Abstract
RNA-binding proteins (RBPs) modulate the expression level of several target RNAs (such as mRNAs) post-transcriptionally through interactions with unique binding sites in the 3′-untranslated region. There is mounting information that suggests RBP dysregulation plays a significant role in carcinogenesis. However, the function of FMR1 autosomal homolog 1(FXR1) in malignancies is just beginning to be unveiled. Due to the diversity of their RNA-binding domains and functional adaptability, FXR1 can regulate diverse transcript processing. Changes in FXR1 interaction with RNA networks have been linked to the emergence of cancer, although the theoretical framework defining these alterations in interaction is insufficient. Alteration in FXR1 expression or localization has been linked to the mRNAs of cancer suppressor genes, cancer-causing genes, and genes involved in genomic expression stability. In particular, FXR1-mediated gene regulation involves in several cellular phenomena related to cancer growth, metastasis, epithelial-mesenchymal transition, senescence, apoptosis, and angiogenesis. FXR1 dysregulation has been implicated in diverse cancer types, suggesting its diagnostic and therapeutic potential. However, the molecular mechanisms and biological effects of FXR1 regulation in cancer have yet to be understood. This review highlights the current knowledge of FXR1 expression and function in various cancer situations, emphasizing its functional variety and complexity. We further address the challenges and opportunities of targeting FXR1 for cancer diagnosis and treatment and propose future directions for FXR1 research in oncology. This work intends to provide an in-depth review of FXR1 as an emerging oncotarget with multiple roles and implications in cancer biology and therapy.
Similar content being viewed by others
Facts
-
FXR1s modulate RNA metabolism and are correlated with the development of malignancy.
-
Alteration in FXR1 expression and function enhance the activities of cancer driver genes, promote tumor development, and trigger malignant behavior.
-
New technologies, in conjunction with genetically engineered animal models, are assisting in the discovery of FXR1 molecular pathways in cancer.
-
Understanding the FXR1 functions in cancer cells will help develop biomarkers for prognosis, as well as possibly reveal new targets for the design of therapies.
Open questions
-
What role does FXR1 play in cancer progression through the regulation of RNA stability?
-
How to decipher the network of intricate connections between FXR1 and cancer-related mRNA?
-
How can the dynamic function of FXR1 in cancer development be overcome?
-
What role does FXR1 play in the coordination of cancer phenotypes across various genetic backgrounds?
-
How can we best target FXR1 as a molecular biomarker or therapeutic target in the field of tumor biology?
Introduction
RNA binding proteins (RBPs) carry out a wide-ranging and critical function in RNA metabolism. RBPs interact with specific RNAs’ forming ribonucleoprotein (RNP) compounds. Hence, regulating gene expression processes like cleavage and polyadenylation, RNA splicing, export, stability, translation, and the degradation of coding- and non-coding- RNAs, as well as their precursors [1,2,3,4,5]. RBPs execute their role by interacting with proteins and various classes of RNA (mRNAs, snoRNA, snRNA, tRNAs, ribosomal RNAs, and non-coding RNAs), regulating their fate and functions [6, 7]. So far, Over 1500 RBPs have been discovered in the entire human genome using recent high-throughput screening, which accounts for ~7–8% of all proteins encoded by genes: though only a few of them have been functionally described [8]. RBPs have important functions in various stages of gene regulation; therefore, alterations in their expression or mutations can frequently affect various pathological and physiological processes that lead to diseases, including malignancies [9,10,11,12]. RBPs interact with their respective RNA targets via RNA binding domains (RBDs). Approximately 50 RBDs have been discovered, including RNA-interacting protein domain, zinc finger domain, PAZ domains, heterogeneous nuclear RNP K-homology domains, RNA recognition motifs, and dsRNA-binding domains, among others, which allow RBPs to be generically categorized [13].
Firm evidence exists that RBP dysregulation, specifically FXR’s occurs in various human cancers where it promotes tumorigenesis. FXR genes family encode very similar RBPs, like Fragile X mental retardation 1 (FMR1), FMR1 autosomal homolog 1 (FXR1), and FMR1 autosomal homolog 2 (FXR2), which have been identified on chromosomes Xq27.3, 3q26.33, and 17p13.1, respectively [13, 14]. Several cancer-related genes are up/downregulated by FXR1 by modulating post-transcriptional and translational gene expression levels. FXR1 dysregulation and mutations are associated with several disease pathogenesis, including cancer [10, 14]. FXR1 induces carcinomas in multiple tissues more than the other two proteins in the family. Many studies have found that FXR1 overexpression promotes carcinogenesis and inhibits senescence in squamous cell carcinoma (SCC) of the lung and head and neck squamous cell carcinoma (HNSCC), resulting in a poor prognosis and survival [15, 16]. Several studies have discovered that FXR1 overexpression at both the RNA and protein levels is strongly associated with a poor prognosis in several cancers.
Structure and function of FXR1
The sequencing of FMR1 enabled the fast discovery of human FXR1 (3q28) via sequence homology [14]. FXR1 genes, unlike FMR1 genes, are autosomal. FXR1 protein exhibits a similar structure to other family members, with 60% identical amino acid sequences; however, their C-termini vary from one another, suggesting that they have distinct roles [17]. The N-terminus of the FXRIP protein comprises Tandem agent-like domain followed by a non-classical nuclear localization signal (NLS), three protein K homology (KH) domain, a nuclear export signal (NES) and an RGG box at C-terminus. Due to their KH and RGG domains, FXR1 was classed as RBP. Apart from FXRI’s ability to bind to RNA, they interact with other members through the NTD domain, which is similar to the 40S and 60S ribosome subunits [18, 19]. NLS enhances FXR1-RNA interaction and affinity among FXRs [20]. Most FXR1 isoforms have nucleolar targeting signals (NoS) on their C-termini [21].
Exportin-1 shuttles FXR1 to the cytoplasm from the nucleus, although its shuttling depends on its isoform [21, 22]. RGG motifs of FXR1 bind to DNA or RNA structures such as G-quadruplex RNA and secondary RNA structures with four guanines; however, this binding affinity can be altered by targeted RNA arginine methylation [18, 23]. FXR1 KH domain binds targets RNA, and a single base change may affect FXR1 structures, which leads to disease development [24,25,26]. These interactions of FXR1 suggest that FXR1 co-regulates and affects RNA and target mRNA expression.
FXR1 expression and localization
Although variations in expression levels among tissues, FXR1 is ubiquitously expressed. FXR1 is widely expressed in the heart, skeletal muscles, brain, and testes [26, 27]. Even in the same tissues, FXR1 is expressed differently across cellular compartments and cell types, demonstrating its targeting and function diversity [26]. FXR1 is present pre-synaptically in axonal regions of the brain’s Olfactory Blub, thalamus, and CA3 area [28, 29]. Within the cytoplasm, FXR1 is observed in ribonuclear particles, ribosomes, and RNA [18, 30, 31].
Post-translational modifications
Since FXR1 plays numerous roles and exists in many cells, it is not surprising that they are regulated by different post-translational modifications (PTMs). Among these include sumoylation, ubiquitylation, acetylation, methylation, and phosphorylation [32, 33].
The association between phosphorylated FXR1 with RISC and polyribosomes represses the translation of target mRNAs. The well-studied FXR1 PTM is serine 420 phosphorylation (S420), which is probably regulated by the constitutive active casein kinase II (CK2) and essential for its involvement in translation regulation. Moreover, the recruitment of FXR1 to arsenite-induced stress granules increases this PTM, suggesting that it plays a role in cellular stress responses [34, 35]. Protein phosphatase 2A (PP2A) dephosphorylates FXR1 and releases it from ribosomes and AGO2, consequently promoting translation. It has been established that FXR1 phosphorylation at S420 is necessary for the development of distinct RNA granules involved in RNA transport and stress responses. The process of sumoylation probably triggers the release of FXR1 from RNA granules. Furthermore, FXR1 ubiquitylation and destruction by the ubiquitin-proteasome system (UPS) require dephosphorylation of S420 [32, 33]. By methylating the FXR1s RGG domains, their ability to form homo- and heterodimers with other FXPs and their association with polyribosomes may be compromised. While methylation of FXR1 has the potential to ultimately diminish the RNA-binding activity of FXR1 [32, 33]. Multiple more PTMs of FXR1 were discovered by proteome-wide techniques [36, 37], but their origins and effects have not yet been investigated.
Mechanistic role of FXR1
FXR1 regulates miRNA processing
RBPs regulate miRNA biogenesis and maturation via regulating canonical proteins, including Drosha, Dicer, and RISC (miRNA-induced silencing complex). FXR1 protein has four RBDs: coiled-coil domain, three K Homology domains (KH1-2), NLS and a C-terminal domain including RNA-binding Arginine-Glycine-Glycine (RGG) domain. FXR1 proteins require these domains to recognize various miRNAs [38].
Post-transcriptional and transcriptional factors influence miRNA expression. FXR1 is essential for efficiently processing neuronal miRNAs and impacts pre-miRNAs’ processing, transport, and stability. In the DT40 cell line, Dicer knockdown enhances FXR1 expression and miRNA-mediated target silencing [39]. FXR1 increases brain-specific miRNA processing by raising mature miR-9 and possibly decreasing pre-miR-9-2. FXR1 forms complex with pre-miRNAs and Dicer and processes pre-miR-124 and pre-miR-9 in vitro. These findings suggest that FXR1 regulates brain-specific miRNA expression [40]. Translational suppression of specific mRNAs via the RISC is thought to be accelerated by the FXR1’s will-recognized interaction with the miRNA machinery [41, 42]. Gessert et al. found that FMR1/FXR1 regulates the stability and translation of Rx1, Pax6, and FoxD3 mRNA through binding to RISC and miRNAs (miR-130a, -219, -23b, -200b, -96, and -196a). FMR1/FXR1 depletion reduces mRNA expression impairs eye development and cranial cartilage formation [43]. FXR1 lack in skeletal muscle embryos reduces miR-1, a cardiovascular development-miRNA, by 45% [43], suggesting that FXR1’s role is not limited to neuronal miRNAs. Muscular defects may result from FXR1 deficiency-induced miR-1 dysregulation [44].
In mammals, miRNAs are degraded by miRNases such as XRN1, XRN2, RRP41, and PNPT1 [45,46,47]. RBPs bind to miRNAs by forming miRNA complexes and protecting them from degradation. miR-144 is stabilized by RBPs ILF3 and BUD13, as does RBP QKI stabilize miR-20 by forming a complex [48], suggesting that RBP-miRNA interactions stabilize miRNAs. FXR1 inhibits PNPT1’s exonuclease activity by binding to miR301a-3p and protects it from exoribonuclease PNPT1-mediated degradation. The PNPT1 3′-5′ exonuclease activity is inhibited by the presence of RNA secondary structure or RBPs at the 3′-end [49]. Target-specific miRNA and AGO2 interact with FXR1 to regulate post-transcriptional gene expression. These findings reveal how mammalian FXR1 protein regulates the stability of mammalian miRNAs (Fig. 1).
