Abstract
Antisense RNAs (asRNAs) represent an underappreciated yet crucial layer of gene expression regulation. Generally thought to modulate their sense genes in cis through sequence complementarity or their act of transcription, asRNAs can also regulate different molecular targets in trans, in the nucleus or in the cytoplasm. Here, we performed an in-depth molecular characterization of NFYC Antisense 1 (NFYC-AS1), the asRNA transcribed head-to-head to NFYC subunit of the proliferation-associated NF-Y transcription factor. Our results show that NFYC-AS1 is a prevalently nuclear asRNA peaking early in the cell cycle. Comparative genomics suggests a narrow phylogenetic distribution, with a probable origin in the common ancestor of mammalian lineages. NFYC-AS1 is overexpressed pancancer, preferentially in association with RB1 mutations. Knockdown of NFYC-AS1 by antisense oligonucleotides impairs cell growth in lung squamous cell carcinoma and small cell lung cancer cells, a phenotype recapitulated by CRISPR/Cas9-deletion of its transcription start site. Surprisingly, expression of the sense gene is affected only when endogenous transcription of NFYC-AS1 is manipulated. This suggests that regulation of cell proliferation is at least in part independent of the in cis transcription-mediated effect on NFYC and is possibly exerted by RNA-dependent in trans effects converging on the regulation of G2/M cell cycle phase genes. Accordingly, NFYC-AS1-depleted cells are stuck in mitosis, indicating defects in mitotic progression. Overall, NFYC-AS1 emerged as a cell cycle-regulating asRNA with dual action, holding therapeutic potential in different cancer types, including the very aggressive RB1-mutated tumors.
Similar content being viewed by others
Introduction
High-throughput sequencing has expanded our knowledge of the non-coding portion of human transcriptome, unearthing numerous novel transcripts [1, 2]. Among these, antisense RNAs (asRNAs), which originate from the antisense DNA strand and can overlap with the sense transcript of genes, are one of the most underappreciated classes [3]. Quantitative measurements of nascent transcripts support antisense transcription for up to ∼70% of protein-coding genes [4]. Despite the growing recognition for contributing to genome regulation, most asRNAs remain poorly characterized due to their very low abundance, which hinders accurate annotation [3]. From a functional perspective, asRNAs are thought to act in cis on their sense gene by transcription-dependent mechanisms and/or sequence complementarity [5,6,7]. Nevertheless, examples exist of asRNAs acting in trans and targeting genomic sequences distal to their locus in an RNA-dependent manner [3].
AsRNAs have been recognized as regulators of gene expression in multiple biological processes [7,8,9,10], and their aberrant expression/function is associated with tumorigenesis [11, 12]. AsRNAs may thus represent a rich and yet underexplored environment for the identification of cancer-relevant biomarkers and therapeutic targets. In this regard, the design of RNA-based therapies involving sequence-specific antisense oligonucleotide (ASOs) to target a pathogenic asRNA of interest (and in general long non-coding RNAs, lncRNAs) is quite straightforward [13]. In addition, as lncRNAs fold into tertiary structures forming domains with specific pockets, it is possible to target them using small molecules [14], which makes lncRNAs ideal entities to modulate processes linked to undruggable protein targets [14,15,16].
NFYC Antisense 1 (NFYC-AS1, Supplementary Fig. S1A) is the asRNA transcribed head-to-head to NFYC, a subunit of the trimeric NF-Y transcription factor (TF). NF-Y has widely been described as a master regulator of proliferation in normal and tumor cells [17, 18]. In contrast, the information on NFYC-AS1 is scanty. While it was shown upregulated in lung adenocarcinoma (LUAD), with its silencing able to induce cell death in LUAD cells via both apoptosis and autophagy [19, 20], crucial aspects of its biology remain to be elucidated. Here, we provide the first comprehensive characterization of NFYC-AS1 expression pancancer, accompanied by a refined annotation of its transcript and an initial characterization of its cell cycle-regulated expression, role in supporting cell growth, and dual in cis/in trans action.
Materials and Methods
Cell culture
Established human tumor cell lines from American Type Culture Collection (ATCC, Rockville, MD, USA) were cultured in standard conditions, routinely tested for mycoplasma contamination using N-GRADE Mycoplasma PCR Reagent set (Euroclone S.p.A., Pero, Italy) and authenticated by STR profiling (Eurofins MWG-Biotech, Ebersberg, Germany). The NCI-H520 RB1-wildtype cell line (H520) is derived from a lung squamous carcinoma patient; these cells are adherent and have an epithelial morphology. The NCI-H82 RB1-mutated cell line (H82) is derived from a small cell lung carcinoma patient; H82 are epithelial cells that grow in suspension forming disordered aggregates. These cell lines were selected to model tumors where NFYC-AS1 is up-regulated (including both RB1 mutational backgrounds), since they show high levels of the asRNA (>4 TPM).
Both cell lines were grown in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 2 mM L-Glutamine and 25 mM Hepes (Euroclone S.p.A., Pero, Italy), at 37 °C in a 5% CO2 atmosphere. For H82 cells, the medium was also supplemented with 4500 mg/L D-Glucose (GeneSpin Srl, Milan, Italy) and 1 mM Sodium Pyruvate (NaPyr, Sigma-Aldrich, Saint Louis, MI, USA).
5’ and 3’ RACE
The 5’ and 3’ RACE System for Rapid Amplification of cDNA Ends Version 2.0 (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used to characterize NFYC-AS1 5’ and 3’ ends in H520 cells, according to manufacturer’s instructions. RACE products were sequenced-verified by Eurofins MWG-Biotech (Ebersberg, Germany).
Gapmer ASO and siRNA treatment
Six different Gapmer ASOs (QIAGEN, Hilden, Germany), GAP1 to GAP6, were designed to target different portions of NFYC-AS1 putative transcript (relative positions are shown in Supplementary Fig. S3A and sequences reported in Supplementary Table S9), making sure that they did not overlap any annotated repetitive regions. Gapmers were transfected at 5-25 nM concentration in Opti-MEM™ (Thermo Fisher Scientific Inc., Waltham, MA, USA) using Lipofectamine™2000 (Thermo Fisher Scientific Inc., Waltham, MA, USA). 7 ×105 H520 cells were seeded the day before transfection, transfected for 6 h with Gapmers or the Negative Control Gapmer (NEG), then incubated in complete medium for 48/72 h. For H82 cells, the day of transfection, 1.0 ×106 H82 cells were transfected in their medium and incubated for 48/72 h. The same protocols were applied to transfect 40 nM NFYB siRNA and relative control siRNA (siCT).
NFYC-AS1 Transcription Start Site (TSS) deletion by CRISPR/Cas9
Two sgRNAs targeting NFYC-AS1 TSS region were designed and cloned into PLV-Cas9-T2A-GFP plasmid (Addgene #53190 - Cambridge, MA, USA). H520 cells were transfected with 2.5 µg plasmids using Lipofectamine™2000. GFP+ cells were sorted using BD FACSAria II flow cytometer and cultured as single clones. Genomic DNA from single clones was extracted using QuickExtractTM DNA Extraction Solution (Lucigen by Biosearch Technologies, Hoddesdon, UK) and 2x Master Mix Standard GL kit (GeneSpin Srl, Milan, Italy) was used to perform end-point PCR, and PCR products verified for the deletion on agarose gel.
Cell-cycle synchronization and re-entry
2 × 106 H520 cells per time point were seeded in complete medium. The day after, cells were left in a serum-deprived medium (0% FBS) for 48 h. Synchronous cell-cycle re-entry was stimulated by adding complete medium (10% FBS). Cells were harvested at different time points after serum addition, and cyclin mRNA levels measured by qRT-PCR to define cell-cycle phases.
