (DEG) [19] did not highlight any differences between iPD and control, indicating that the disease is not associated with a pronounced mitochondrial defect in blood cells. To unravel more subtle differences, we took advantage of gene set enrichment analysis (GSEA) [20], which rather than focusing on a single significant DEG calculates aggregated statistical significance in a set of related genes with common biological function, i.e. a pathway.
We first analyzed PD patients as a single group, i.e. without stratifying for disease progression rate. Here, GSEA detected the downregulation of mitochondrial pathways involved in bioenergetic processes and mitochondrial-related macromolecular synthesis (Fig. 1B).
We next stratified patients based on the rate of disease progression. At visit 1, patients who subsequently developed more severe motor signs (i.e. severe cases) displayed downregulation in more mitochondrial pathways than patients with more benign disease progression (i.e. mild cases) (Fig. 1B, C). Deregulation pathways included bioenergetic processes, mitophagy, mitochondrial biogenesis, and mitochondrial-related macromolecular synthesis. Interestingly, at visit 8 no deregulation of mitochondrial pathways was detected and processes related to reactive oxygen species were instead upregulated (Fig. 1B). Collectively, these elements obtained in a large longitudinal PD dataset indicate that mitochondrial alterations are detectable at earlier rather than later stages of the PD symptomatic phase, and also inform on disease progression.
Blood cells
Encouraged by the supportive bioinformatic evidence obtained in the PPMI cohort, we next sought to determine alterations in mitochondrial function in blood cells. Because of the different metabolic layout of blood cell types [17], bioenergetic analyses in this tissue should be performed in homogeneous and characterized populations. We followed a novel strategy and investigated mitochondrial function in immature erythroid progenitors (erythroblasts) obtained from peripheral blood mononuclear cells [21]. This approach does not require preliminary antibody-based cell sorting and allows easy expansion of erythroblasts, with consequent generation of a large and very homogeneous population from a limited amount of starting material [21, 22]. Erythroblasts retain functional mitochondria, which will be lost during terminal differentiation [23].
We successfully isolated and expanded erythroblasts from blood human erythroid progenitors from PD patients and age-matched donors (Fig. 2). The erythroblast phenotype was confirmed by high-level expression of the specific markers CD44 and CD71, which, as expected, displayed a significantly lower expression level in iPSc. MitoTracker and TMRM staining confirm that erythroblasts have functional, polarized mitochondria (Fig. 2B).
A As expected, isolated cells express the specific markers CD44 and CD71.No mortality was observed among cell populations. Negative control experiments in iPSCs fail to detect the expression of the specific markers as expected. B In erythroblasts, mitochondria are polarized, as indicated by the TMRM signal.
We performed bioenergetic characterization of 35 erythroblasts lines of from PD patients with different disease severity [14] and from 18 lines from age and sex matched controls (Supplementary Table 1).
As in our previous work, bioenergetic analyses were performed in a standard glucose-containing medium, i.e. in conditions allowing both glycolysis and oxidative phosphorylation, and in non-permissive conditions, where glucose was replaced by galactose. Because the use of galactose as a substrate in the glycolytic pathways is highly unfavored from the kinetic standpoint, the substrate cannot be metabolized through this pathway. Replacing glucose with galactose therefore forces bioenergetics through oxidative phosphorylation allowing detection of even subtle defects in mitochondrial function.
When we investigated bioenergetics in a glucose-containing medium, we detected a significant reduction in both basal and maximal oxygen consumption (OCR) rates in PD erythroblasts (compare dark and light blue boxes in Fig. 3). PD erythroblasts, however, can increase mitochondrial respiration if oxidative phosphorylation is the only available bioenergetic pathway, as it happens in galactose medium. Under these conditions, in fact, PD erythroblasts display higher basal respiration, maximal respiration, and respiration dedicated to ATP production than observed in glucose medium. (Fig. 3A). Conversely, increased mitochondrial activity in galactose medium was not observed in control erythroblasts (Fig. 3A). As expected, medium acidification (ECAR), which reflects glycolysis, is significantly reduced in galactose when compared to glucose medium in both healthy controls and PD patients (Fig. 3B). These results reveal reduced respiration in PD specimens cultured in conditions that permit glycolysis. The comparison between glucose and galactose conditions, moreover, may discriminate between PD patients, who display different oxygen consumption rates in the two conditions, and healthy subjects.