FXR1 involved in the localization of RNAs
The mRNA and lncRNA stability and translation rely on subcellular localization, as the cancer-related RBPs frequently bind to the subcellular compartments where mRNA and lncRNA are localized and translated [50, 51]. FXR1 are new cytoplasmic RNA-binding proteins with basic architecture like FMRP and are associated with cytoplasmic RNPs [52]. The FXR1 protein family is involved in several RNA processes, such as the subcellular localization of RNA by cytoplasmic shuttling [13] and interaction with motor proteins [53,54,55]. FXR1 is linked to mRNP structures that contain poly(A)+ mRNA in polyribosomes that are actively translating. It has NLS and an export signal, making it a potential mRNA carrier (chaperone) to the cytoplasm from the nucleus [52].
Nuclear pore complexes (NPCs) within the nuclear envelope (NE) facilitate macromolecule transport across the nucleus and cytosol. At the end of mitosis and during interphase, nucleoporins (Nups) assemble into NPCs in mammalian cells. In the absence of FXR1 or microtubule-based transfer, Nups improperly localize to the cytosol and form cytoplasmic nucleoporin granules, impeding the potential of NPCs to export protein. Furthermore, considering the well-established functions in translation pathways, FXR1 may transport Nups to the NE and govern their translation during interphase [56,57,58].
RNA sequences and trans-acting elements regulate mRNA’s translation, stability, and localization in the cytoplasm [59]. The 3′-UTR of oncogenes, cytokines, and growth factors contains well-studied AU-rich elements (AREs), which may trigger immunological disorders and cancers [60, 61]. AREs regulate mRNA export and translation in addition to their role in decay (Fig. 1) [60, 62, 63].
FXR1 involved in the stability of the RNAs
The 3′-poly(A) tail and the 5′-terminal 7-methylguanosine (m7G) prevent mRNA from decay and promote translation initiation. Deadenylation of the poly (A) tail, 5′-3′ exonucleolytic decay, decapping of the 5′-terminal m7G cap, and exosome-mediated decay contribute to mRNA degradation. mRNA that is slated for degradation are transported to stress granules or P-bodies for degradation [64, 65].
RNA sequences and trans-acting elements regulate cytoplasmic mRNA localization, translation, and stability [59, 66]. The well-studied AREs in the 3′-UTR of cytokines, oncogenes, and growth factors may trigger immunological diseases and malignancies [60, 61]. In addition to being involved in mRNA decay, AREs regulate translation and export [60, 62, 63]. To stabilize mRNA, FXR1 associates with the miRNA complex or AREs through their KH domains [67, 68]. FXR1 and AGO2 bind to miR369-3 and Let-7, respectively, and inhibit translation during the cell cycle [69]. Even though FXR1 regulates the stability of p21 mRNA via AREs within the 3′-UTR, it is not yet explicitly associated with the RISC pathway [70]. FXR1 binds to the AREs elements (AUUUA) in the 3′-UTR of cMYC mRNA and upregulates their protein levels [71]. FXR1 has also been shown to bind to numerous inflammatory mRNAs and reduce their stability [72]. Mechanistically, it was proposed that mRNA decay was caused by competition between the RNA-destabilizing FXR1 protein and the RNA-stabilizing HuR protein on ARE-containing transcripts. Previous research has shown that HAdV-infected cells benefit from HuR protein stabilization of canonical ARE-containing reporter gene transcripts [73]. As a result, we considered the possibility that ARE-containing transcripts could be the target of an FXR1-HuR interaction that regulates MLTU mRNA turnover.
Both myoblast proliferation and cancer quiescence are regulated by p21 through FXR1 [74]. Some miRNAs suppress p21 mRNA expression and accelerate cell cycle development and progression [74]. FXR1 is known to control the cell cycle in proliferating cells, such as neural stem cells (NSCs) [75]. FMR1/FXR1 depletion decreases Rx1, Pax6, and FoxD3 mRNA expression through interactions with RISC and miRNAs (miR-130a, -219, -23b, -200b, -96, and -196a), resulting in aberrant eye development and cranial cartilage formation [43, 76]. FMRP/FXR1 regulates many synaptic proteins involved in NMDA receptor activation by interacting with GluN1, GluN2A, and GluN2B mRNA [77, 78]. Previous research has demonstrated that FXR1 regulates p21 by interacting with the G-quadruplex (G4) RNA sequence in p21 mRNA (Fig. 1) [79, 80]. We have recently identified that FXR1 overexpression destabilizes PDZK1IP1 and ATOH8 mRNA expression and promotes the development of esophageal cancer. Mechanistically, FXR1 negatively regulates PDZK1IP1 or ATOH8 transcripts by promoting mRNA degradation via direct interaction with its 3′-UTRs. Our findings show that FXR1 has oncogenic activities through the PDZK1IP1/ATOH8 pathway, which might have potential diagnostic or therapeutic implications (unpublished data).
FXR1 involved in the regulation/translation of RNAs
mRNA translation is among the numerous posttranscriptional processes that are significantly impacted by RBPs [81]. Translation is the cellular activity that requires the most energy and precision in regulation, regulated by both cis-acting RNA elements like terminal oligopyrimidine motifs and CA-rich regions [82], and trans-acting factors like mRNA binding proteins (mRBPs) [81]. The latter impacts every stage of translation, whether it is transcript-specific or global [81]. A considerable number of mRBPs and (m)RNA binding domains have been identified via mRNA interactome capture’ techniques based on mass spectrometry [83, 84]. These approaches identify mRBPs physically linked to sequence- or structure-specific regions in mRNAs that influence gene expression at the posttranscriptional level.
FXR1 regulates mRNA translation efficiency in many ways. FXR1 exploits the miRNA pathway to inhibit translation initiation or elongation. Although the regulatory mechanisms are thought to be the same, the FXR family of proteins may have different targets. FMRP interacts with CYFIP1, binds to the translation-initiating factor eIF4E, and inhibits the translation-initiating complex assembly to regulate MAP1B, APP, and CaMKII levels in neurons [85, 86]. Since they bind to CYFIP2 rather than CYFIP1, it is not the exact process by which FXR1 operates; nonetheless, an alternative approach is possible [87]. Elongation stalling is the predominant translation regulatory mechanism through which FXR1 regulates translation. When phosphorylated, FXR1 interacts with polyribosomes since it binds to the entire open reading frame and the UTRs [88]. FXR1 regulates mRNA translation by interacting with miRNAs and the RNAi pathway through the RISC protein AGO2. AREs in the 3′-UTR of mRNA are key post-transcriptional regulatory signals that may modify mRNA translation and stability instantly, thereby modifying gene expression with clinical and physiological implications. The interaction of FXR1 with the 3′-UTR AREs regions of cMYC mRNA promotes translation. FXR1 enhances cMYC translation by binding to the 60S ribosomal subunit, promoting polysome accumulation, and maintaining the mRNA [71, 89].
TNFα-ARE regulates translation activation in human cell lines through FXR1 and AGO2. FXR1 form the FXR1/AGO2/microRNA complex interacts with DAP5/p97 in the quiescent state, promoting non-canonical translation. The distinct contributions of FXR1 and AGO2 in cell-growth-dependent translation activation give insight into ARE-mediated regulation and link two fundamental post-transcriptional regulatory mechanisms [90, 91].
Several studies have indicated that miRNAs act as ARE-containing mRNA regulators. First, RISC contains two ARE-associated proteins, PAI-RBP1 and FXR1 are ARE-associated [67]. Second, miRNAs and ARE-binding proteins share the same cytoplasmic bodies [92, 93]. Third, Tristetraprolin (TTP) through RISC via AGO2 and miR16-1 [94] regulates TNFα 3′-UTR levels.
FXR1, TTP family members, and HuR interact with TNFα and other AREs in response to cell signaling pathways to alter translation or stability [95,96,97]. Moreover, in response to TNFα translational upregulation, FXR1 inhibition induces muscle atrophy, reduced growth, and neonatal mortality [98]. According to another study, the FXR1 RGG motif binds with eIF4A1 and eIF4E to recruit the eIF4F complex to the cMYC translation initiation site. The lack of the RGG motif blocked FXR1’s interaction with the eIF4A1, eIF4E, and eIF4G1 proteins. Our finding shows that the RGG-box domain of FXR1 recruits eIF4F to the mRNA translation start site (Fig. 1) [71].
FXR1 has recently been identified as a new m6A reader protein [99]. Importantly, Price and coworkers [100] showed that knocking down METTL3 (an m6A catalytic enzyme) greatly decreased capsid protein accumulation and dramatically reduced infectious HAdV-5 progeny by reducing splicing of MLTU pre-mRNAs. Unexpectedly, they observed that the elimination of cytoplasmic m6A readers YTHDF1, YTHDF2, and YTHDF3 had a negligible impact on the infectious cycle of HAdV-5 in their investigation [100]. These findings imply that an additional cytoplasmic m6A reader protein, like FXR1, targets m6A-modified MLTU mRNAs and regulates their stability and translation. Endogenous FXR1 binds selectively to m6A-modified MLTU transcripts and protects the m6A signal on pVII and fiber mRNAs. These findings may suggest that FXR1 regulates the translation of mRNAs, especially in m6A-dependent manner.
FXR1 and defects in RNA editing
Interestingly, FXR1, in particular, is connected to RNA editing mechanisms. Exquisite studies in Drosophila found that FXR1 physically interacts with and regulates the activity of dADAR, an A-to-I- editing enzyme in RNA. Here, aberrant RNA editing was caused by deletion or overexpression of dFXR1, particularly of synaptic transmission and neuromuscular junction architecture-related mRNAs [101]. In humans, FXR1 interacts with the functional active A-to-I RNA editing enzyme ADAR1. The fact that differential RNA editing sites in several diseases are near FXR1-binding sites indicates that FXR1 plays an immediate role in the recruitment of ADAR enzymes to mRNA-editing sites. It is interesting to note that FXR1 may inhibit the editing of certain mRNA sites [102], probably impacting the spinal cord motoneurons in patients [103], which may have implications for synaptic transmission, the integrity of neuromuscular junctions, and motoneuron survival.
Other function of FXR1
In addition to its role in RNA metabolism, FXR1 also plays important roles in regulating chromatin dynamics and the DNA damage response, the cell cycle, ribosome biogenesis, and mitochondrial organization [32, 104, 105]. FXR1 has a key function in modulating ion channels at numerous stages in neurons. In addition to mediating the regional translation of mRNAs which encode for different ion channels, FXR1 also plays a vital function in the trafficking and gating of channels through interactions between proteins [106]. Furthermore, FXR1 plays a significant role in cellular stress responses and stress granule production. Stress granules are membrane-free assemblies of proteins and RNAs that form in the cytoplasm as a result of protein/RNA phase separation, and facilitate the majority of cell types to survive under stressful environments [107]. All three FXPs, like many other RBPs, are known to be found in stress granules [108], but FXR1 is particularly important for stress granule assembly [109]. Interestingly, the expression of FXR1 is induced by various stressors in different cell types, providing additional evidence for its involvement in cellular stress responses. It is worth noting that lower and greater levels of FXR1 expression are related to reduced and enhanced cell viability, respectively [110].