RNA-sequencing
RNA-sequencing was performed in quadruplicates on H520 cells treated with GAP2/3/4 compared to NEG at 48 h after transfection and on four independent ΔTSS H520 clones compared to three independent Wild-Type (WT) clones. Polyadenylated RNA was purified through oligo-dT-based RNA capturing, randomly fragmented, and transformed into cDNA using random hexamers with NEB library preparation protocol. Library preparation, paired-end sequencing, and data quality control were performed by Novogene, UK. Gene expression levels were computed by RSEM-1.3.1 software using NCBI RefSeq (GRCh38/hg38) as a reference. Bioinformatic analyses are described in Supplementary Methods.
Statistics and reproducibility
The results are presented as mean values ± standard deviation (sd) or standard error (se). p-values were calculated by unpaired two-tailed t-test, one sample t-test, Jonckheere-Terpstra test, or log-rank test as highlighted in captions (where the number of replicates for the different experiments is indicated) and considered statistically significant if p < 0.05.
Additional Materials and Methods
Additional methods are described in Supplementary Methods. Sequences of primers, Gapmers and siRNAs, and sgRNAs are reported in Supplementary Tables S1-S3. Original data of qRT-PCR and western blot are reported as Original Data 1 and 2, respectively.
Results
NFYC-AS1 is upregulated in cancer with preferential association with RB1 mutation
Across healthy human tissues, NFYC-AS1 expression levels are high in cerebellum, uterus, prostate, thyroid, cervix, ovary (Supplementary Fig. S1B), overall mirroring those of its sense gene NFYC (Supplementary Fig. S1C), though remaining invariably lower (NFYC-AS1/NFYC ratio=0.0923-0.365). Analysis of The Cancer Genome Atlas (TCGA) [21] showed a systematic upregulation of NFYC-AS1 in most human tumors as compared to normal tissues, including the main histotypes of non-small cell lung cancer (NSCLC), lung adenocarcinoma (LUAD) and lung squamous carcinoma (LUSC) (Fig. 1A). Conversely NFYC modulation is not reproducible across cancer types, with some tumors showing concomitant NFYC-AS1 upregulation and NFYC downregulation (Supplementary Fig. S2A). Accordingly, NFYC-AS1/NFYC expression ratio (calculated in individual samples) is increased in most human cancers (Fig. 1B), suggesting a tumor-specific asRNA overexpression. Moreover, NFYC-AS1 levels are sufficient to discriminate between normal and tumor samples in NSCLC cohorts and pancancer (Supplementary Fig. S2B). NFYC-AS1 overexpression in NSCLC was confirmed in an independent dataset (GSE81089), with superimposable tumor/normal fold-changes (Supplementary Fig. S2C). No major differences were found in NFYC-AS1 expression across NSCLC molecular subtypes, with the exception of markedly higher levels in LUSC primitive subtype (Supplementary Fig. S2D, E). Instead, a significant association was found with selected mutational profiles: lower asRNA levels were observed in KRAS-mutated (-mut) LUAD and in NF1/MYCBP2-mut LUSC (Supplementary Tables S4, 5). Mutation in RB1, a prototypical tumor suppressor gene [22], was found significantly associated with increased NFYC-AS1 expression in both LUAD and LUSC (Fig. 1C–E), where it affects 5% and 7% of cases, respectively. Notably, 14% of primitive LUSC, the form with the worse prognosis [23], are RB1-mut (Supplementary Fig. S2E), consistent with the particularly high NFYC-AS1 levels observed in this subtype.
A Boxplots of NFYC-AS1 expression and B NFYC-AS1/NFYC expression level ratios in tumor and matched control tissues (TCGA). C Venn diagram showing the intersection between the top significant mutations (sorted by p-value) associated with higher NFYC-AS1 expression level (mut/wt > 1) in LUAD and LUSC (TCGA). D Boxplots of NFYC-AS1 expression level in RB1-wild-type (RB1-wt) and RB1-mutated (RB1-mut) tumors compared to normal tissues in LUAD and E LUSC (TCGA). F Boxplots of NFYC-AS1 expression in SCLC tumors compared with normal tissues (GSE60052). G Boxplots of NFYC-AS1 expression in lung cancer CCLE cell lines according to the lung cancer histotype of origin or H the RB1 mutational status. Throughout the figure, the gene expression level is expressed as logarithm in base 2 of the normalized counts (norm) or TPM plus one [log2(norm or TPM + 1)], depending on the available data. The tumor-normal fold-change (FCT/N) is calculated as the ratio between the average normalized counts or TPM for NFYC-AS1 in tumors and in normal tissues, in SCLC and in the other lung cancer histotypes, or in presence and absence of RB1 mutation. The NFYC-AS1/NFYC ratio is calculated as the ratio between the normalized counts for NFYC-AS1 and for NFYC in individual samples and then averaged. Two-tailed unpaired t-test p-values are reported, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns (non-significant).
RB1 gene mutations are generally rare but reach 90% in the highly aggressive small cell lung cancer (SCLC) histotype [24], where they drive tumor cell hyperproliferation and increased lineage plasticity towards a neuroendocrine phenotype [25]. We indeed found that NFYC-AS1 is markedly upregulated in SCLC (Fig. 1F), with a tumor-normal fold-change of 4.97, the highest among the lung cancer histotypes analyzed in this study. This finding was corroborated by the analysis of 208 lung cancer cell lines from the Cancer Cell Line Encyclopedia (CCLE) [26], where a major upregulation of NFYC-AS1 is observable in SCLC compared to LUAD, LUSC and large cell lung carcinoma (LCLC) cells (Fig. 1G), and in RB1-mut vs -wt cells (Fig. 1H). Considering SCLC cell line classification into molecular subtypes [25], higher NFYC-AS1 levels were found in neuroendocrine ASCL1 and NEUROD1 groups (Supplementary Fig. S2F), which are characterized by 71% and 90% of RB1-mut cell lines, respectively, as compared to the YAP1 subtype, which has low/absent expression of neuroendocrine markers [27] and comprises only 15% of RB1-mut cell lines. Moreover, NFYC-AS1 levels were sufficient to discriminate between RB1-wt and RB1-mut NSCLC tumors and lung cancer cell lines, with good levels of accuracy (Supplementary Fig. S2G).
Accurate transcript annotation reveals a dominant long NFYC-AS1 isoform and prevalently nuclear expression
A monoexonic transcript is reported for NFYC-AS1 both in the Refseq and Gencode annotations, but marked differences exist in the designation of the TSS, termination site (TTS), and RNA length (Supplementary Fig. S1A). By analyzing the profile of RNA-seq reads from cell lines with high levels (>4 TPM) of the asRNA (H520 LUSC cells and H82 SCLC cells), we observed a good support for the Gencode TSS (proximal TSS, w.r.t. the NFYC TSS) (Fig. 2A). Two FANTOM CAGE [28] peaks were found next to only the proximal TSS, which also corresponded to the peak of DNase I hypersensitivity signal (Fig. 2A). Furthermore, 5’RACE experiments indicated the proximal TSS as the true 5’ end (Fig. 2A, B, Supplementary Fig. S3A).
A NFYC(-AS1) locus at chr1p34.2 as from UCSC Genome Browser (GRCh38/hg38 assembly). From the top to the bottom: NCBI RefSeq and GENCODE V42 annotations are reported together with CAGE peaks (FANTOM5 project) on the minus strand for NFYC-AS1 (blue) and plus strand for NFYC (red), polyadenylation sites on the minus strand (red) (polyA site database, 3’-seq data), polyadenylation signal (PAS), our NFYC-AS1 reconstructed annotation, and BigWig profile for H520 and H82 cell lines. B Electrophoretic gel of 5’RACE PCR products. C Electrophoretic gel of 3’RACE PCR products. D Bar plot showing the relative abundance of NFYC-AS1 isoforms in different cell lines from the CCLE and in H520 cell line as from our RNA-seq. E Bar plot showing the relative percentage of NFYC-AS1 measured through qRT-PCR using primer 4 (Pr4) in the nuclear and cytoplasmic fractions of H520 and H82 cells. MALAT1 and GAPDH are used as control for the nuclear and cytoplasmic fractions, respectively. F Expression levels of NFYC-AS1, NFYC and C-MYC (qRT-PCR) in H520 and H82 cell lines, after Actinomycin-D treatment. Data are normalized to time 0 for every gene and represent the mean ± sd, as from n = 3 independent qRT-PCR measurements.