Galactose medium was used to force energetic metabolism through OXPHOS and to unmask mitochondrial defects. A In glucose conditions, PD patients display reduced basal and maximal oxygen consumption rate (compare dark and light blue boxes). Galactose medium increases OCR in PDerythroblasts (compare light blue and orange boxes), but not in cells from healthy controls (compare dark blue and red boxes). B ECAR is reduced in galactose medium. *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA followed by Tukey’s multiple comparison test.
In our previous studies in fibroblasts, we reported a correlation between the magnitude of bioenergetic defects and clinical severity, particularly with regard to non-dopaminergic symptoms [14]. We, therefore, investigated whether biochemical parameters paralleled clinical measures also in erythroblasts originated from the very same patients we examined in our previous work. Patients were divided into four groups of increasing and comparable disease severity based on motor (UPDRS-III) and non-dopaminergic (SENS-PD) clinical scales, and upregulation of mitochondrial respiration in galactose conditions was higher in patients with more severe presentation [14] (Supplementary Table 2).
When we compared mitochondrial bioenergetics among the four experimental groups, we found that maximal respiration changed with disease severity more than basal respiration (Fig. 4). In glucose medium, maximal respiration was progressively reduced in the three groups with higher disease severity when compared to the control group, while it was unchanged patients with the mildest clinical presentation (Fig. 4A). When maximal respiration was compared among patients’ severity groups, we found almost significant differences between patients with the mildest and most severe presentation (i.e. severity 1 vs. severity 4) (p = 0.05281) and between patients in severity groups 3 and 4 (p = 0.05429) (Supplementary Table 3). No differences were detected in basal respiration among the four groups (data not shown). Culturing cells in galactose medium caused an increase in maximal respiration in PD cells, as indicated by the galactose/glucose OCR ratio, which reached statistical significance in the three groups with more severe presentation when compared to healthy controls (Fig. 4B). Higher values of the galactose/glucose OCR ratio in PD cells show, consistently with our previous data in fibroblasts [14], that respiration in PD cells can be increased in conditions forcing metabolism through OXPHOS and is therefore reversible. These elements support the concept that mitochondrial respiration suppression might reflect an adaptative, protective strategy to counteract pathogenic processes.
A Maximal respiration reveals differences between groups with different disease severity and healthy control subjects. Maximal respiration in glucose displays a reduction close to statistical significance in severity group 1 vs. 4 (p = 0.05281) and in severity group 3 vs. 4 (p = 0.05429). B The ratio of OCR in galactose and glucose does not increase in PD specimens. *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA followed by Dunnett’s multiple comparison test.
Collectively, these results confirm that mitochondrial respiration is reduced in PD patients’ peripheral cells and that these alterations parallel disease severity.
iPSC derived dopaminergic neurons
We next extended our examination to dopaminergic neurons differentiated from inducible pluripotent stem cells derived (iDAN). We differentiated iDAN from iPS reprogrammed from fibroblasts from the same patients’ erythroblasts were originated from using small molecules [24]. iPS expressed the expected phenotypic markers (Fig. 5A). We analyzed iDAN from 17 idiopathic PD patients, thus a relatively large number of cell lines. As expected, iDAN expressed neuronal (MAP2) and dopaminergic (TH) markers (Fig. 5B).
Bioenergetic analysis revealed significantly reduced basal mitochondrial respiration in PD lines when compared to healthy controls. Reserve capacity, however, was comparable between PD and healthy subjects (Fig. 6A). As expected, galactose medium remarkably reduced glycolysis, as indicated by reduced medium acidification (ECAR). Contrary to erythroblasts and fibroblasts, however, galactose medium did not lead to increased mitochondrial respiration. On the contrary, galactose medium induced a significant reduction in the reserve capacity levels in PD neurons (Fig. 6A). As expected, ECAR was reduced in galactose medium (Fig. 6B).