Recently, a study has demonstrated that FXR1 binds to proteasomes, and that proteasome activity increases in the absence of FXR1, indicating that FXR1 plays a significant role in proteasome regulation. Further evidence pointed to a role for it in intracellular ubiquitination. Because little was known about the association between FXR1 and the ubiquitin-proteasome system (UPS), these results are interesting and provide novel insights into FXR1’s functioning mechanism. Moreover, by reassessing FXR1 in the context of UPS, a novel understanding of the pathogenesis of diseases associated with FXR1 may be attained [111]. In FXR1-associated diseases, for instance, problems with protein quality control may be caused by FXR1 dysregulation. Future research and investigations are anticipated to reveal novel functional pathways of FXR1 as a UPS-related mediator in biological activities and disease.
FXR1 modulation of cancer phenotypes
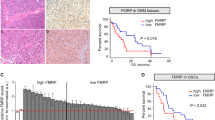
FXR1 has been related to almost every step leading to tumor formation. This altered FXR1 activity seems to be present in all cancer types, and it appears to be associated with the dysregulation of the corresponding mRNA targets. An alternative way of thinking about FXR1 roles in cancer is to divide them into different categories: prolonged proliferative potential, evading cell death and senescence. Below are examples of FXR1 involved in each phase of these fundamental biological processes (Fig. 2) and (Table 1). Moreover, Fig. 3 schematically illustrates how nanoparticles can be employed to deliver drugs or siRNA that interfere with FXR1 expression or activity/stability in esophageal cancer cells.
Proliferation
Uncontrolled cell proliferation can cause a transformed cell population to expand and lead to cancer. A potentially effective cancer therapy must be able to stop or limit the unchecked proliferation of cancer cells. Most RBPs are involved in tumor cell proliferation, making them potential cancer progression drivers [112]. FXR1 is upregulated in numerous malignancies, suggesting it is involved in cancer development. FXR1 expression levels were increased in SCC of the lung, Non-small cell lung cancer, HNSCC, and prostate cancer [113, 114]. FXR1 is an oncogene because it targets and stabilizes many cancer-related mRNAs, and its dysregulation in oncogenesis has a complicated molecular mechanism that varies depending on the cancer type. FXR1 regulates p21 and c-Myc mRNA levels to promote cell proliferation [15]. FXR1 is also involved in regulating TRAF1, FBXO4, COX2, TNF-α, eIF4E-BP2, CDK4, CCNE1, CCND1, CDK2, and CDK1 by binding to the 3′-UTR AREs [69, 115,116,117,118,119,120,121,122,123,124]. MiRNAs and ribonucleases promote cell proliferation by binding to their 3′-UTRs [123, 125, 126].
RBPs prevent miRNAs from degradation by forming miRNP complexes. RBPs QKI and ILF3 stabilize miR-20 and miR-144, suggesting that mRNA stability is associated with RBP-miRNA interactions [126, 127]. According to the TCGA analysis, patients with oral cancer who overexpressed FXR1 had poorer outcomes. FXR1 targets the tumor suppressor p21 3′-UTR, thereby promoting the progression of HNSCC and preventing cellular senescence. MiR301a-3p and FXR1 bind to the 3′-UTR of p21 mRNA, promoting its degradation and accelerating the progression of HNSCC. In laryngeal squamous cell carcinoma (LSCC), miR301a-3p acts as a cancer-promoting gene, targeting multiple tumor suppressor genes, such as Smad4 [128]. FXR1 specifically binds to miR301a-3p to form a miRNP complex that protects it from exoribonuclease PNPT1-mediated degradation, supporting the notion that it stabilizes miRNAs and enhances their oncogenic properties. FXR1 and miR301a-3p are upregulated in NSLSC and HNSCC cancers, suggesting a distinct regulatory mechanism for miR301a-3p stabilization by FXR1. Targeting FXR1-miRNA-mediated p21 regulation can inhibit the growth and proliferation of oral cancer [15]. It is necessary to conduct additional research to investigate how the FXR1-miR301a-3p axis regulates the mRNA stability of other genes, which may drive cancer progression [15]. FXR1 knockdown alters the expression of several miRNAs in both positive and negative oral cancer cells, inhibiting the expression of miR301a-3p and miR29b-3p in different oral carcinoma cells. Through deadenylation, decapping, and degradation, both RBPs and miRNAs can target and modulate particular mRNA transcripts [129]. CCR4-NOT mRNA deadenylase complex, DCP1/2 decapping enzymes, and XRN1/2 and PNPT1 exonucleases have all been associated with mRNA decay mediated by sequence-binding proteins and miRNAs [130,131,132,133,134].
FXR1 is highly expressed in advanced and high-grade ovarian tumors. According to the clinical outcome study, FXR1 mRNA high expression is linked with decreased overall and recurrence-free survival [135]. CRISPR/Cas9-mediated deletions show that FXR1’s oncogenic effects require the AREs in the 3′-UTR of the cMYC gene. Overexpression of cMYC by FXR1 increased cell-cycle regulators such as cyclin E1, D1, and CDKs, promoting the growth of ovarian cancer. FXR1 promotes the translation of the cMYC oncoproteins by binding to the AREs within cMYC mRNA, which is essential for ovarian cancer progression and aggressiveness [71]. This demonstrates that cMYC mediates FXR1’s oncogenic effects, such as cell proliferation and metastasis.
FXR1 regulates NSC cell-cycle proteins (p21) during adult neurogenesis to maintain adult NSC proliferation [78]. FXR1 deficiency increases p21 mRNA and its protein expression while inhibiting cell proliferation. This proliferation deficit can be reversed by restoring p21 to wild-type levels in NSCs. FXR1 promotes myoblast (muscle stem cell) proliferation by accelerating cell cycle progression and decreasing p21 mRNA stability [136, 137].
Epithelial-mesenchymal transition, invasion, and metastasis
During embryogenesis, tissue regeneration, wound healing, tumor progression, and metastasis, healthy cells undergo epithelial–mesenchymal transition (EMT). EMT and cell structure and function modifications are regulated by transcriptional and post-transcriptional gene expression. During EMT, FXR1 regulates mRNA translation, alternative splicing, polyadenylation, and stability [138]. In a loss of function study, FXR1 knockdown reduces hepatocellular carcinoma (HCC) cell invasion and migration via TGF-β modulation, whereas upregulation in HCC cells increases cell invasion which is abrogated by inhibiting SMAD2/3. TGF-β-SMAD signaling promotes metastasis and EMT of HCC. The EMT gene Slug is a popular SMAD3 target [139]. Environmental stressors include reactive oxygen species, hypoxia, inflammation, and extracellular mediators such as epidermal growth factor, fibroblast growth factor-2, and TGF-β [140,141,142,143,144], which can cause EMT. FXR1 depletion inhibited SMAD2/3 expression, whereas SMAD2/3 knockdown decreased the production of EMT-related proteins [112].
Apoptosis
Cancer cells replicate and grow indefinitely, in addition to their ability to evade cell death. Apoptosis is a critical process that allows healthy cells to determine whether to die under extreme conditions. Cancer cells avoid apoptosis to accelerate tumor growth. RBPs regulate the expression of mRNAs involved in apoptosis, including PARP, Bcl, p53, Fas, Caspases, and others [145,146,147,148].
FXR1 is highly expressed in many cancers, including SCC of the lung and HNSCC [16]. Loss of FXR1 induces apoptosis and induces cellular Senescence in HNSCC [15].
George et al. 2021 investigated apoptosis in FXR1 knocked-out OVCAR5, Kuramochi, and HeyA8 cells. They observed that inhibiting FXR1 accelerates the death of these cells. Following TCGA datasets, GSEA results showed that FXR1 depletion upregulates mRNA levels of CDKN1A and CDKN1B while downregulates that of CDK2, RAD51, BCL2L11, MCM2, CCNB1, and HMGA1, all of which are essential genes in apoptosis functional annotation. FXR1 deficiency may activate apoptosis biomarkers cleaved caspase-3 and caspase-7 [71].
FXR1 deletion decreased pro-survival proteins such as Survivin and HSP-60 while increasing pro-apoptotic proteins levels like cytochrome-C, BAX, and death receptors like p21, p27, FADD, phospho-p53, and FAS. FXR1 depletion decreases the levels of several oncogenic proteins, such as cyclin B1, cMYC, CHK1, EVI1, FOXM1, and CDC6, consistent with previous findings that FXR1 suppresses apoptosis and promotes oncogenesis. When FXR1 is depleted, the expression level of proteins BAX, p21, p27, FAK1, DUSP4, and PAI1 increases while BCL2 decreases, all of which are involved in cancer cell death pathways [71]. This finding shows that FXR1 silencing inhibits cancer cell growth, stops the cell cycle, and activates cell death pathways.
Senescence
Genetic changes, telomere length, reactive oxygen species, chemotherapy, and radiotherapy can all lead to cellular senescence in healthy and malignant cells. It is a permanent G1 cell cycle arrest characterized by metabolically active and viable cells. These cells typically activate the RB/p16 tumor suppressor pathways and the p53/p21 stress response [149,150,151].
According to recent research, senescence suppresses tumors in vivo in premalignant tumors such as naevi, neurofibromas, and lung adenomas [152]. Most transcriptionally active genes, including p21, p27, p16, and PTEN, promote cellular senescence by activating p53 or p16-mediated pathways [69]. Although transcriptional alterations affect cellular senescence, post-transcriptional modifications are poorly understood. During Senescence, RBPs and ncRNAs frequently interact to modulate gene expression post-transcriptionally [26]. In mammalian cells, many RBPs regulate mRNA processing, transport, translation, and stability of senescence-related genes. Because of their growing involvement in DDR, RBPs are the primary genomic instability regulators [153].
FXR1 has upregulated in HNSCC; inhibiting FXR1 causes deactivation of the phosphatidylinositol 3-kinase/Akt signaling pathway and induces the expression of genes associated with senescence such as PTEN, p53, p21, and p27. Overexpression of FXR1 regulates the cycle of p21 and TERC mRNA to avoid senescence. FXR1 binds to and regulates TERC RNA, suppressing cellular senescence via telomerase activity. Deficient FXR1 in cancer cells activates p53 causing DNA damage and eventually senesce. FXR1-mediated senescence is irreversible, and cells deficient in FXR1 cannot colonize or proliferate. FXR1’s unique p53-dependent regulation of p21 expression inhibits cellular senescence in oral cancer cells [15].
FXR1 and miR301a-3p promote oral and lung cancer by decreasing p21 expression. In FXR1-deficient cells, PNPT1 degrades miR301a-3p, increasing p21 protein translation and consequent oral cancer cell senescence [15]. miR301a-3p is an oncogene targeting many tumor suppressor genes, such as Smad4 in LSCC [128].