Three different polyadenylated 3’ ends were identified by 3’RACE and confirmed by Sanger sequencing (Fig. 2A, C, Supplementary Fig. S3A), corresponding to the RefSeq, the Gencode and an additional TTS, named NFYC-AS1.1 (long), NFYC-AS1.2 (medium) and NFYC-AS1.3 (short). A canonical AAUAAA/AUUAAA polyadenylation signal (PAS) was indeed found upstream of the long and short 3’ ends (Fig. 2A), while 11 A’s were found at the intermediate TTS. To exclude mispriming in 3’RACE, oligo-dT and random hexamer retrotranscribed RNA were compared using different primers (Supplementary Fig. S3A): similar oligo-dT/random hexamer ratios were observed for primers in proximity of all the putative TTSs (Supplementary Fig. S3B), confirming them as true 3’ ends. Re-analyzing RNA-seq data from highly expressing cells using our refined annotation, long NFYC-AS1 isoform resulted to be the most abundant overall, nevertheless low levels of expression of short and medium isoforms were observed in some samples (Fig. 2D), consistent with 3’-seq data from the PolyASite database [29] (Fig. 2A).
NFYC-AS1 subcellular localization was assessed in H520 and H82 cells, revealing a prevalently nuclear expression (Fig. 2E). Although five complete ORFs of > 75 nt in size can be detected within NFYC-AS1 sequence, computational prediction, and interrogation of publicly available RIBO-seq data consistently classified the transcript as devoid of protein-coding potential (Supplementary Fig. S3C and Supplementary Tables S6–10), ultimately indicating that NFYC-AS1 is a lncRNA. Decay experiments showed that NFYC-AS1 transcript half-life is less than one hour, similarly to the short-lived transcript C-MYC [30]; however, detectable levels were observed at later time points, suggesting that NFYC-AS1 turnover is rapid, though a pool of stable transcripts may exist (Fig. 2F). In contrast, NFYC mRNA revealed to be quite stable with a lower decay (Fig. 2F).
NFYC-AS1 is regulated by NF-Y and conserved throughout mammals
ENCODE DNase I hypersensitivity data were inspected to annotate NFYC-AS1 and NFYC regulatory elements, revealing two peaks corresponding to NFYC TSS and NFYC-AS1 proximal TSS (Fig. 3A). According to ENCODE candidate cis-regulatory elements (cCREs), two clearly distinct promoters insist on this genomic region, indicating that the two genes are transcribed independently (Fig. 3A). Three equidistant CCAAT boxes – a typical feature of promoters bound by NF-Y TF [17] – and NF-Y ChIP-seq signal were found just upstream of NFYC-AS1 TSS (Fig. 3A); consistent with this, abrogation of NF-Y activity through NFYB silencing resulted in a dramatic downregulation of NFYC-AS1 in both H520 and H82 cells (Fig. 3B). In contrast, none of these features is present immediately upstream of NFYC TSS (Fig. 3A), and, accordingly, NFYC was not repressed upon NFYB silencing (Fig. 3B). Although NFYC has an alternative TSS, approximately 17 kb downstream of NFYC-AS1 (Fig. 3A), this TSS was shown to be induced only upon DNA damage [31] and is considerably less used in basal conditions compared with the canonical TSS, as exemplified by the cumulative fraction of NFYC transcripts originating from either TSS in H520 and H82 cells (Fig. 3C). Curiously, the alternative TSS resulted to be directly under the control of NF-Y, as NFYB silencing markedly abrogated the expression of alternative NFYC transcript in both cell lines (Fig. 3B), in line with the presence of CCAAT boxes and NF-Y ChIP-seq peaks in its proximity (Fig. 3A).
A NFYC(-AS1) locus at chr1p34.2 as from UCSC Genome Browser (GRCh38/hg38 assembly). Top: CRISPR/Cas9 single guide (sg)RNAs and genomic PCR primers are shown together with the long NFYC-AS1 isoform. Middle: transcription regulatory elements, including CCAAT boxes (green rectangles), NF-Y ChIP-seq peaks (ENCODE), ENCODE candidate Cis-Regulatory Elements (cCREs), and DNase hypersensitivity tracks. Bottom: a detailed overview of conservation profiles according to the Vertebrate Multiz Alignment & Conservation (100 Species) track in the Genome Browser. Blue: phyloP conservation score. Green: PhastCons conservation score. Alignment of a manual selection of representative genome sequences for different taxa is represented at the bottom, together with the Repeating Element by RepeatMasker track; mammalian-wide interspersed repeats (MIRs) are highlighted by red rectangles. B Bar plot showing NFYB, NFYC total, NFYC canonical, NFYC alternative, and NFYC-AS1 expression levels (qRT-PCR) at 48 h after transfection with NFYB siRNA (siNFYB) in H520 RB1-wt cells and H82 RB1-mut cells. Data are siCT-normalized and reported as mean ± sd, as from n = 3 independent biological replicates. One sample t-test p-values are reported. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns (non-significant). C Pie charts showing the cumulative fraction of NFYC transcripts originating from either TSS (NFYC canonical and alternative) as from RNA-seq data of H520 and H82 cells. D PhyloP conservation score at exons of lncRNAs (lncRNAs), exons of protein coding genes (protCod), protein coding exons of protein coding genes (CDS), compared with different NFYC-AS1 segments/regions and NFYC regulatory elements: AS1body, NFYC-AS1 3’ end segment not overlapped with NFYC; promYC_can, canonical NFYC isoform promoter; interProm, NFYC-AS1 segment spanning the genomic region in between NFYC canonical promoter and NFYC-AS1 promoter; promAS1, NFYC-AS1 promoter; promYC_alt, alternative NFYC isoform promoter. Promoters were defined as the genomic region spanning 250 bp upstream of annotated transcription start sites.
NFYC-AS1 sequence is highly conserved in primates and moderately conserved in other mammals, with the exception of mouse/rodents (Fig. 3A). An orthologue asRNA with similar tissue expression pattern as in humans has been described in dog [32]. Inspection of conservation tracks and multiple alignments of genome sequences showed that NFYC alternative TSS has high levels of conservation across vertebrates; conversely, the canonical TSS is conserved only in mammals (Fig. 3A, D). NFYC-AS1 TSS displayed levels of sequence conservation comparable to those of NFYC first exon and canonical TSS, with a score in line with that of protein-coding exons (Fig. 3D). Lower levels of conservation were instead observed for NFYC-AS1 3’ terminal portion, which were however comparable with those of other lncRNAs (Fig. 3D).
Interestingly, both NFYC-AS1 and NFYC first intron are punctuated by Mammalian-wide Interspersed Repeats (MIRs) (Fig. 3A), a family of transposable elements specific to mammals that have been linked with the dissemination of novel promoters and enhancer elements throughout mammalian and human genomes [33]. This arrangement and the observed patterns of sequence conservation might indicate that NFYC canonical TSS/first intron and NFYC-AS1 originated in mammals, while the broader phylogenetic distribution and higher levels of conservation would position NFYC alternative TSS as ancestral.