Galactose medium was used to force energetic metabolism through OXPHOS and to unmask mitochondrial defects. A In glucose conditions, there are no differences in both basal respiration and reserve capacity between PD patients and healthy control subjects. Galactose medium unmasked differences in basal respiration between patients and healthy subjects (compare light blue and orange boxes), even though it did not result in increased mitochondrial respiration parameters. B Galactose medium causes a reduction in several ECAR parameters. P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA followed by Tukey’s multiple comparison test.
Basal respiration in glucose did not differ between iDAN from PD and healthy subjects. Galactose medium, however, unmasked differences and revealed reduced basal respiration in iDAN from patients with more severe clinical presentation (Fig. 7A). We found that also in iDAN maximal OCR in glucose increased with disease severity when compared to healthy subjects (Fig. 7B, C). Galactose, however, was not able to increase respiration in iDAN, as indicated by the galactose/glucose OCR ratio (Fig. 7D).
Galactose medium was used to force energetic metabolism through OXPHOS and to unmask mitochondrial defects. A Basal respiration in glucose medium is unchanged between PD and healthy subjects. Galactose medium unmasks differences and reveals reduced respiration in PD iDAN. B Reserve capacity is higher in PD cases with more severe clinical presentation in both glucose and galactose medium. C Maximal respiration in glucose is higher in PD patients with different disease severity when compared to healthy subjects but is unchanged between patients’ severity groups. D Galactose medium does not cause in an increase in maximal mitochondrial respiration as indicated by the ratio of OCR in galactose and glucose. *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA followed by Tukey’s multiple comparison test.
Collectively, these data show mitochondrial alterations in idiopathic PD at the neuronal level and point to different bioenergetic features of cultured neurons versus peripheral cells such as erythroblasts and fibroblasts.
Discussion
In this study, we present evidence confirming that bioenergetic defects in PD occur systemically, in peripheral cells, and are also detectable in dopamine neurons differentiated from iPS cells. PD erythroblasts display reduced mitochondrial respiration in culturing conditions allowing glycolysis, which can be however increased when cellular bioenergetics relies exclusively on mitochondrial respiration. These elements indicate that suppression of mitochondrial respiration in iPD is reversible and is consistent with previous findings in fibroblasts from our laboratory [14].
Peripheral alterations in mitochondrial function are also confirmed by transcriptome canonical pathway analysis in the longitudinal PPMI cohort. Interestingly, the downregulation of mitochondrial pathways was more pronounced in patients who showed more severe disease progression in the 36-month period of observation. As expected, PD patients also displayed alteration in mitophagy, consistently with the key role of PINK1 and PRKN in mitochondrial quality control [25, 26], and our findings offer further support to the role of mitophagy in idiopathic PD as well [27].
Mitochondrial biology in PD has been investigated in several cell types, including different PBMC subpopulations, fibroblasts, and human neurons [28,29,30,31,32,33]. A strength of our study is that it analyzes and compares blood cells and iDAN derived from the same patient; additionally, it investigates an easily accessible blood cell type that to our best knowledge has been never studied in PD, i.e. erythroblasts. Moreover, studies in human neurons largely focused on PD familial forms so far, while we focused on idiopathic patients analyzing a rather large number of iDAN lines (N = 17).
As expected, our data confirmed altered mitochondrial function in PD specimens and therefore substantiated the role of these organelles in PD pathobiology. Moreover, our results are consistent with recent clinical findings obtained using 31-phosphorus magnetic resonance spectroscopy describing mitochondrial impairment in the posterior putamen of PD patients [34].
When we analyzed erythroblasts from patients with different clinical severity we found almost significant differences in maximal respiration in the groups with the mildest and most severe presentation (i.e. severity 1 vs. severity 4) (p = 0.05281) and between the severity groups 3 and 4 (p = 0.05429) (Supplementary Table 3). While the results do not reach the canonical criteria for statistical significance (i.e. p < 0.05), they point to a correlation between peripheral bioenergetics and clinical severity, consistent with data previously reported by us and other groups [14, 16]. The proximity to statistical significance indicates the groups size lacks statistical power, which can however be easily reached in future confirmatory studies. The fact that in our previous study, we found significant differences among severity groups by analyzing patients’ fibroblasts points to metabolic and bioenergetic differences between this cell types and erythroblasts, which can however be expected given the different biological role of these cells. We did not found differences in basal respiration in patients with different clinical severity. Here, a possible explanation could be that mitochondrial defects in PD are modest and these organelles can therefore properly function in basal conditions, while anomalies emerge under stress. This hypothesis is consistent with our previous data demonstrating the metabolic challenge of PD cells unmasks bioenergetic alterations [14] and also fits with the double-hit hypothesis positing that the etiology of PD lays in gene-environment interactions [35]. Overall, our results corroborate the concept that peripheral cells may recapitulate neuronal pathology.