FXR1: a potential therapeutic target for cancer therapy
Drug resistance
Cancer treatment aims to eliminate tumors and improve patient survival. The complicated genetic landscape of most malignancies makes it challenging to get the optimum treatment responses and leads to therapeutic failure. Drug resistance is a complex issue and a significant factor in the failure of anticancer therapy [154]. It is mostly controlled by the tumor microenvironment, consisting of stromal population, immunological, and cancer cells. FXR1 and treatment resistance have been examined in pancreatic, breast, brain, and lung cancer [155]. FXR1 post-transcriptionally regulate multidrug resistance (MDR)-related genes, supporting them as therapeutic targets.
FXR1 decreased KEAP mRNA stability, and FXR1 knockdown caused cell death and limited axitinib resistance. Even in clear cell renal cell carcinoma (ccRCC) cells with downregulated FXR1, KEAP1 knockdown increased apoptosis, suppressed autophagy, oxidative stress, and axitinib resistance. FXR1 altered the KEAP/Nrf2 pathway, causing ccRCC cells to resist axitinib [156].
Through regulating CDC6, FXR1 overexpression boosted tumor growth and oxaliplatin resistance in colorectal cells, while knockdown improved their sensitivity [157]. FXR1 knockdown reacts similarly to epirubicin. FXR1 knockdown increased ROS generation and ROS-FXR1-TGF-b-mediated epirubicin sensitivity. siFXR1’s galectin-3/b-catenin signaling suppression inhibited MDR protein expression in colorectal cancer cells [158]. Many studies have correlated FXR1 knockdown to enhanced radiation sensitivity. FXR1 upregulated caspase-2 and inhibited thioredoxin reductase in breast and colorectal cancer tissues, making them more radiation-sensitive. FXR1-mediated post-transcriptional modulation of ARID1A also increased radiation therapy resistance in breast cancer cells [159]. Finally, FXR1 in tumors affects treatment sensitivity and resistance. FXR1’s role in resistance and sensitivity may vary due to the genetic makeup of various malignancies. FXR1-based targeted therapy might have more options if the specific mechanism by which FXR1 influences treatment responses in animals and clinical studies is elucidated.
Approaches for targeting FXR1
As previously stated, FXR1 has been associated with tumor development and resistance to anticancer drugs. FXR1 is an attractive target for cancer treatment due to its widespread expression in nearly all cancers and its important function in the post-transcriptional controls of key genes involved in tumor growth and survival. Therefore, it is essential to develop new therapeutic approaches to block the biological actions of FXR1. Several inhibitors, like shRNAs or siRNAs, have been recommended to target FXR1 mRNA, which is discussed briefly in the following sections. Inhibitors, antisense oligonucleotides (ASOs), Small-molecule medicines, and short therapeutic peptides are among the future treatment strategies that are being designed and examined in clinical trials [160,161,162,163]. Fig. 4 illustrates the diverse strategies that can interfere with FXR1 expression or activity in various cancer models. These strategies aim to modulate the interaction of FXR1 with its target RNAs and alter the expression of oncogenes and anti-oncogenes. However, Fig. 5 outlines the steps involved in the development and application of FXR1-targeted therapies for different cancers, from basic research to clinical trials. It also discusses some of the challenges such as reliable biomarkers, optimal drug delivery systems, drug resistance, and long-term outcomes, as well as some of the opportunities that need to be addressed in order to translate FXR1 research into effective and safe treatments for different cancer patients.
RNA interference-based approach
RNA interference (RNAi) lowers gene expression of disease-causing genes. They also modulate “druggable” targets and, more significantly, “non-druggable” targets that drugs or small-molecular inhibitors cannot regulate. RNAi affects cells in two ways. The first adds commercially produced siRNA to the biological system and then integrates it into the RISC complex. Once the siRNA guide strand binds to its complementary strand, the Argonaute proteins cleave the target mRNA to shut down the gene expression [164].
shRNA and siRNA were used to inhibit FXR1 in mice ovarian cancer cells. In ovarian cancer cells, transient and constitutive FXR1 inhibition by shRNA and siRNA reduced cell proliferation, colony formation, migratory, and invasion ability and suppressed tumor development in vivo. Instead of inducing tumor cell death, this decreased cancer cell proliferation. In addition, siFXR1 therapy reduces cMYC and Ki67, the primary target of FXR1s. In tumor tissues, siFXR1 administration upregulates p21, p27, and cleaved caspase-3. Hence, FXR1 may be a promising target for cMYC reduction in ovarian and other cancers. FXR1 siRNAs in nanoliposomes composed of 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC) reduced ovarian cancer growth and metastasis. These findings suggest that targeting FXR1 to lower its expression level through siRNA/shRNA reduces cancer cell growth, proliferation, and metastasis.
Genome editing approaches
CRISPR-Cas9 system for genome editing is a widespread method for genetic alteration. This approach examines gene function using precise genomic editions. The utility of CRISPR-Cas9 technology in diabetes, obesity, neurodegenerative, and ophthalmic illnesses is supported by data [165,166,167]. CRISPR-Cas9 suppresses the FXR1 gene expression (U2OSFFF and delACAG) in several cancers. In vitro, FXR1 knockdown led to a decrease in malignancy, higher apoptosis rate, and lower sphere formation than wild-type cells, indicating their significance in cell proliferation and growth. FXR1 deletion in mice reduced tumor development by down-regulating oncogenic gene expression. Others have verified that FXR1 depletion using CRISPR-Cas9 is beneficial in cancer treatment [160].
Nanomedicine-based approaches
Several studies have shown that employing nanomedicine-based techniques has a considerable advantage over traditional therapies. Some of the most frequent nanomaterials employed in the treatment of cancer include liposomes, metallic nanoparticles, polymeric nanoparticles, dendrimers, and solid–lipid nanoparticles [168, 169]. Different targeting ligands have previously been utilized to adorn the surface of nanoparticles to investigate the effective targeting of nanocarriers. To achieve targeted siRNA distribution and effectively execute mRNA suppression, conjugating siRNA to nanocarriers like dendrimers and liposomes would help minimize siRNA degradation [170]. Recently, researchers have focused on the use of extracellular vesicles (EVs), dendrimers, liposomes, and ASOs to improve FXR1s siRNA/shRNA distribution in cancer models [171, 172].
Extracellular vesicles (EVs)
Various techniques are now available for producing and characterizing EVs. EVs may also be utilized as biomarker molecules or potential candidates for liquid biopsies of clinical disorders since their composition is substantially impacted by the cell type from which they arise [173, 174]. When isolated from various cell types, tissues, and body fluids (including amniotic fluid, serum/blood, breast milk, saliva, and urine), there is evidence that EVs may be utilized to detect diseases [175, 176]. Natural drug carriers influence cell-cell communication by crossing physiological barriers, including the blood-brain barrier [177], while conventional drugs cannot access them. Because of these properties, EVs are important carriers to target FXR1 for cancer treatment and diagnostics.
Antisense oligonucleotides (ASOs)
Antisense oligonucleotides have been used in cancer therapies in the context of drug delivery and angiogenesis [178]. FXR1 has been implicated in inflammation by altering the expression of important pro-inflammatory molecules such as TNF-a, IL-1b, and IL-6. Therefore, suppression of FXR1, specifically using phosphorothioate phosphate-modified ASOs can be applied to reduce disease severity, at least in mice models. To improve cellular uptake and stability, ASOs may be incubated with cationic lipids (DOTAP) and administered through intranasal and intrathecal channels.
FXR1 expression is high in various human malignancies, including cervical, SCC of the lung, and HNSCC [71]. As a result, FXR1-inhibiting therapeutic interventions may have widespread applicability to other malignancies that contain FXR1 CNV. An unbiased genome-wide study examined the possibility that FXR1 can directly regulate numerous additional mRNAs as its targets. The use of pro-senescence techniques in the treatment of cancer is a promising alternative to conventional chemotherapy treatments [179]. FXR1 has the potential to function as diagnostic and prognostic markers for several malignancies due to their aberrant expression and mRNA regulatory functions. For instance, FXR1 is significantly overexpressed in the majority of cancers and is linked to an aggressive phenotype or a bad prognosis [180].
Furthermore, a high FXR1 expression level is associated with more severe features and worse survival outcomes in ductal breast cancer patients [159]. In the absence of FXR1, PNPT1 degrades matured oncogenic miR301a-3p. In addition, it has been shown that p21 is a target of miR301a-3p and that when FXR1 is knocked out, miR301a-3p is downregulated while p21 mRNA and protein expression levels are elevated in different oral cancer patients. The downregulation of p21 signaling in an HNSCC cohort with overexpressed FXR1 and miR301a-3p might be explained by this pathway. According to these findings, FXR1 inhibitors combined with anti-miR oligonucleotide intervention and chemotherapy could be an effective therapy for HNSCC patients [15]. Besides, research is being conducted to classify RBPs as diagnostic and predictive biomarkers that reveal cancer-specific expression using modern bioinformatics methods such as microarray or newly processed RNA-seq data from the TCGA data [181, 182].
Conclusion and future prospective
The regulation of RNA metabolism is an essential component of gene expression because it allows for the fine-tuning of transcript levels under physiological circumstances as well as the quick and dramatic changes in global gene expression associated with inflammation and immune responses. Long-term dysregulation of RNA metabolism is often associated with disease states, including cancer. FXR1 protein has emerged as an essential regulator of several aspects of RNA metabolism, with significant therapeutic potential.
FXR1 participates in almost every stage of post-transcriptional regulation, determining the fate and function of each transcript within the cells and maintaining cell equilibrium. They create ribonucleoprotein complexes that control RNA splicing, translation, localization, stability, polyadenylation, and degradation through dynamic interactions with different proteins and coding RNAs and ncRNAs [183]. This now becomes obvious that FXR1 is dysregulated in various types of cancers, affecting the production and functioning of cancer-causing and tumor-suppressor proteins. Therefore, deciphering the intricate relationships between FXR1 and its RNA targets associated with cancer could help improve our understanding of tumor development and possibly lead to the discovery of novel targets for cancer therapy [10]. According to the information in hand, FXR1 primarily affects the occurrence of cancer following a major carcinogenic activity by influencing many cancer-related downstream targets, thereby enhancing the biological implication of the initial transforming hit(s) via a “ripple effect”. In this case, FXR1 predominantly functions as amplifiers in oncogenic driver mutation [184].