NFYC-AS1 knockdown impairs proliferation of both RB1-wt and -mut cancer cells
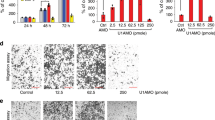
Six different Gapmer ASOs were used to knockdown NFYC-AS1 in RB1-wt H520 cells, three of which (GAP2, GAP3, and GAP4) targeted all NFYC-AS1 isoforms, two (GAP5 and GAP6) only the long isoform, and one (GAP1) the sequence in between the proximal and distal TSS (Supplementary Fig. S3A). Gapmers from 2 to 6 effectively downmodulated NFYC-AS1 (Fig. 4A), with a degree of repression that paralleled transcript accessibility (Supplementary Fig. S4A, B). GAP1, despite the high accessibility of its target region, failed to repress NFYC-AS1, again supporting the use of the proximal TSS (Fig. 4A, Supplementary Fig. S4A). NFYC-AS1 silencing resulted in a time-dependent reduction of cell growth, which was proportional to the extent of knockdown exerted by the different Gapmers (Fig. 4B). As a control, GAP1 did not induce any significant decline of cell number (Supplementary Fig. S4C). The other way around, we found that NFYC-AS1 levels decreased endogenously in H520 cells as they reduced their growth in response to confluency, in trend with cyclin D1 levels (Fig. 4C). Altogether, these results suggest a role of NFYC-AS1 in supporting cell proliferation.
A Bar plot showing NFYC-AS1 expression measured through qRT-PCR using primer 4 (Pr4) and primer 6 (Pr6) at 48 h after 5 nM Gapmer transfection in H520 RB1-wt cells. Data are NEG-normalized and reported as mean ± sd as from multiple independent biological replicates (n indicated in Supplementary Table S11). B Cell counts at different timepoints after NFYC-AS1 Gapmer-knockdown, normalized against the NEG at 72 h in H520 cells. Data reported as mean ± se, as from n = 3 independent biological replicates. C Bar plot showing NFYC-AS1 and CCND1 expression levels (qRT-PCR) in H520 cells seeded at different cell densities, normalized against the lowest confluency. Data reported as mean ± sd, as from n = 3 independent biological replicates. Jonckheere-Terpstra test p-values are shown. D Heatmap reporting NES (FDR < 0.10 for all three Gapmers) of cancer hallmarks (GSEA) for genes differentially expressed in Gapmer-transfected H520 cells (allGap = all Gapmers vs NEG). E Heatmap reporting NES (FDR < 0.10) of cancer hallmarks (GSEA) for genes ranked for correlation with NFYC-AS1 in LUSC cells (CCLE). F GSEA plots of genes related to lung cancer vulnerabilities [34] or G synthetic lethal in RB1-mut cells [35] in genes modulated upon NFYC-AS1 Gapmer-silencing. H Bar plot showing NFYC-AS1 expression (qRT-PCR) at 48 h after 25 nM Gapmer transfection in H82 RB1-mut cells. Data are NEG-normalized and reported as mean ± sd, as from n = 4 independent biological replicates. I Cell counts at different time points after NFYC-AS1 Gapmer-knockdown, normalized against NEG at 72 h in H82 cells. Data reported as mean ± se, as from n = 6 independent biological replicates. J Progression-free survival curve stratified according to Cutoff Finder-determined threshold (63.72) for NFYC-AS1 in LUSC patients (TCGA). Log-rank test p-value and hazard risk (HR) are shown. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns (non-significant). One sample t-test p-values are reported for panels A, B, H, and I.
To frame transcriptome changes induced by NFYC-AS1 silencing, RNA-seq was performed on cells treated with GAP2, GAP3, and GAP4. GSEA analysis performed on ranked gene lists from individual Gapmers and all Gapmers together (compared to NEG) revealed significant enrichment of proliferation/cell cycle-related gene sets, such as E2F targets, G2/M checkpoint and mTORC1 signaling in downregulated genes (Fig. 4D). Following a guilt-by-association approach, the same gene sets were markedly enriched in genes positively correlated with NFYC-AS1 in LUSC cells (Fig. 4E), suggesting a physiological link between the asRNA and proliferation-related programs.
Interestingly, genes downregulated upon NFYC-AS1 knockdown were enriched in possible actionable therapeutic targets for all lung cancer histotypes, as identified from DepMap vulnerabilities [34] (Fig. 4F). A significant enrichment in genes that are synthetic lethal in RB1-mut SCLC cells was also observed (signatures from Oser et al. [35].) (Fig. 4G), suggesting that NFYC-AS1 inhibition may have therapeutic effects in SCLC.
To test this hypothesis, we used Gapmers to silence NFYC-AS1 in RB1-mut H82 cells. The in-suspension growth pattern and the poor transfectability of these cells allowed only very modest knockdown with 5 nM Gapmers (Supplementary Fig. S4D), whereas 25 nM of the most effective Gapmers, GAP5 and GAP6, recapitulated NFYC-AS1 repression obtained in H520 cells (Fig. 4H). In this setting, both Gapmers significantly reduced H82 cell growth at all time points, even if at a lesser extent as compared to H520 cells (Fig. 4I).
In accord with the observed growth-supporting function of NFYC-AS1, survival analyses revealed that its higher expression in tumors is associated with significantly increased risk of disease progression in LUSC patients (Fig. 4J) and a trend for decreased overall survival in SCLC patients (Supplementary Fig. S4E).
NFYC-AS1 genetic editing recapitulates growth impairment phenotype revealing a role in mitotic progression
Given that NFYC-AS1 knock-down by Gapmers results from RNA cleavage and that asRNAs may also work in a transcription- rather than RNA-dependent manner [3], we attempted to block NFYC-AS1 transcription through a CRISPR/Cas9-based approach. Since the removal of the whole NFYC-AS1 sequence would disrupt NFYC promoter, we designed two sgRNAs to selectively delete the −independently regulated as from our previous assessments (Fig. 3A)− NFYC-AS1 TSS from NFYC first intron (Fig. 5A) in H520 cells. Several edited heterozygous clones (ΔTSS) were isolated and confirmed to express markedly lower levels of the asRNA as compared to non-edited clones (WT) (Fig. 5B). ΔTSS cells showed impaired cell growth (Fig. 5C), recapitulating the phenotype observed in Gapmer-transfected cells. Similarly, comparison of transcriptional profiles of four independent ΔTSS clones with three independent WT clones revealed significant downregulation of E2F targets, G2/M checkpoint genes, and mTORC1 signaling (Fig. 5D). The most concordant NES among ASO and genetic editing experiments was found for G2/M checkpoint and UV response down gene sets within down- and upregulated genes, respectively (Fig. 5E). Notably, leading edge genes of both gene sets (Supplementary Table S12) showed inverse patterns of expression in the comparison between tumor and normal samples in LUAD, LUSC and SCLC cohorts (Fig. 5F). In particular, up to 98% of G2/M genes downregulated upon NFYC-AS1 silencing showed significant upregulation in all cancer histotypes, suggesting that NFYC-AS1 impacts on cancer-relevant targets. Reactome pathways analyses highlighted that downregulated genes in both comparisons were clearly enriched for gene sets related to mitotic segregation (Supplementary Fig. S5B). In line with these findings, FACS analysis showed that ΔTSS clones tend to accumulate in G2/M cell cycle phase, as compared to parental cells or WT clones, suggesting defects in mitotic progression (Fig. 5G). Accordingly, deleted clones showed increased cyclin B1 protein levels, which is considered a marker for cells stuck in G2/M phase (Fig. 5H).