Blood samples are a highly convenient source of patient material given their accessibility and the minimally invasive procedures required to obtain the specimens. While several other studies analyzed the bioenergetic profile in PD blood cells, primarily in PBMC, to our best knowledge this is the first case that investigates mitochondrial function in erythroblasts. This approach offers some important conceptual and practical advantages. First, bioenergetics greatly differs among the various blood cells, and even among their subtypes. For instance, T-cell subtypes display large metabolic differences, being effector T-cells more reliant on glycolysis than regulatory and memory T-cells. The same applies to monocytes in their different polarized forms [17]. Moreover, erythroid cells express high level of a-syn – unlike other blood cells and fibroblasts - and may therefore be an amenable system to study the interplay between a-syn, bioenergetics, and other factors relevant for PD [36]. Finally, erythroblasts can be prepared by amplifying in cell cultures a very small amount of starting material, generating a rather uniform population that does not require laborious and expensive cell sorting. Of note, other studies emphasized that the small number of obtainable blood cells limits the number of analyses that can be performed on a single sample [16]; the use of expandable erythroblasts may obviate this issue, opening to further analytical options.
The evidence that PD erythroblasts can potentiate mitochondrial respiration when cultured in conditions that do not permit glycolysis, i.e. in galactose medium, replicates our previous findings in fibroblasts. Potentiation of mitochondrial respiration in galactose medium in PD peripheral cells suggests that in idiopathic PD, at least in a sub-group of cases, impaired mitochondrial function is reversible and may represent an adaptive protective response. Indeed, in our previous work we have shown that augmented mitochondrial respiration may favor alpha-synuclein stress [14]. The possibility that suppression of mitochondrial respiration is a protective adaptation may be consistent with evidence obtained by other laboratories and by our group as well. An independent study has in fact shown a protective effect of the reversible inhibitor Mitochondrial Division Inhibitor 1 (Mdivi-1) in PD animal models [37]. Moreover, we have shown that reversible and mild inhibition of complex I caused by its S-nitrosation is protective in multiple models of PD and improves bioenergetic efficiency in PD fibroblasts [14]. A protective role for mitochondrial respiration suppression may also be consistent with recent hypotheses positing that in Alzheimer’s disease dysfunctional neurons increase mitochondrial respiration in a pathogenic mechanism that might be interpreted as an inverse-Warburg effect [38, 39]. We deem the observation that reduction of mitochondrial respiration in PD particularly relevant because it might represent a paradigm shift from the general idea that this biochemical defect in PD is constitutive.
Differently than in peripheral cells, in iDANs potentiation of respiration in galactose medium was instead not detected. This finding might indicate that PD neurons may be more susceptible to mitochondrial defects, accordingly to a large body of literature showing that mild mitochondrial inhibition is particularly impactful on the dopaminergic system [40]. It should be noted, however, that iDAN cultures do not contain glial cells; because neurons are metabolically coupled to astrocytes [41], this experimental setup is necessarily incomplete. Biological processes such as astrocytes-neurons lactate exchange to fuel OXPHOS [42] may be necessary to increase mitochondrial respiration in iDANs. The lack of potentiation of respiration in galactose in iDAN is also consistent with multiple lines of evidence indicating that neurons reliance on glycolysis is modest, differently than in astrocytes. Glycolysis inhibition in neurons may therefore result in modest effects, as observed in our studies. Alternatively, neuronal metabolic plasticity, i.e. the ability to switch in the use of metabolic substrates, particularly under stressful conditions, might be reduced when compared to other cell types, particularly when these cells are extrapolated from their physiological environment, i.e. in culture dishes. Additional experiments should be performed in co-cultures—and possibly also in vivo—to determine whether this condition may permit potentiation of respiration when glycolysis is not permitted.