A lot of outstanding research leaves little question regarding the role of FXR1 in cancer etiology. Here we have the strongest evidence linking FXR1 to carcinogenesis, from studies of human tissue, animal models, and mechanistic investigations. Furthermore, there are several suggestions in the literature that the FXR1 may have a role in cancer associated with cMYC, PNPT1, p21, p27, TERC, and p53. The number of potential FXR1-binding oncogenes/tumor suppressor mRNAs might increase this list even more. Since most, if not all, patients share similar disease processes, dysregulated expression and/or function of the FXR1 may constitute a pathogenic event in cancer. Mutations in various genes are expected to have distinct effects on FXR1, although they may all lead to the same result. Therefore, it is more probable that loss of function and/or expression of FXR1 in cancer reflect one of the several “hits” required for cancer development. FXR1 deserves to be in the limelight and shift from stand-in roles to regular participants in carcinogenesis, given its scaffolding ability in developing RNP networks that regulate the expression of transcripts encoding proteins implicated in malignant procedures [10]. Numerous studies have repeatedly emphasized that FXR1 could be a useful biomarker for predicting the prognosis and treatment response of cancer patients. Few studies have demonstrated that small-molecule inhibitors or oligoribonucleotides can be used in vitro to selectively inhibit FXR1 or FXR1–RNA interactions, as previously established for HuR and LIN28, with positive functional outcomes [185, 186].
Due to recent advancements in employing more biologically accurate cellular models, such as patient-derived tumor xenografts, biomimetic microfluidic culture methods, and human tissue organoids, the cancer RBPome will soon be studied in unprecedented detail. In addition, these models will help in the identification of altered signaling combined through FXR1 in cancer and assist in identifying altered signaling pathways and PTMs that regulate FXR1. In the meantime, the development of synthetic FXR1s as molecular weapons is getting closer to being a reality. To control the regulation of individual or functionally related sets of cancer-associated transcripts with common recognizing patterns, these drugs might be designed to integrate distinct effector domains with particular RBDs [187]. Therefore, a more thorough investigation is needed to ascertain the specific role of the FXR1 and establish customized approaches for targeted FXR1 cancer therapy strategies without damaging nearby healthy cells. In addition, drug delivery systems should be optimized for target specificity to maximize the advantages of the identified drugs and enable future therapeutic applications of these different approaches [188, 189].
FXR1 is the key molecule that regulates the progression of cancer. Its transient or stable inhibition can significantly decrease cell survival and tumor development in vitro and in vivo through inhibitors such as siRNAs, shRNAs, ASOs, small molecules, and CRISPR/Cas9. These FXR1-targeting therapies based on RNAi, gene edition, and pharmacologic inhibition show considerable therapeutic effects in pre-clinical models; however, there are several challenges that must be overcome before they can be successfully implemented in clinical settings for the benefit of patients. First, the route of administration has a significant impact on the effectiveness of siRNA delivery. Second, siRNAs’ small size, short half-life, negative charge, difficulty in penetrating cell membranes, instability in the bloodstream, and susceptibility to nuclease degradation are the primary factors limiting their ability to travel to a particular target site. Thirdly, establishing cell- or tissue-specific delivery is an additional key impediment to the therapeutic application of siRNAs. Finally, genome editing using CRISPR/Cas-9-based technology in a therapeutic setting also confronts significant challenges. Its use is presently restricted due to restrictions on site-specific CRISPR/Cas-9 system delivery with minimal off-target implications. Therefore a recent study proposes using novel nanomedicine-based drug delivery approaches to address the issues associated with siRNAs and CRISPR/Cas-9, including off-target effects, site-specific delivery, and degradation. Designing efficient carrier molecules that facilitate site-specific delivery with little damage is critical to translate shRNA, siRNA, and CRISPR-Cas9-based strategies successfully.
In conclusion, we must gain a deeper understanding of FXR1 in cancers because they indicate great promise as potential therapeutic targets in the foreseeable future for cancer treatment. In contrast to targeting the expression of altered genes using antisense oligonucleotides or other approaches, targeting FXR1 could lead to shifts in the choice of therapy for the majority of patients, even those who do not have a genetic mutation in a commonly associated gene. Although the possibility of curing the respective disease through the restoration of FXR1 function and/or expression is unlikely, any advancement in currently available therapies is considered a success. We hope that in-depth new research will improve the applicability of developing FXR1-based diagnostic and treatment techniques in the coming days. This review might help to accelerate the expansion of FXR1 research and therapeutic applications in cancer clinics.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Fu XD, Ares M Jr. Context-dependent control of alternative splicing by RNA-binding proteins. Nat Rev Genet. 2014;15:689–701. https://doi.org/10.1038/nrg3778.
Martin KC, Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell. 2009;136:719–30. https://doi.org/10.1016/j.cell.2009.01.044.
Castello A, Fischer B, Frese CK, Horos R, Alleaume AM, Foehr S, et al. Comprehensive identification of RNA-binding domains in human cells. Mol Cell. 2016;63:696–710. https://doi.org/10.1016/j.molcel.2016.06.029.
Moore MJ, Proudfoot NJ. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell. 2009;136:688–700. https://doi.org/10.1016/j.cell.2009.02.001.
Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–45. https://doi.org/10.1016/j.cell.2009.01.042.
Re A, Joshi T, Kulberkyte E, Morris Q, Workman CT. RNA-protein interactions: an overview. Methods Mol Biol. 2014;1097:491–521. https://doi.org/10.1007/978-1-62703-709-9_23.
Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet. 2014;15:829–45. https://doi.org/10.1038/nrg3813.
Mohibi S, Chen X, Zhang J. Cancer the’RBP’eutics-RNA-binding proteins as therapeutic targets for cancer. Pharm Ther. 2019;203:107390. https://doi.org/10.1016/j.pharmthera.2019.07.001.
Neelamraju Y, Gonzalez-Perez A, Bhat-Nakshatri P, Nakshatri H, Janga SC. Mutational landscape of RNA-binding proteins in human cancers. RNA Biol. 2018;15:115–29. https://doi.org/10.1080/15476286.2017.1391436.
Pereira B, Billaud M, Almeida R. RNA-binding proteins in cancer: old players and new actors. Trends Cancer. 2017;3:506–28. https://doi.org/10.1016/j.trecan.2017.05.003.
Brinegar AE, Cooper TA. Roles for RNA-binding proteins in development and disease. Brain Res. 2016;1647:1–8. https://doi.org/10.1016/j.brainres.2016.02.050.
Kechavarzi B, Janga SC. Dissecting the expression landscape of RNA-binding proteins in human cancers. Genome Biol. 2014;15:R14. https://doi.org/10.1186/gb-2014-15-1-r14.
Wang ZL, Li B, Luo YX, Lin Q, Liu SR, Zhang XQ, et al. Comprehensive genomic characterization of RNA-binding proteins across human cancers. Cell Rep. 2018;22:286–98.
Hentze MW, Castello A, Schwarzl T, Preiss T. A brave new world of RNA-binding proteins. Nat Rev Mol Cell Biol. 2018;19:327–41. https://doi.org/10.1038/nrm.2017.130.
Majumder M, House R, Palanisamy N, Qie S, Day TA, Neskey D, et al. RNA-binding protein FXR1 regulates p21 and TERC RNA to bypass p53-mediated cellular senescence in OSCC. PLoS Genet. 2016;12: https://doi.org/10.1371/journal.pgen.1006306.
Qian J, Hassanein M, Hoeksema MD, Harris BK, Zou Y, Chen H, et al. The RNA binding protein FXR1 is a new driver in the 3q26-29 amplicon and predicts poor prognosis in human cancers. Proc Natl Acad Sci USA. 2015;112:3469–74.
Kirkpatrick LL, McIlwain KA, Nelson DL. Comparative genomic sequence analysis of the FXR gene family: FMR1, FXR1, and FXR2. Genomics. 2001;78:169–77. https://doi.org/10.1006/geno.2001.6667.
Mazroui R, Huot ME, Tremblay S, Boilard N, Labelle Y, Khandjian EW. Fragile X Mental Retardation protein determinants required for its association with polyribosomal mRNPs. Hum Mol Genet. 2003;12:3087–96. https://doi.org/10.1093/hmg/ddg335.
Adinolfi S, Ramos A, Martin SR, Dal Piaz F, Pucci P, Bardoni B, et al. The N-terminus of the fragile X mental retardation protein contains a novel domain involved in dimerization and RNA binding. Biochemistry. 2003;42:10437–44. https://doi.org/10.1021/bi034909g.
Kim M, Bellini M, Ceman S. Fragile X mental retardation protein FMRP binds mRNAs in the nucleus. Mol Cell Biol. 2009;29:214–28. https://doi.org/10.1128/MCB.01377-08.
Tamanini F, Kirkpatrick LL, Schonkeren J, van Unen L, Bontekoe C, Bakker C, et al. The fragile X-related proteins FXR1 and FXR2P contain a functional nucleolar-targeting signal equivalent to the HIV-1 regulatory proteins. Hum Mol Genet. 2000;9:1487–93. https://doi.org/10.1093/hmg/9.10.1487.
Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997;17:1539–47. https://doi.org/10.1523/JNEUROSCI.17-05-01539.1997.
Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–99. https://doi.org/10.1016/s0092-8674(01)00566-9.
Blackwell E, Ceman S. Arginine methylation of RNA-binding proteins regulates cell function and differentiation. Mol Reprod Dev. 2012;79:163–75. https://doi.org/10.1002/mrd.22024.
Tamanini F, Meijer N, Verheij C, Willems PJ, Galjaard H, Oostra BA, et al. FMRP is associated to the ribosomes via RNA. Hum Mol Genet. 1996;5:809–13. https://doi.org/10.1093/hmg/5.6.809.
Tamanini F, Willemsen R, van Unen L, Bontekoe C, Galjaard H, Oostra BA, et al. Differential expression of FMR1, FXR1 and FXR2 proteins in human brain and testis. Hum Mol Genet. 1997;6:1315–22. https://doi.org/10.1093/hmg/6.8.1315.
Bakker CE, Kooy RF, D’Hooge R, Tamanini F, Willemsen R, Nieuwenhuizen I, et al. Introduction of a FMR1 transgene in the fragile X knockout mouse. Neurosci Res Commun. 2000;26:265–77.
Akins MR, Leblanc HF, Stackpole EE, Chyung E, Fallon JR. Systematic mapping of fragile X granules in the mouse brain reveals a potential role for presynaptic FMRP in sensorimotor functions. J Comp Neurol. 2012;520:3687–706. https://doi.org/10.1002/cne.23123.
Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32:37–48. https://doi.org/10.1016/j.mcn.2006.02.001.
Khandjian EW, Huot ME, Tremblay S, Davidovic L, Mazroui R, Bardoni B. Biochemical evidence for the association of fragile X mental retardation protein with brain polyribosomal ribonucleoparticles. Proc Natl Acad Sci USA. 2004;101:13357–62. https://doi.org/10.1073/pnas.0405398101.
Chyung E, LeBlanc HF, Fallon JR, Akins MR, Fragile X. granules are a family of axonal ribonucleoprotein particles with circuit-dependent protein composition and mRNA cargos. J Comp Neurol. 2018;526:96–108. https://doi.org/10.1002/cne.24321.
Majumder M, Johnson RH, Palanisamy V. Fragile X-related protein family: a double-edged sword in neurodevelopmental disorders and cancer. Crit Rev Biochem Mol Biol. 2020;55:409–24. https://doi.org/10.1080/10409238.2020.1810621.