A Schematic representation of the CRISPR/Cas9 strategy employed for NFYC-AS1 TSS deletion (primers used to check for the deletion are shown in Fig. 3A and PCR results in Supplementary Fig. S5A). B Bar plot showing NFYC-AS1 expression measured through qRT-PCR using primer 4 (Pr4) in four non-deleted (WT) and six deleted (ΔTSS) H520 clones. Data are reported as mean ± se, as from n = 3 independent qRT-PCR measurements. Clones subjected to RNA-seq (chosen among those having the most significant down-regulation of NFYC-AS1) are indicated in bold. One sample t-test p-values are reported. C Cell counts of WT and ΔTSS H520 clones at 72 h, 96 h and 120 h after plating, normalized against the average cell number in WT clones at 72 h. Data are reported as mean ± se, as from n = 3 independent biological replicates. Two-tailed unpaired t-test p-values are reported. D Heatmap of significant NES (FDR < 0.10) of cancer hallmarks (GSEA) for genes differentially expressed in ΔTSS clones compared with WT clones. E Comparative bubble plot of significant NES (FDR < 0.10) of cancer hallmarks (GSEA) for differentially expressed genes in Gapmer-treated H520 and in ΔTSS clones. Bubble size is proportional to the FDR (-log10FDR) of all Gapmers vs NEG comparison. F Heatmap of the fold-change (log2FC) of common leading-edge genes of UV response DN and G2/M checkpoint gene sets in all the Gapmers (vs NEG) and in ΔTSS clones (vs WT) and the relative tumor/normal ratio (expressed as log2FC) in LUAD, LUSC (TCGA), and SCLC (GSE60052). G Cell cycle analysis by FACS of parental H520 cells, ΔTSS and WT clones. Data are reported as mean ± sd, as from n = 2 independent biological replicates. Two-tailed unpaired t-test p-values are reported. H Representative western blot analysis of cyclin B1 in ΔTSS (n = 3) and WT (n = 3) clones. Vinculin was used as a loading control. Unpaired two-tailed t-test p-values are reported. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns (non-significant).
Different knockdown approaches shed light on NFYC-AS1 dual mode of action
Cell growth impairment consequent to NFYC-AS1 silencing could be compatible with in cis interference with its overlapping sense gene NFYC, since NF-Y inhibition was shown to trigger proliferation-related defects, including repression of G2/M genes [36]. However, no major effect on NFYC expression was found upon Gapmer treatment of H520 cells either at the mRNA (Fig. 6A) or protein level (Supplementary Fig. S6A), regardless of the extent of NFYC-AS1 repression by the different Gapmers or time points (Supplementary Fig. S6B). The same result was recapitulated in H82 cells treated with Gapmers at either dose (Supplementary Fig. S6C). In contrast, NFYC mRNA was significantly upregulated in ΔTSS clones (2 to 3-fold increase), as measured by RNA-seq and qRT-PCR (Fig. 6B), though no substantial changes of protein levels were observed (Supplementary Fig. S6A). When NFYC transcripts originating from the two main TSSs were analyzed separately, only those transcribed from the canonical NFYC TSS (i.e., proximal to NFYC-AS1) resulted significantly upregulated in ΔTSS clones, whereas no changes were recorded for those transcribed from the alternative NFYC TSS in either clones or Gapmer-transfected cells (Fig. 6C, D). Increase of NFYC mRNA in ΔTSS clones was ascribable to enhanced transcription, as evidenced by the analysis of NFYC primary transcript (Fig. 6E).
A Bar plot showing NFYC expression level (qRT-PCR and RNA-seq) at 48 h after transfection with GAP2-GAP4 in H520 RB1-wt cells. Data are NEG-normalized and reported as mean ± sd, as from n = 4 independent biological replicates. B Bar plot showing NFYC expression level (qRT-PCR and RNA-seq) in ΔTSS H520 clones normalized against WT H520 clones. Data are reported as mean ± sd, as from n = 4 WT clones and n = 4 ΔTSS clones in qRT-PCR and n = 3 WT clones and n = 4 ΔTSS clones in RNA-seq. C Bar plot showing NFYC canonical and alternative expression levels (RNA-seq) at 48 h after transfection with GAP2-GAP4 in H520 RB1-wt cells. Data are NEG-normalized and reported as mean ± sd, as from n = 4 independent biological replicates. D Bar plot showing NFYC canonical and alternative expression levels (RNA-seq) in ΔTSS H520 clones normalized against WT H520 clones. Data are reported as mean ± sd, as from n = 3 WT clones and n = 4 ΔTSS clones. E Bar plot showing NFYC primary transcript expression measured using an intronic primer through qRT-PCR in ΔTSS H520 clones normalized against WT H520 clones. Data are reported as mean ± sd, as from n = 3 WT clones and n = 4 ΔTSS clones. F Scatter plot of effect sizes of eQTLs shared by NFYC and NFYC-AS1. G Time course of NFYC-AS1, NFYC, and CMYC expression levels (qRT-PCR) after re-entry of H520 cells into the cell-cycle. Data are reported as mean ± sd, as from n = 3 independent biological replicates. H Time course of NFYC-AS1/NFYC relative induction ratio after re-entry of H520 cells into the cell-cycle. Data are reported as mean ± sd, as from n = 3 independent biological replicates. Two-tailed unpaired t-test p-values are reported for panels A–E. deseq2 adjusted p-values are reported for panels A and B. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns (non-significant).
Altogether, these findings would suggest that in cis regulation of NFYC may rely on transcription-dependent mechanisms. Consistent with this, orthogonal approaches inducing NFYC-AS1 transcriptional knockdown, such as impairment of NF-Y activity by NFYB silencing, resulted in markedly increased NFYC levels, mainly due to upregulation of canonical TSS-derived isoforms (Fig. 3B).
Indirect evidence supporting the transcriptional interference existing between NFYC-AS1 and NFYC was obtained by the analysis of expression quantitative trait loci (eQTL). We leveraged significant variant-gene associations in different tissues (GTEx v8) [37] and identified 231 variants that are eQTLs for NFYC-AS1. Interestingly, 60.2% were eQTLs also for NFYC and all of them consistently showed an opposite effect on NFYC expression (Fig. 6F).
To get further validation of the transcriptional interference effect in a physiological setting, we assessed the reciprocal expression of NFYC-AS1 and NFYC during the cell-cycle. We found that, upon re-entry after synchronization, cells correctly progressed through the cell cycle, as shown by modulations in cyclin levels (Supplementary Fig. S6D). NFYC-AS1 expression peaked in the early G1 phase, similar to the early spike gene C-MYC [38], then decreased to remain stable across the following cell cycle phases (Fig. 6G). NFYC mRNA decreased concomitantly to NFYC-AS1 induction (Fig. 6G), then started to increase immediately after NFYC-AS1 peak with reversal of sense/antisense fold-change ratio at the G1/S boundary (Fig. 6H), compatible with a transcriptional interference. Notably, this pattern was recapitulated when cells synchronized in the different cell cycle phases as from Hao et al. [39]. (Supplementary Fig. S6E) were analyzed.
The observation that cell growth decline invariably occurred upon NFYC-AS1 silencing with both Gapmers and TSS deletion suggests that this phenotype is i) at least in part NFYC-independent and ii) mediated by RNA- rather than transcription-dependent mechanisms. In support of the first hypothesis, TransCistor tool [40] predicted a predominantly in trans function from both Gapmer and ΔTSS RNA-seq data (Supplementary Fig. S6F). Independent validation of NFYC-AS1 RNA-dependent role in the cell cycle was obtained by interrogating an RNAi-based screening for lncRNAs involved in cell division [41]. When analyzing the mitotic index (i.e., number of cells stuck in mitosis), z-score of NFYC-AS1 appeared in line with (or superior to) that of other lncRNAs demonstrated to regulate mitotic progression, such as NORAD1 [42] or LY6K-AS [43] (Fig. 7A).
A Bar plot showing the average mitotic index z-score ± sd of the two screenings as from Stojic et al. [41] for a panel of selected lncRNAs. B GSEA enrichment plots of FISCHER G1/S and G2/M CELL CYCLE, and WHITFIELD G1/S and G2/M CELL CYCLE gene sets in genes modulated upon NFYC-AS1 Gapmer-silencing. NES and FDR values are indicated in each plot. C GSEA enrichment plots of FOXM1 and E2F4 bound genes (defined as described in Supplementary Materials and Methods section) in genes modulated upon NFYC-AS1 Gapmer-silencing. NES and FDR values are indicated in each plot. D Schematic representation of the in cis and in trans possible effects, mechanism(s) of action and biological role of NFYC-AS1.