In conclusion, this offers additional arguments in favor of the use of peripheral cell in biomarker research for PD and confirms mitochondrial defects in human neurons derived from idiopathic patients. Altogether, these elements reinforce the possibility that—at least in some subgroups of PD patients—mitochondrial function could be an amenable target for disease modification.
Data availability
Data will be made available on request.
References
Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, et al. Intersecting pathways to neurodegeneration in Parkinson’s disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006;22:404–20.
Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci. 2017;18:101–13.
Bose A, Beal MF. Mitochondrial dysfunction in Parkinson’s disease. J Neurochem. 2016;139:216–31.
Mortiboys H, Macdonald R, Payne T, Sassani M, Jenkins T, Bandmann O. Translational approaches to restoring mitochondrial function in Parkinson’s disease. Febs Lett. 2018;592:776–92.
Surmeier DJ, Halliday GM, Simuni T. Calcium, mitochondrial dysfunction and slowing the progression of Parkinson’s disease. Exp Neurol. 2017;298:202–9.
Quansah E, Peelaerts W, Langston JW, Simon DK, Colca J, Brundin P. Targeting energy metabolism via the mitochondrial pyruvate carrier as a novel approach to attenuate neurodegeneration. Mol Neurodegener. 2018;13:28.
Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269.
Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119:866–72.
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–6.
González-Rodríguez P, Zampese E, Stout KA, Guzman JN, Ilijic E, Yang B, et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature. 2021;599:650–6.
Beilina A, Cookson MR. Genes associated with Parkinson’s disease: regulation of autophagy and beyond. J Neurochem. 2016;139:91–107.
Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–13.
Macdonald R, Barnes K, Hastings C, Mortiboys H. Mitochondrial abnormalities in Parkinson’s disease and Alzheimer’s disease: can mitochondria be targeted therapeutically? Biochem Soc Trans. 2018;46:891–909.
Milanese C, Payan-Gomez C, Galvani M, Molano Gonzalez N, Tresini M, Nait Abdellah S, et al. Peripheral mitochondrial function correlates with clinical severity in idiopathic Parkinson’s disease. Mov Disord. 2019.
Robinson BH, Petrova-Benedict R, Buncic JR, Wallace DC. Nonviability of cells with oxidative defects in galactose medium: a screening test for affected patient fibroblasts. Biochem Med Metab Biol. 1992;48:122–6.
Smith AM, Depp C, Ryan BJ, Johnston GI, Alegre-Abarrategui J, Evetts S, et al. Mitochondrial dysfunction and increased glycolysis in prodromal and early Parkinson’s blood cells. Mov Disord. 2018;33:1580–90.
O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–65.
Reinhardt P, Glatza M, Hemmer K, Tsytsyura Y, Thiel CS, Hoing S, et al. Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PLoS One. 2013;8:e59252.
Boyle EI, Weng S, Gollub J, Jin H, Botstein D, Cherry JM, et al. GO::TermFinder—open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics. 2004;20:3710–5.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
van den Akker E, Satchwell TJ, Pellegrin S, Daniels G, Toye AM. The majority of the in vitro erythroid expansion potential resides in CD34− cells, outweighing the contribution of CD34+ cells and significantly increasing the erythroblast yield from peripheral blood samples. Haematologica. 2010;95:1594–8.
Leberbauer C, Boulmé F, Unfried G, Huber J, Beug H, Müllner EW. Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood. 2005;105:85–94.
Liang R, Menon V, Qiu J, Arif T, Renuse S, Lin M, et al. Mitochondrial localization and moderated activity are key to murine erythroid enucleation. Blood Adv. 2021;5:2490–504.
Grochowska MM, Carreras Mascaro A, Boumeester V, Natale D, Breedveld GJ, Geut H, et al. LRP10 interacts with SORL1 in the intracellular vesicle trafficking pathway in non-neuronal brain cells and localises to Lewy bodies in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. 2021;142:117–37.