Prieto M, Folci A, Martin S. Post-translational modifications of the fragile X mental retardation protein in neuronal function and dysfunction. Mol Psychiatry. 2020;25:1688–703. https://doi.org/10.1038/s41380-019-0629-4.
Say E, Tay HG, Zhao ZS, Baskaran Y, Li R, Lim L, et al. A functional requirement for PAK1 binding to the KH(2) domain of the fragile X protein-related FXR1. Mol Cell. 2010;38:236–49. https://doi.org/10.1016/j.molcel.2010.04.004.
Fan Z, He Y, Sun W, Li Z, Ye C, Wang C. Clinical characteristics, diagnosis and management of Sweet syndrome induced by azathioprine. Clin Exp Med. 2023; https://doi.org/10.1007/s10238-023-01135-9.
Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43:D512–20. https://doi.org/10.1093/nar/gku1267.
Liu Y, Li H, Wang X, Huang J, Zhao D, Tan Y, et al. Anti-Alzheimers molecular mechanism of icariin: insights from gut microbiota, metabolomics, and network pharmacology. J Transl Med. 2023;21:277. https://doi.org/10.1186/s12967-023-04137-z.
Van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer. 2011;11:644–56.
Cheever A, Blackwell E, Ceman S. Fragile X protein family member FXR1 is regulated by microRNAs. RNA. 2010;16:1530–9. https://doi.org/10.1261/rna.2022210.
Xu XL, Zong R, Li Z, Biswas MH, Fang Z, Nelson DL, et al. FXR1P but not FMRP regulates the levels of mammalian brain-specific microRNA-9 and microRNA-124. J Neurosci. 2011;31:13705–9. https://doi.org/10.1523/JNEUROSCI.2827-11.2011.
Banerjee A, Ifrim MF, Valdez AN, Raj N, Bassell GJ. Aberrant RNA translation in fragile X syndrome: from FMRP mechanisms to emerging therapeutic strategies. Brain Res. 2018;1693:24–36. https://doi.org/10.1016/j.brainres.2018.04.008.
Zhang Z, Wang L, Zheng W, Yin L, Hu R, Yang B. Endoscope image mosaic based on pyramid ORB. Biomed Signal Process Control. 2022;71:103261. https://doi.org/10.1016/j.bspc.2021.103261.
Gessert S, Bugner V, Tecza A, Pinker M, Kühl M. FMR1/FXR1 and the miRNA pathway are required for eye and neural crest development. Dev Biol. 2010;341:222–35. https://doi.org/10.1016/j.ydbio.2010.02.031.
Van’t Padje S, Chaudhry B, Severijnen LA, van der Linde HC, Mientjes EJ, Oostra BA, et al. Reduction in fragile X related 1 protein causes cardiomyopathy and muscular dystrophy in zebrafish. J Exp Biol. 2009;212:2564–70. https://doi.org/10.1242/jeb.032532.
Bail S, Swerdel M, Liu H, Jiao X, Goff LA, Hart RP, et al. Differential regulation of microRNA stability. Rna. 2010;16:1032–9. https://doi.org/10.1261/rna.1851510.
Ruegger S, Grosshans H. MicroRNA turnover: when, how, and why. Trends Biochem Sci. 2012;37:436–46. https://doi.org/10.1016/j.tibs.2012.07.002.
Galardi S, Mercatelli N, Giorda E, Massalini S, Frajese GV, Ciafre SA, et al. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem. 2007;282:23716–24. https://doi.org/10.1074/jbc.
Chen AJ, Paik JH, Zhang H, Shukla SA, Mortensen R, Hu J, et al. STAR RNA-binding protein Quaking suppresses cancer via stabilization of specific miRNA. Genes Dev. 2012;26:1459–72. https://doi.org/10.1101/gad.189001.112.
Liu X, Fu R, Pan Y, Meza-Sosa KF, Zhang Z, Lieberman J. PNPT1 release from mitochondria during apoptosis triggers decay of poly(A) RNAs. Cell. 2018;174:187–201 e12. https://doi.org/10.1016/j.cell.2018.04.017.
Jonas K, Calin GA, Pichler M. RNA-binding proteins as important regulators of long non-coding RNAs in cancer. Int J Mol Sci. 2020;21:2969. https://doi.org/10.3390/ijms21082969.
Degrauwe N, Suvà ML, Janiszewska M, Riggi N, Stamenkovic I. IMPs: an RNA-binding protein family that provides a link between stem cell maintenance in normal development and cancer. Genes Dev. 2016;30:2459–74. https://doi.org/10.1101/gad.287540.116.
Khandjian EW. Biology of the fragile X mental retardation protein, an RNA-binding protein. Biochem Cell Biol. 1999;77:331–42.
Ling SC, Fahrner PS, Greenough WT, Gelfand VI. Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proc Natl Acad Sci USA. 2004;101:17428–33. https://doi.org/10.1073/pnas.0408114101.
Davidovic L, Jaglin XH, Lepagnol-Bestel AM, Tremblay S, Simonneau M, Bardoni B, et al. The fragile X mental retardation protein is a molecular adaptor between the neurospecific KIF3C kinesin and dendritic RNA granules. Hum Mol Genet. 2007;16:3047–58. https://doi.org/10.1093/hmg/ddm263.
Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14:926–39. https://doi.org/10.1016/j.devcel.2008.04.003.
Agote-Arán A, Lin J, Sumara I. Fragile X–related protein 1 regulates nucleoporin localization in a cell cycle–dependent manner. Front Cell Dev Biol 2021;9:755847. https://doi.org/10.3389/fcell.2021.755847.
Agote-Aran A, Schmucker S, Jerabkova K, Jmel Boyer I, Berto A, Pacini L, et al. Spatial control of nucleoporin condensation by fragile X-related proteins. EMBO J. 2020;39:e104467. https://doi.org/10.15252/embj.2020104467.
Holzer G, Antonin W. FXR proteins bring new perspectives to nucleoporins’ homeostasis. EMBO J. 2020;39:e106510. https://doi.org/10.15252/embj.2020106510.
Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–24. https://doi.org/10.1101/gad.1399806.
Wilusz CJ, Wormington M, Peltz SW. The cap-to-tail guide to mRNA turnover. Nat Rev Mol Cell Biol. 2001;2:237–46. https://doi.org/10.1038/35067025.
Lapucci A, Donnini M, Papucci L, Witort E, Tempestini A, Bevilacqua A, et al. AUF1 Is a bcl-2 A+ U-rich element-binding protein involved in bcl-2 mRNA destabilization during apoptosis. J Biol Chem. 2002;277:16139–46.
Bakheet T, Frevel M, Williams BR, Greer W, Khabar KS. ARED: human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res. 2001;29:246–54.
Espel E. The role of the AU-rich elements of mRNAs in controlling translation. Semin Cell Dev Biol. 2005;16:59–67. (Academic Press).
Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8:113–26. https://doi.org/10.1038/nrm2104.
Nsengimana B, Khan FA, Ngowi EE, Zhou X, Jin Y, Jia Y, et al. Processing body (P-body) and its mediators in cancer. Mol Cell Biochem. 2022. https://doi.org/10.1007/s11010-022-04359-7.
Khan FA, Nsengimana B, Khan NH, Huang J, Guo H, Awan UA, et al. Differential expression profiles of circRNAs in cancers: Future clinical and diagnostic perspectives. Gene Protein Dis. 2022;138:1. https://doi.org/10.36922/gpd.v1i2.138.
Jin P, Zarnescu DC, Ceman S, Nakamoto M, Mowrey J, Jongens TA, et al. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat Neurosci. 2004;7:113–7. https://doi.org/10.1038/nn1174.
Plante I, Provost P. Hypothesis: a role for fragile X mental retardation protein in mediating and relieving microRNA-guided translational repression? J Biomed Biotechnol. 2006; 16806. https://doi.org/10.1155/JBB/2006/16806.
Vasudevan S, Steitz JA. AU-rich-element-mediated upregulation of translation by FXR1 and argonaute 2. Cell. 2007;128:1105–18. https://doi.org/10.1016/j.cell.2007.01.038.
Davidovic L, Durand N, Khalfallah O, Tabet R, Barbry P, Mari B, et al. A novel role for the RNA-binding protein FXR1P in myoblasts cell-cycle progression by modulating p21/Cdkn1a/Cip1/Waf1 mRNA stability. PLoS Genet. 2013;9:e1003367. https://doi.org/10.1371/journal.pgen.1003367.
George J, Li Y, Kadamberi IP, Parashar D, Tsaih SW, Gupta P, et al. RNA-binding protein FXR1 drives cMYC translation by recruiting eIF4F complex to the translation start site. Cell Rep. 2021;37:109934. https://doi.org/10.1016/j.celrep.2021.109934.
Herman AB, Vrakas CN, Ray M, Kelemen SE, Sweredoski MJ, Moradian A, et al. FXR1 is an IL-19-responsive RNA-binding protein that destabilizes pro-inflammatory transcripts in vascular smooth muscle cells. Cell Rep. 2018;24:1176–89. https://doi.org/10.1016/j.celrep.2018.07.002.
Jehung JP, Kitamura T, Yanagawa-Matsuda A, Kuroshima T, Towfik A, Yasuda M, et al. Adenovirus infection induces HuR relocalization to facilitate virus replication. Biochem Biophys Res Commun. 2018;495:1795–1800. https://doi.org/10.1016/j.bbrc.2017.12.036.
Ivanovska I, Ball AS, Diaz RL, Magnus JF, Kibukawa M, Schelter JM, et al. MicroRNAs in the miR-106b family regulate p21/CDKN1A and promote cell cycle progression. Mol Cell Biol. 2008;28:2167–74. https://doi.org/10.1128/MCB.01977-07.
Patzlaff NE, Nemec KM, Malone SG, Li Y, Zhao X. Fragile X related protein 1 (FXR1P) regulates proliferation of adult neural stem cells. Hum Mol Genet. 2017;26:1340–52. https://doi.org/10.1093/hmg/ddx034.
Muddashetty RS, Nalavadi VC, Gross C, Yao X, Xing L, Laur O, et al. Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol Cell. 2011;42:673–88. https://doi.org/10.1016/j.molcel.2011.05.006.
Schütt J, Falley K, Richter D, Kreienkamp HJ, Kindler S. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities. J Biol Chem. 2009;284:25479–87. https://doi.org/10.1074/jbc.M109.042663.
Patzlaff NE, Shen M, Zhao X. Regulation of adult neurogenesis by the fragile X family of RNA binding proteins. Brain Plast. 2018;3:205–23. https://doi.org/10.3233/BPL-170061.
Gabay M, Li Y, Felsher DW. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med. 2014;4:a014241. https://doi.org/10.1101/cshperspect.a014241.
Li Y, Tang W, Zhang LR, Zhang CY. FMRP regulates miR196a-mediated repression of HOXB8 via interaction with the AGO2 MID domain. Mol Biosyst. 2014;10:1757–64. https://doi.org/10.1039/c4mb00066h.