Consistent with this, genes repressed by Gapmer treatment (the optimum to selectively visualize in trans-mediated effects) were significantly enriched in G2/M and not in G1/S genes (Fig. 7B) and displayed a highly significant enrichment of TF binding sites (defined based on ChIP-seq signal and presence of consensus motif in the promoter) of complexes involved in G1/S gene repression (i.e., E2F4/5-DREAM) and G2/M gene activation (i.e., MYBL2, FOXM1) [44], with moderate or no enrichment for activator E2Fs or NF-Y, respectively (Fig. 7C and Supplementary Fig. S6G).
Discussion
The identification of asRNAs that could serve as molecular targets in cancer therapy requires the careful study of their expression in tumors and association with molecular/clinical features, as well as a deep understanding of their function. Here, we showed that NFYC-AS1 is an asRNA overexpressed pancancer, with striking upregulation in the very aggressive RB1-mut tumors. Significant discriminative power in distinguishing tumor from normal tissues as well as molecular subtypes (RB1-mut vs -wt) may suggest NFYC-AS1 as a potential cancer biomarker.
We found that NFYC-AS1 depletion by either ASOs or CRISPR/Cas9 results in cell growth impairment in both RB1-wt and -mut cancer cells, mainly due to downregulation of G2/M cell cycle phase genes. The cancer-specific NFYC-AS1 upregulation, which can be partly explained by transcriptional activation by NF-Y or E2Fs as a consequence of RB1 mutations, may indeed exacerbate cell proliferation by facilitating mitotic progression.
From a therapeutic perspective, NFYC-AS1 knockdown simultaneously repressed genes that represent vulnerabilities of all lung cancer histotypes. Consistent with this model, NFYC-AS1 silencing impairs cell growth in both LUSC and SCLC cells, as reported in this study, and induces apoptosis in LUAD cells, as shown in the work by Song and colleagues [19]. Moreover, NFYC-AS1 knockdown inhibited genes that are synthetic lethal in RB1-mut cells, suggesting a therapeutic potential in the very aggressive RB1-mut tumors, for which current treatments are often unsuccessful [45]. Moreover, defects in mitotic progression arising from NFYC-AS1 depletion may create new vulnerabilities, which can be particularly deleterious in RB1-defective cancers, as already shown for Aurora B kinase inhibitors [35]. At this stage, we cannot conclude whether the apparently lower sensitivity of H82 cells to NFYC-AS1 knockdown as compared to H520 cells is the result of an intrinsically higher resistance of RB1-mut cells or is rather related to the different cell growth pattern. In this regard, studies using isogenic models of RB1-mut and -wt cells are warranted to address this point.
From a mechanistic point of view, our data suggest that NFYC-AS1 may have a dual mode of action (Fig. 7D): it represses its sense gene NFYC in cis in a transcription-dependent manner, while it regulates G2/M cell cycle phase genes in trans in a transcription- and apparently NFYC-independent manner. In this regard, being completely in line with those of other lncRNAs, the observed levels of conservation of NFYC-AS1 locus would support the relevance of sequence-based mechanisms. It is not surprising that asRNAs overlapping with sense genes may also (or even only) work in trans, as shown for GNG12-AS1 which represses its sense gene in cis via transcriptional interference, while it regulates other genes involved in cell proliferation and migration in trans through an RNA-dependent mechanism [46].
Regarding NFYC-AS1 in cis activity, we conclude it is mediated by a transcription-dependent mechanism as it becomes evident only when asRNA transcription is affected (e.g., CRISPR/Cas9-editing, NF-Y depletion, or cell cycle re-entry assay). Specifically, NFYC-AS1 seems to antagonize NFYC expression, likely through transcriptional interference. An opposite expression pattern between the two transcripts is also evident in different human tumors as compared to matched healthy tissues and in eQTL analysis. At this stage, mechanisms relying on NFYC-AS1 transcript, such as the formation of R-loops with DNA elements in NFYC promoter [47], pairing with NFYC pre-mRNA [48], or interaction with protein factors are unlikely to occur, as the in cis effect was not recapitulated by any of the ASOs spanning the whole asRNA sequence. Transcriptional overlap mechanism proposed for Airn [49] is also improbable, as cleavage of NFYC-AS1 just downstream of its TSS by GAP2 does not relieve repression on NFYC. Only mechanisms strictly associated with the act of transcription per se, such as RNA pol-II dislodgement/occlusion/collision/roadblock events (reviewed in Zhao et al. [11]), the creation of a repressive chromatin state by epigenetic histone modifications [10, 50] or nucleosome re-positioning [51], might be compatible with our findings. In light of our ΔTSS model, DNA-dependent or topological effects, as shown for HASTER promoter on HNF1A expression [52], cannot be completely excluded. However, approaches commonly used to manipulate endogenous lncRNA transcription, such as CRISPR-activation or inhibition, are not applicable to NFYC-AS1, as unintended effects on the adjacent NFYC TSS are likely to bias results [53]. Biologically, NFYC-AS1 may serve to finely tune NFYC transcription timing at the beginning of the cell cycle and/or contribute to the negative feedback loop existing between NFYB and NFYC [54] (Fig. 7D). It was indeed shown that NFYB knockdown results in increased NFYC mRNA levels (also evident in Fig. 3B), which could be explained by direct activation of NFYC-AS1 by NF-Y and subsequent impaired NFYC transcription. Observed patterns of sequence conservation and stretches of MIR at the NFYC-AS1 locus are suggestive of a mammalian evolutionary origin, with similar considerations applying to the canonical NFYC TSS. This arrangement might represent an additional layer of regulation of NFYC transcription, which instead should lack in other species having only the ancestral TSS, regulated directly by NF-Y.
Regarding NFYC-AS1 in trans mode of action, it appears to converge on the regulation of G2/M cell cycle phase genes, in trend with the mitotic arrest phenotype observed in ΔTSS cells and in Stojic’s RNAi-based screening [41]. Further investigation will be required to define i) which among these genes are direct or indirect targets, ii) which is the exact amount of the asRNA in the cell (i.e. copies per cell) and its subnuclear distribution, and iii) whether RNA-dependent effects are mediated by NFYC-AS1 sequence or 3D structure, as well as the nature of its interactors. In this regard, the insertion of repeated elements, such as the presence of SINE/Alu at NFYC-AS1 3’ end (Fig. 3A), may have endowed it with a novel in trans function. Transposable elements have been proven to act as functional modules for a number of lncRNAs [7, 55], including MIR205HG, which we showed to use an Alu element to physically interact with Alu elements in target gene promoters [56, 57]. NFYC-AS1 may interact with direct targets through RNA/RNA pairing to affect mRNA processing/stability or RNA/DNA interaction with their regulatory regions, in the form of triplexes or R-loops. Indirect targets may instead be regulated via an intermediate factor (e.g. TF or RBP), itself physically bound/stabilized/sequestered by NFYC-AS1. LncRNAs can indeed directly interact with TFs, as shown for PANDA that acts as a molecular decoy for NF-YA, thereby titrating the TF away from its targets [58]. LncRNAs can also function as RNA guides to facilitate TF interaction with specific genes, as is the case of SLC16A1-AS1, which simultaneously acts as guide and chaperone/coactivator for E2F1 [59]. All the scenarios could be interrogated to dissect NFYC-AS1 mechanism of action in trans. Speculatively speaking, NFYC-AS1 might interact with E2F4/5 for the correct assembly/stability/activity of the DREAM complex to silence G2/M cell cycle genes during G1/S phase or sequester FOXM1 in G1/S phase until its expression decreases releasing FOXM1 to allow activation of G2/M genes (Fig. 7D). Given that NF-Y cooperates with these TFs in the regulation of common targets [60], NFYC-AS1 may also tune NF-Y function by altering TF multicomplex assembly, cooperativity, and genome-wide co-occupancy, especially on selected subsets of targets.