Narendra DP, Jin SM, Tanaka A, Suen D-F, Gautier CA, Shen J, et al. PINK1 is selectively stabilized on impaired mitochondria to activate parkin. PLOS Biol. 2010;8:e1000298.
Pickles S, Vigié P, Youle RJ. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol. 2018;28:R170–R185.
Malpartida AB, Williamson M, Narendra DP, Wade-Martins R, Ryan BJ. Mitochondrial dysfunction and mitophagy in Parkinson’s disease: from mechanism to therapy. Trends Biochem Sci. 2021;46:329–43.
Carling PJ, Mortiboys H, Green C, Mihaylov S, Sandor C, Schwartzentruber A, et al. Deep phenotyping of peripheral tissue facilitates mechanistic disease stratification in sporadic Parkinson’s disease. Prog Neurobiol. 2020;187:101772.
Cooper O, Seo H, Andrabi S, Guardia-Laguarta C, Graziotto J, Sundberg M, et al. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Sci Transl Med. 2012;4:141ra190.
Mortiboys H, Johansen KK, Aasly JO, Bandmann O. Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology. 2010;75:2017–20.
Mortiboys H, Thomas KJ, Koopman WJ, Klaffke S, Abou-Sleiman P, Olpin S, et al. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann Neurol. 2008;64:555–65.
Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D. Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci. 2011;31:5970–6.
Zambon F, Cherubini M, Fernandes HJR, Lang C, Ryan BJ, Volpato V, et al. Cellular alpha-synuclein pathology is associated with bioenergetic dysfunction in Parkinson’s iPSC-derived dopamine neurons. Hum Mol Genet. 2019;28:2001–13.
Payne T, Burgess T, Bradley S, Roscoe S, Sassani M, Dunning MJ, et al. Multimodal assessment of mitochondrial function in Parkinson’s disease. Brain. 2024;147:267–80.
Greenamyre JT, Hastings TG. Biomedicine. Parkinson’s-divergent causes, convergent mechanisms. Sci (N. Y, NY). 2004;304:1120–2.
Scherzer CR, Grass JA, Liao Z, Pepivani I, Zheng B, Eklund AC, et al. GATA transcription factors directly regulate the Parkinson’s disease-linked gene alpha-synuclein. Proc Natl Acad Sci USA. 2008;105:10907–12.
Bordt EA, Clerc P, Roelofs BA, Saladino AJ, Tretter L, Adam-Vizi V, et al. The putative Drp1 inhibitor mdivi-1 is a reversible mitochondrial complex I inhibitor that modulates reactive oxygen species. Dev Cell. 2017;40:583–94.e586.
Demetrius LA, Magistretti PJ, Pellerin L. Alzheimer’s disease: the amyloid hypothesis and the Inverse Warburg effect. Front Physiol. 2014;5:522.
Demetrius LA, Simon DK. An inverse-Warburg effect and the origin of Alzheimer’s disease. Biogerontology. 2012;13:583–94.
Greenamyre JT, Cannon JR, Drolet R, Mastroberardino PG. Lessons from the rotenone model of Parkinson’s disease. Trends Pharm Sci. 2010;31:141–2.
Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86:883–901.
Bélanger M, Allaman I, Magistretti PierreJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–38.
Acknowledgements
This study was funded by the Michael J. Fox Foundation (PGM). DS was supported by a fellowship from the Fondazione Umberto Veronesi.
Author information
Authors and Affiliations
Contributions
Study design: PGM, SB, CM, MG. Experiments: SB, TL, LD, SF, MS. Data analysis PGM, SB, CM, DS, CPG. PGM wrote the initial draft of the manuscript with input from all the authors. PGM conceived and directed the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the medical ethics committee of the Leiden University Medical Center (P12.194/NV/ib), and written informed consent was obtained from all PD patients. Healthy control samples were obtained from Sanquin Rotterdam (NVT0585.00 Mantel, NVT0585.01 Annex).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Mauro Piacentini
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barnhoorn, S., Milanese, C., Li, T. et al. Orthogonal analysis of mitochondrial function in Parkinson’s disease patients. Cell Death Dis 15, 243 (2024). https://doi.org/10.1038/s41419-024-06617-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-024-06617-6