Harvey RF, Smith TS, Mulroney T, Queiroz RML, Pizzinga M, Dezi V, et al. Trans-acting translational regulatory RNA binding proteins. Wiley Interdiscip Rev RNA. 2018;9:e1465. https://doi.org/10.1002/wrna.1465.
Leppek K, Das R, Barna M. Functional 5’ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat Rev Mol Cell Biol. 2018;19:158–74. https://doi.org/10.1038/nrm.2017.103. Erratum in: Nat Rev Mol Cell Biol. 2018 Oct;19(10):673.
Kastelic N, Landthaler M. mRNA interactome capture in mammalian cells. Methods. 2017;126:38–43. https://doi.org/10.1016/j.ymeth.2017.07.006.
Wang Y, Zhai W, Yang L, Cheng S, Cui W, Li J. Establishments and evaluations of post-operative adhesion animal models. Adv Ther. 2023; https://doi.org/10.1002/adtp.202200297.
Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134:1042–54. https://doi.org/10.1016/j.cell.2008.07.031.
Schenck A, Bardoni B, Moro A, Bagni C, Mandel JL. A highly conserved protein family interacting with the fragile X mental retardation protein (FMRP) and displaying selective interactions with FMRP-related proteins FXR1P and FXR2P. Proc Natl Acad Sci USA. 2001;98:8844–9. https://doi.org/10.1073/pnas.151231598.
Schenck A, Bardoni B, Langmann C, Harden N, Mandel JL, Giangrande A. CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron. 2003;38:887–98. https://doi.org/10.1016/s0896-6273(03)00354-4.
Darnell RB. HITS-CLIP: panoramic views of protein-RNA regulation in living cells. Wiley Interdiscip Rev RNA. 2010;1:266–86. https://doi.org/10.1002/wrna.31.
Ruggero D. Translational control in cancer etiology. Cold Spring Harb Perspect Biol. 2013;5:a012336. https://doi.org/10.1101/cshperspect.a012336. Erratum in: Cold Spring Harb Perspect Biol. 2012; 4(11). doi:10.1101/cshperspect.a015891.
Bukhari SIA, Truesdell SS, Lee S, Kollu S, Classon A, Boukhali M, et al. A specialized mechanism of translation mediated by FXR1a-associated MicroRNP in cellular quiescence. Mol Cell. 2016;61:760–73. https://doi.org/10.1016/j.molcel.2016.02.013.
Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can upregulate translation. Science. 2007;318:1931–4. https://doi.org/10.1126/science.1149460.
Jakymiw A, Lian S, Eystathioy T, Li S, Satoh M, Hamel JC, et al. Disruption of GW bodies impairs mammalian RNA interference. Nat Cell Biol. 2005:1267–74. https://doi.org/10.1038/ncb1334. Erratum in: Nat Cell Biol. 2006; 8(1):100.
Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, et al. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–84. https://doi.org/10.1083/jcb.200502088.
Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, et al. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120:623–34. https://doi.org/10.1016/j.cell.2004.12.038.
Stoecklin G, Ming XF, Looser R, Moroni C. Somatic mRNA turnover mutants implicate tristetraprolin in the interleukin-3 mRNA degradation pathway. Mol Cell Biol. 2000;20:3753–63. https://doi.org/10.1128/MCB.20.11.3753-3763.2000.
Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001;58:266–77. https://doi.org/10.1007/PL00000rna4.
Mazan-Mamczarz K, Galbán S, López de Silanes I, Martindale JL, Atasoy U, et al. RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc Natl Acad Sci USA. 2003;100:8354–9. https://doi.org/10.1073/pnas.1432104100.
Garnon J, Lachance C, Di Marco S, Hel Z, Marion D, Ruiz MC, et al. Fragile X-related protein FXR1P regulates pro-inflammatory cytokine tumor necrosis factor expression at the post-transcriptional level. J Biol Chem. 2005;280:5750–63. https://doi.org/10.1074/jbc.M401988200.
Baquero-Perez B, Antanaviciute A, Yonchev ID, Carr IM, Wilson SA, Whitehouse A. The Tudor SND1 protein is an m6A RNA reader essential for replication of Kaposi’s sarcoma-associated herpesvirus. Elife. 2019;8:e47261. https://doi.org/10.7554/eLife.47261.
Price AM, Hayer KE, McIntyre ABR, Gokhale NS, Abebe JS, Della Fera AN, et al. Direct RNA sequencing reveals m6A modifications on adenovirus RNA are necessary for efficient splicing. Nat Commun. 2020;11:6016. https://doi.org/10.1038/s41467-020-19787-6.
Bhogal B, Jepson JE, Savva YA, Pepper AS, Reenan RA, Jongens TA. Modulation of dADAR-dependent RNA editing by the Drosophila fragile X mental retardation protein. Nat Neurosci. 2011;14:1517–24. https://doi.org/10.1038/nn.2950.
Tran SS, Jun HI, Bahn JH, Azghadi A, Ramaswami G, Van Nostrand EL, et al. Widespread RNA editing dysregulation in brains from autistic individuals. Nat Neurosci. 2019;22:25–36. https://doi.org/10.1038/s41593-018-0287-x.
Freischmidt A, Goswami A, Limm K, Zimyanin VL, Demestre M, Glaß H, et al. A serum microRNA sequence reveals fragile X protein pathology in amyotrophic lateral sclerosis. Brain. 2021;144:1214–29. https://doi.org/10.1093/brain/awab018.
Richter JD, Zhao X. The molecular biology of FMRP: new insights into fragile X syndrome. Nat Rev Neurosci. 2021;22:209–22. https://doi.org/10.1038/s41583-021-00432-0.
Taha MS, Haghighi F, Stefanski A, Nakhaei-Rad S, Kazemein Jasemi NS, Al Kabbani MA, et al. Novel FMRP interaction networks linked to cellular stress. FEBS J. 2021;288:837–60. https://doi.org/10.1111/febs.15443.
Deng PY, Klyachko VA. Channelopathies in fragile X syndrome. Nat Rev Neurosci. 2021;22:275–89. https://doi.org/10.1038/s41583-021-00445-9.
Glauninger H, Wong Hickernell CJ, Bard JAM, Drummond DA. Stressful steps: progress and challenges in understanding stress-induced mRNA condensation and accumulation in stress granules. Mol Cell. 2022;82:2544–56. https://doi.org/10.1016/j.molcel.2022.05.014.
Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell. 2016;164:487–98. https://doi.org/10.1016/j.cell.2015.12.038.
Didiot MC, Subramanian M, Flatter E, Mandel JL, Moine H. Cells lacking the fragile X mental retardation protein (FMRP) have normal RISC activity but exhibit altered stress granule assembly. Mol Biol Cell. 2009;20:428–37. https://doi.org/10.1091/mbc.e08-07-0737.
Jeon SJ, Han SH, Yang SI, Choi JW, Kwon KJ, Park SH, et al. Positive feedback regulation of Akt-FMRP pathway protects neurons from cell death. J Neurochem. 2012;123:226–38. https://doi.org/10.1111/j.1471-4159.2012.07886.x.
Shimizu H, Hohjoh H. FMRP, FXR1 protein and Dlg4 mRNA, which are associated with fragile X syndrome, are involved in the ubiquitin-proteasome system. Sci Rep. 2023;13:1956. https://doi.org/10.1038/s41598-023-29152-4.
Zhao K, Gao J, Shi J, Shi C, Pang C, Li J, et al. FXR1 promotes proliferation, invasion and migration of hepatocellular carcinoma in vitro and in vivo. Oncol Lett. 2023;25:1–1.
Yang C, Ströbel P, Marx A, Hofmann I. Plakophilin-associated RNA-binding proteins in prostate cancer and their implications in tumor progression and metastasis. Virchows Arch. 2013;463:379–90. https://doi.org/10.1007/s00428-013-1452-y.
Qie S, Majumder M, Mackiefwicz K, Howley BV, Peterson YK, Howe PH, et al. Fbxo4-mediated degradation of Fxr1 suppresses tumorigenesis in head and neck squamous cell carcinoma. Nat Commun. 2017;8:1534. https://doi.org/10.1038/s41467-017-01199-8.
Deng M, Wang N, Li Z, Chen R, Duan J, Peng Y, et al. FXR1 can bind with the CFIm25/CFIm68 complex and promote the progression of urothelial carcinoma of the bladder by stabilizing TRAF1 mRNA. Cell Death Dis. 2022;13:170. https://doi.org/10.1038/s41419-022-04614-1.
Cao S, Zheng J, Liu X, Liu Y, Ruan X, Ma J, et al. FXR1 promotes the malignant biological behavior of glioma cells via stabilizing MIR17HG. J Exp Clin Cancer Res. 2019;38:1–22.
Cao H, Gao R, Yu C, Chen L, Feng Y. The RNA-binding protein FXR1 modulates prostate cancer progression by regulating FBXO4. Funct Integr Genom. 2019;19:487–96. https://doi.org/10.1007/s10142-019-00661-8.
Fan Y, Yue J, Xiao M, Han-Zhang H, Wang YV, Ma C, et al. FXR1 regulates transcription and is required for growth of human cancer cells with TP53/FXR2 homozygous deletion. Elife 2017;6:e26129. https://doi.org/10.7554/eLife.26129.
Majumder M, Palanisamy V. RNA binding protein FXR1-miR301a-3p axis contributes to p21WAF1 degradation in oral cancer. PLoS Genet. 2020;16:e1008580. https://doi.org/10.1371/journal.pgen.1008580.
Ban Y, Tan Y, Li X, Li X, Zeng Z, Xiong W, et al. RNA-binding protein YBX1 promotes cell proliferation and invasiveness of nasopharyngeal carcinoma cells via binding to AURKA mRNA. J Cancer. 2021;12:3315–24. https://doi.org/10.7150/jca.56262.
Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–95. https://doi.org/10.1038/s41556-018-0045-z. Erratum in: Nat Cell Biol. 2018; 20(9):1098. Erratum in: Nat Cell Biol. 2020; 22(10):1288.
Li Y, Soendergaard C, Bergenheim FH, Aronoff DM, Milne G, Riis LB, et al. COX-2–PGE2 signaling impairs intestinal epithelial regeneration and associates with TNF inhibitor responsiveness in ulcerative colitis. EBioMedicine. 2018;36:497–507.
Wang Z, Tong D, Han C, Zhao Z, Wang X, Jiang T, et al. Blockade of miR-3614 maturation by IGF2BP3 increases TRIM25 expression and promotes breast cancer cell proliferation. EBioMedicine. 2019;41:357–69. https://doi.org/10.1016/j.ebiom.2018.12.061. Erratum in: EBioMedicine. 2023; 88:104423.
Mizutani R, Imamachi N, Suzuki Y, Yoshida H, Tochigi N, Oonishi T, et al. Oncofetal protein IGF2BP3 facilitates the activity of proto-oncogene protein eIF4E through the destabilization of EIF4E-BP2 mRNA. Oncogene. 2016;35:3495–502. https://doi.org/10.1038/onc.2015.410.