A hypothesis conciliating NFYC-AS1 dual modes of action could be that NF-Y (and/or RB1/E2F axis) induces NFYC-AS1 expression to i) finely tune NFYC transcription in cis in a negative feedback loop circuit and ii) simultaneously in trans stimulate the activity of known NF-Y partners on G2/M-specific genes via chaperoning DREAM/MYBL2/FOXM1 activity (Fig. 7D). Intriguing could be to evaluate whether the in cis fine-tuning effect may prevail in normal cells at low NFYC-AS1 concentrations, whereas the in trans effect may become predominant in tumor cells to support excessive proliferation and mitotic progression.
Overall, we showed that NFYC-AS1 is an optimal candidate to be evaluated in the long term as a new target entity in different cancer types, including the very aggressive RB1-mut tumors. Moreover, the dissection of NFYC-AS1 mechanism of action and the identification of its interactors may provide a rich environment of actionable protein-coding genes that can be targeted with already existing drugs.
Data availability
All data used in this work can be acquired from the TCGA database (http://cancergenome.nih.gov/), ENCODE database (http://encodeproject.org/), CCLE database (http://sites.broadinstitute.org/ccle), Gene Expression Omnibus (GEO) datasets (https://www.ncbi.nlm.nih.gov/geo/), GTEx project (https://gtexportal.org/home/). Raw and processed RNA-seq data of H520 cells transfected with Gapmer ASOs or deleted for NFYC-AS1 TSS have been deposited at GEO under accession number GSE240468 and are available from the corresponding author upon reasonable request.
References
Amaral P, Carbonell-Sala S, De La Vega FM, Faial T, Frankish A, Gingeras T, et al. The status of the human gene catalogue. Nature. 2023;622:41–7.
Frankish A, Carbonell-Sala S, Diekhans M, Jungreis I, Loveland JE, Mudge JM, et al. GENCODE: reference annotation for the human and mouse genomes in 2023. Nucleic Acids Res. 2023;51:D942–9.
Pagani G, Pandini C, Gandellini P. Navigating the multiverse of antisense RNAs: The transcription- and RNA-dependent dimension. Non-Cod RNA. 2022;8:74.
Wery M, Gautier C, Descrimes M, Yoda M, Vennin-Rendos H, Migeot V, et al. Native elongating transcript sequencing reveals global anti-correlation between sense and antisense nascent transcription in fission yeast. RNA. 2018;24:196–208.
Gil N, Ulitsky I. Regulation of gene expression by cis-acting long non-coding RNAs. Nat Rev Genet. 2020;21:102–17.
Pelechano V, Steinmetz LM. Gene regulation by antisense transcription. Nat Rev Genet. 2013;14:880–93.
Zucchelli S, Cotella D, Takahashi H, Carrieri C, Cimatti L, Fasolo F, et al. SINEUPs: A new class of natural and synthetic antisense long non-coding RNAs that activate translation. RNA Biol. 2015;12:771–9.
Rey F, Pandini C, Messa L, Launi R, Barzaghini B, Zangaglia R, et al. α-Synuclein antisense transcript SNCA-AS1 regulates synapses- and aging-related genes suggesting its implication in Parkinson’s disease. Aging Cell. 2021;20:e13504.
Rey F, Maghraby E, Messa L, Esposito L, Barzaghini B, Pandini C, et al. Identification of a novel pathway in sporadic Amyotrophic Lateral Sclerosis mediated by the long non-coding RNA ZEB1-AS1. Neurobiol Dis. 2023;178:106030.
Cipriano A, Macino M, Buonaiuto G, Santini T, Biferali B, Peruzzi G, et al. Epigenetic regulation of Wnt7b expression by the cis-acting long noncoding RNA Lnc-Rewind in muscle stem cells. Lee JT, Struhl K, Khalil AS, editors. eLife. 2021 Jan 12;10:e54782.
Zhao S, Zhang X, Chen S, Zhang S. Natural antisense transcripts in the biological hallmarks of cancer: powerful regulators hidden in the dark. J Exp Clin Cancer Res. 2020;39:187.
Balbin OA, Malik R, Dhanasekaran SM, Prensner JR, Cao X, Wu YM, et al. The landscape of antisense gene expression in human cancers. Genome Res. 2015;25:1068–79.
Mercer TR, Munro T, Mattick JS. The potential of long noncoding RNA therapies. Trends Pharm Sci. 2022;43:269–80.
Tassinari M, Richter SN, Gandellini P. Biological relevance and therapeutic potential of G-quadruplex structures in the human noncoding transcriptome. Nucleic Acids Res. 2021;49:3617–33.
Warner KD, Hajdin CE, Weeks KM. Principles for targeting RNA with drug-like small molecules. Nat Rev Drug Discov. 2018;17:547–58.
Khorkova O, Stahl J, Joji A, Volmar CH, Zeier Z, Wahlestedt C. Natural antisense transcripts as drug targets. Front Mol Biosci. 2022;9:978375.
Dolfini D, Gatta R, Mantovani R. NF-Y and the transcriptional activation of CCAAT promoters. Crit Rev Biochem Mol Biol. 2012;47:29–49.
Gurtner A, Manni I, Piaggio G. NF-Y in cancer: Impact on cell transformation of a gene essential for proliferation. Biochim Biophys Acta BBA - Gene Regul Mech. 2017;1860:604–16.
Song Y, Du J, Lu P, Zou Q, Zeng S, Liu M, et al. LncRNA NFYC-AS1 promotes the development of lung adenocarcinomas through autophagy, apoptosis, and MET/c-Myc oncogenic proteins. Ann Transl Med. 2021;9:1621.
Tong F, Xu L, Xu S, Zhang M. Identification of an autophagy-related 12-lncRNA signature and evaluation of NFYC-AS1 as a pro-cancer factor in lung adenocarcinoma. Front Genet. 2022;13:834935.
Weinstein JN, Collisson EA, Mills GB, Shaw KRM, Ozenberger BA, Ellrott K, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113–20.
Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–7.
Wilkerson MD, Yin X, Hoadley KA, Liu Y, Hayward MC, Cabanski CR, et al. Lung squamous cell carcinoma mRNA expression subtypes are reproducible, clinically important and correspond to different normal cell types. Clin Cancer Res. 2010;16:4864–75.
George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47–53.
Ireland AS, Micinski AM, Kastner DW, Guo B, Wait SJ, Spainhower KB, et al. MYC drives temporal evolution of small cell lung cancer subtypes by reprogramming neuroendocrine fate. Cancer Cell. 2020;38:60–78.e12.
Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012;483:603–7.
Sonkin D, Thomas A, Teicher BA Are neuroendocrine negative small cell lung cancer and large cell neuroendocrine carcinoma with WT RB1 two faces of the same entity? Lung Cancer Manag. 8:LMT13.
Lizio M, Harshbarger J, Shimoji H, Severin J, Kasukawa T, Sahin S, et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol. 2015;16:22.
Herrmann CJ, Schmidt R, Kanitz A, Artimo P, Gruber AJ, Zavolan M. PolyASite 2.0: a consolidated atlas of polyadenylation sites from 3′ end sequencing. Nucleic Acids Res. 2020;48:D174–9.
Miller DM, Thomas SD, Islam A, Muench D, Sedoris K. c-Myc and cancer metabolism. Clin Cancer Res. 2012;18:5546–53.
Ceribelli M, Benatti P, Imbriano C, Mantovani R. NF-YC complexity is generated by dual promoters and alternative splicing. J Biol Chem. 2009;284:34189–200.
Le Béguec C, Wucher V, Lagoutte L, Cadieu E, Botherel N, Hédan B, et al. Characterisation and functional predictions of canine long non-coding RNAs. Sci Rep. 2018;8:13444.
Oliver KR, Greene WK. Transposable elements: powerful facilitators of evolution. BioEssays. 2009;31:703–14.
Sauta E, Reggiani F, Torricelli F, Zanetti E, Tagliavini E, Santandrea G, et al. CSNK1A1, KDM2A, and LTB4R2 are new druggable vulnerabilities in lung cancer. Cancers. 2021;13:3477.
Oser MG, Fonseca R, Chakraborty AA, Brough R, Spektor A, Jennings RB, et al. Cells lacking the RB1 tumor suppressor gene are hyperdependent on Aurora B kinase for survival. Cancer Discov. 2019;9:230–47.
Hu Q, Lu JF, Luo R, Sen S, Maity SN. Inhibition of CBF/NF-Y mediated transcription activation arrests cells at G2/M phase and suppresses expression of genes activated at G2/M phase of the cell cycle. Nucleic Acids Res. 2006;34:6272–85.
Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, et al. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–5.
Morrish F, Neretti N, Sedivy JM, Hockenbery DM. The oncogene c-Myc coordinates regulation of metabolic networks to enable rapid cell cycle entry. Cell Cycle. 2008;7:1054–66.
Hao Q, Zong X, Sun Q, Lin YC, Song YJ, Hashemikhabir S, et al. The S-phase-induced lncRNA SUNO1 promotes cell proliferation by controlling YAP1/Hippo signaling pathway. Davis RJ, Struhl K, editors. eLife. 2020 Oct 27;9:e55102.
Dhaka B, Zimmerli M, Hanhart D, Moser MB, Guillen-Ramirez H, Mishra S, et al. Functional identification of cis-regulatory long noncoding RNAs at controlled false discovery rates. Nucleic Acids Res. 2024 Feb 13:gkae075.
Stojic L, Lun ATL, Mascalchi P, Ernst C, Redmond AM, Mangei J, et al. A high-content RNAi screen reveals multiple roles for long noncoding RNAs in cell division. Nat Commun. 2020;11:1851.
Elguindy MM, Mendell JT. NORAD-induced Pumilio phase separation is required for genome stability. Nature. 2021;595:303–8.
Ali MM, Di Marco M, Mahale S, Jachimowicz D, Kosalai ST, Reischl S, et al. LY6K-AS lncRNA is a lung adenocarcinoma prognostic biomarker and regulator of mitotic progression. Oncogene. 2021;40:2463–78.
Fischer M, Schade AE, Branigan TB, Müller GA, DeCaprio JA. Coordinating gene expression during the cell cycle. Trends Biochem Sci. 2022;47:1009–22.
Jiang Z, Jones R, Liu JC, Deng T, Robinson T, Chung PED, et al. RB1 and p53 at the crossroad of EMT and triple-negative breast cancer. Cell Cycle. 2011;10:1563–70.
Stojic L, Niemczyk M, Orjalo A, Ito Y, Ruijter AEM, Uribe-Lewis S, et al. Transcriptional silencing of long noncoding RNA GNG12-AS1 uncouples its transcriptional and product-related functions. Nat Commun. 2016;7:10406.
Boque-Sastre R, Soler M, Oliveira-Mateos C, Portela A, Moutinho C, Sayols S, et al. Head-to-head antisense transcription and R-loop formation promotes transcriptional activation. Proc Natl Acad Sci. 2015;112:5785–90.
Pan K, Xie Y. LncRNA FOXC2-AS1 enhances FOXC2 mRNA stability to promote colorectal cancer progression via activation of Ca2+-FAK signal pathway. Cell Death Dis. 2020;11:1–14.
Latos PA, Pauler FM, Koerner MV, Şenergin HB, Hudson QJ, Stocsits RR, et al. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science. 2012;338:1469–72.
Wery M, Gautier C, Descrimes M, Yoda M, Migeot V, Hermand D, et al. Bases of antisense lncRNA-associated regulation of gene expression in fission yeast. PLOS Genet. 2018;14:e1007465.
Martens JA, Laprade L, Winston F. Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature. 2004;429:571–4.
Beucher A, Miguel-Escalada I, Balboa D, De Vas MG, Maestro MA, Garcia-Hurtado J, et al. The HASTER lncRNA promoter is a cis-acting transcriptional stabilizer of HNF1A. Nat Cell Biol. 2022;24:1528–40.
Goyal A, Myacheva K, Groß M, Klingenberg M, Duran Arqué B, Diederichs S. Challenges of CRISPR/Cas9 applications for long non-coding RNA genes. Nucleic Acids Res. 2017;45:e12.
Belluti S, Semeghini V, Basile V, Rigillo G, Salsi V, Genovese F, et al. An autoregulatory loop controls the expression of the transcription factor NF-Y. Biochim Biophys Acta BBA - Gene Regul Mech. 2018;1861:509–18.
Johnson R, Guigó R. The RIDL hypothesis: transposable elements as functional domains of long noncoding RNAs. RNA. 2014;20:959–76.
Profumo V, Forte B, Percio S, Rotundo F, Doldi V, Ferrari E, et al. LEADeR role of miR-205 host gene as long noncoding RNA in prostate basal cell differentiation. Nat Commun. 2019;10:307.
Bezzecchi E, Pagani G, Forte B, Percio S, Zaffaroni N, Dolfini D, et al. MIR205HG/LEADR long noncoding RNA binds to primed proximal regulatory regions in prostate basal cells through a triplex- and alu-mediated mechanism. Front Cell Dev Biol. 2022;10:909097.
Puvvula PK, Desetty RD, Pineau P, Marchio A, Moon A, Dejean A, et al. Long noncoding RNA PANDA and scaffold-attachment-factor SAFA control senescence entry and exit. Nat Commun. 2014;5:5323.
Logotheti S, Marquardt S, Gupta SK, Richter C, Edelhäuser BAH, Engelmann D, et al. LncRNA-SLC16A1-AS1 induces metabolic reprogramming during Bladder Cancer progression as target and co-activator of E2F1. Theranostics. 2020;10:9620–43.
Dolfini D, Zambelli F, Pedrazzoli M, Mantovani R, Pavesi G. A high definition look at the NF-Y regulome reveals genome-wide associations with selected transcription factors. Nucleic Acids Res. 2016;44:4684–702.
Acknowledgements
The authors thank Marco Folini, Paola Perego, Nadia Zaffaroni from Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy for sharing the H520 and H82 cell lines; Diletta Dolfini, Nerina Gnesutta and all the members of Roberto Mantovani’s group from the University of Milan for sharing materials and protocols and for the constructive discussion; Federica Rotundo, previous lab member, for helping in the initial phases of the project.
Funding
The research leading to these results has received funding from AIRC under IG 2020 - ID. 24325 – P.I. Gandellini Paolo and from European Union—NextGenerationEU (PNRR M4C2-Investimento 1.4 -CN00000041-PNRR_CN3RNA_SPOKE2; Mantovani Roberto).
Author information
Authors and Affiliations
Contributions
CP, GP acquired and interpreted data, and drafted the manuscript; MT, EV, EB, MKS, VD, GG, MC acquired data and revised the manuscript; RM, AC contributed to data interpretation and revision of the manuscript; PG conceived and supervised the work, acquired, and interpreted data, and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Dr. Alessandro Rufini
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pandini, C., Pagani, G., Tassinari, M. et al. The pancancer overexpressed NFYC Antisense 1 controls cell cycle mitotic progression through in cis and in trans modes of action. Cell Death Dis 15, 206 (2024). https://doi.org/10.1038/s41419-024-06576-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-024-06576-y