Rivera Vargas T, Boudoukha S, Simon A, Souidi M, Cuvellier S, Pinna G, et al. Post-transcriptional regulation of cyclins D1, D3 and G1 and proliferation of human cancer cells depend on IMP-3 nuclear localization. Oncogene. 2014;33:2866–75. https://doi.org/10.1038/onc.2013.252.
Nussbacher JK, Yeo GW. Systematic discovery of RNA binding proteins that regulate MicroRNA levels. Mol Cell. 2018;69:1005–16 e7. https://doi.org/10.1016/j.molcel.2018.02.012.
Zhou X, Lu J, Wu B, Guo Z. HOXA11-AS facilitates the proliferation, cell cycle process and migration of keloid fibroblasts through sponging miR-188–5p to regulate VEGFA. J Dermatol Sci. 2022;106:111–8. https://doi.org/10.1016/j.jdermsci.2022.04.004.
Lu Y, Gao W, Zhang C, Wen S, Huangfu H, Kang J, et al. Hsa-miR-301a-3p acts as an oncogene in laryngeal squamous cell carcinoma via target regulation of Smad4. J Cancer. 2015;6:1260–75. https://doi.org/10.7150/jca.12659.
van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev cancer. 2011;11:644–56. https://doi.org/10.1038/nrc3107.
Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006;20:1885–98. https://doi.org/10.1101/gad.1424106.
Lykke-Andersen J, Wagner E. Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1. Genes Dev. 2005;19:351–61. https://doi.org/10.1101/gad.1282305.
Jiang P, Coller H. Functional interactions between microRNAs and RNA binding proteins. MicroRNA. 2012;1:70–9. https://doi.org/10.2174/2211536611201010070.
Li C, Lin L, Zhang L, Xu R, Chen X, Ji J, et al. Long noncoding RNA p21 enhances autophagy to alleviate endothelial progenitor cells damage and promote endothelial repair in hypertension through SESN2/AMPK/TSC2 pathway. Pharmacol Res. 2021;173:105920. https://doi.org/10.1016/j.phrs.2021.105920.
Zhao H, Tang S, Tao Q, Ming T, Lei J, Liang Y, et al. Ursolic acid suppresses colorectal cancer by down-regulation of Wnt/β-catenin signaling pathway activity. J Agric Food Chem. 2023;71:3981–93. https://doi.org/10.1021/acs.jafc.2c06775.
Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–208. https://doi.org/10.1158/1078-0432.CCR-08-0196.
Zongaro S, Hukema R, D’Antoni S, Davidovic L, Barbry P, Catania MV. et al. The 3’ UTR of FMR1 mRNA is a target of miR-101, miR-129-5p and miR-221: implications for the molecular pathology of FXTAS at the synapse. Hum Mol Genet. 2013;22:1971–82. https://doi.org/10.1093/hmg/ddt044.
Zhu Y, Huang R, Wu Z, Song S, Cheng L, Zhu R. Deep learning-based predictive identification of neural stem cell differentiation. Nat Commun. 2021;12:2614. https://doi.org/10.1038/s41467-021-22758-0.
Bebee TW, Cieply BW, Carstens RP. Genome-wide activities of RNA binding proteins that regulate cellular changes in the epithelial to mesenchymal transition (EMT). Adv Exp Med Biol. 2014;825:267–302. https://doi.org/10.1007/978-1-4939-1221-6_8.
Bai X, Yi M, Jiao Y, Chu Q, Wu K, Blocking TGF-. β signaling to enhance the efficacy of immune checkpoint inhibitor. Onco Targets Ther. 2019;12:9527–38. https://doi.org/10.2147/OTT.S224013.
Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–26. https://doi.org/10.1016/j.tcb.2018.12.001.
Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol. 2020;30:764–76. https://doi.org/10.1016/j.tcb.2020.07.003.
Yuan GJ, Li QW, Shan SL, Wang WM, Jiang S, Xu XM. Hyperthermia inhibits hypoxia-induced epithelial-mesenchymal transition in HepG2 hepatocellular carcinoma cells. World J Gastroenterol. 2012;18:4781–6. https://doi.org/10.3748/wjg.v18.i34.4781.
Peng P, Luan Y, Sun P, Wang L, Zeng X, Wang Y, et al. Prognostic factors in stage iv colorectal cancer patients with resection of liver and/or pulmonary metastases: a population-based cohort study. Front Oncol. 2022; 12. https://doi.org/10.3389/fonc.2022.850937.
Xu H, Li L, Wang S, Wang Z, Qu L, Wang C, et al. Royal jelly acid suppresses hepatocellular carcinoma tumorigenicity by inhibiting H3 histone lactylation at H3K9la and H3K14la sites. Phytomedicine. 2023; 154940. https://doi.org/10.1016/j.phymed.2023.154940.
Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. https://doi.org/10.1186/1756-9966-30-87.
Kang D, Lee Y, Lee JS. RNA-binding proteins in cancer: functional and therapeutic perspectives. Cancers. 2020;12:2699. https://doi.org/10.3390/cancers12092699.
Chen S, Zhao Y, Shen F, Long D, Yu T, Lin X. Introduction of exogenous wild‑type p53 mediates the regulation of oncoprotein 18/stathmin signaling via nuclear factor‑κB in non‑small cell lung cancer NCI‑H1299 cells. Oncol Rep. 2019;41:2051–9. https://doi.org/10.3892/or.2019.6964.
Chen X, Liao Y, Long D, Yu T, Shen F, Lin X. The Cdc2/Cdk1 inhibitor, purvalanol A, enhances the cytotoxic effects of taxol through Op18/stathmin in non-small cell lung cancer cells in vitro. Int J Mol Med. 2017;40:235–42. https://doi.org/10.3892/ijmm.2017.2989.
Lee M, Lee JS. Exploiting tumor cell senescence in anticancer therapy. BMB Rep. 2014;47:51–9. https://doi.org/10.5483/bmbrep.2014.47.2.005.
Lee S, Lee JS. Cellular senescence: a promising strategy for cancer therapy. BMB Rep. 2019;52:35–41. https://doi.org/10.5483/BMBRep.2019.52.1.294.
Khan FA, Nsengimana B, Khan NH, Song Z, Ngowi EE, Wang Y, et al. Chimeric peptides/proteins encoded by circRNA: an update on mechanisms and functions in human cancers. Front Oncol. 2022;12:781270. https://doi.org/10.3389/fonc.2022.781270.
Fernández E, Mallette FA. The rise of FXR1: escaping cellular senescence in head and neck squamous cell carcinoma. PLoS Genet. 2016;12:e1006344. https://doi.org/10.1371/journal.pgen.1006344.
Macfarlane LA, Murphy PR. MicroRNA: biogenesis, function and role in cancer. Curr Genom. 2010;11:537–61. https://doi.org/10.2174/138920210793175895.
Zhang Y, Zeng M, Li B, Zhang B, Cao B, Wu Y, et al. Ephedra Herb extract ameliorates adriamycin-induced nephrotic syndrome in rats via the CAMKK2/AMPK/mTOR signaling pathway. Chin J Nat Med. 2023;21:371–82. https://doi.org/10.1016/S1875-5364(23)60454-6.
Zhou F, Zhang F, Zhou C, Liang M, Cai Z, Lv H, et al. Human antigen R and drug resistance in tumors. Invest N. Drugs. 2019;37:1107–16. https://doi.org/10.1007/s10637-018-00723-x.
Huang H, Zhang J, Jiang P, Xu X, Huang F, Zhao B, et al. FXR1 facilitates axitinib resistance in clear cell renal cell carcinoma via regulating KEAP1/Nrf2 signaling pathway. Anticancer Drugs. 2023;34:248–56. https://doi.org/10.1097/CAD.0000000000001416.
Cai J, Wang H, Jiao X, Huang R, Qin Q, Zhang J, et al. The RNA-binding protein HuR confers oxaliplatin resistance of colorectal cancer by upregulating CDC6. Mol Cancer Ther. 2019;18:1243–54. https://doi.org/10.1158/1535-7163.MCT-18-0945.
Lin GL, Ting HJ, Tseng TC, Juang V, Lo YL. Modulation of the mRNA-binding protein HuR as a novel reversal mechanism of epirubicin-triggered multidrug resistance in colorectal cancer cells. PLoS One. 2017;12:e0185625. https://doi.org/10.1371/journal.pone.0185625.
Andrade D, Mehta M, Griffith J, Oh S, Corbin J, Babu A, et al. HuR reduces radiation-induced DNA damage by enhancing expression of ARID1A. Cancers. 2019;11:2014. https://doi.org/10.3390/cancers11122014.
Raguraman R, Shanmugarama S, Mehta M, Peterson JE, Zhao YD, Munshi A, et al. Drug delivery approaches for HuR-targeted therapy for lung cancer. Adv Drug Deliv Rev. 2022;180:114068.
Lu L, Dong J, Liu Y, Qian Y, Zhang G, Zhou W, et al. etNew insights into natural products that target the gut microbiota: effects on the prevention and treatment of colorectal cancer. Front Pharmacol 2022;13:964793. https://doi.org/10.3389/fphar.2022.964793.
Xu H, Van der Jeught K, Zhou Z, Zhang L, Yu T, Sun Y, et al. Atractylenolide I enhances responsiveness to immune checkpoint blockade therapy by activating tumor antigen presentation. J Clin Investig. 2021;131: https://doi.org/10.1172/JCI146832.
Zhao Y, Chen S, Shen F, Long D, Yu T, Wu M, et al. In vitro neutralization of autocrine IL‑10 affects Op18/stathmin signaling in non‑small cell lung cancer cells. Oncol Rep. 2019;41:501–11. https://doi.org/10.3892/or.2018.6795.
Lambeth LS, Smith CA. Short hairpin RNA-mediated gene silencing. Methods Mol Biol. 2013;942:205–32. https://doi.org/10.1007/978-1-62703-119-6_12.
Bevacqua RJ, Dai X, Lam JY, Gu X, Friedlander MSH, Tellez K, et al. CRISPR-based genome editing in primary human pancreatic islet cells. Nat Commun. 2021;12:2397. https://doi.org/10.1038/s41467-021-22651-w.
Crunkhorn S. CRISPR-engineered fat cells prevent obesity. Nat Rev Drug Discov. 2020;19:672–3.
Wu W, Tang L, D’Amore PA, Lei H. Application of CRISPR-Cas9 in eye disease. Exp Eye Res. 2017;161:116–23. https://doi.org/10.1016/j.exer.2017.06.007.
Caruthers SD, Wickline SA, Lanza GM. Nanotechnological applications in medicine. Curr Opin Biotechnol. 2007;18:26–30. https://doi.org/10.1016/j.copbio.2007.01.006.
García-Fernández C, Fornaguera C, Borrós S. Nanomedicine in non-small cell lung cancer: from conventional treatments to immunotherapy. Cancers. 2020;12:1609. https://doi.org/10.3390/cancers12061609.
Subhan MA, Torchilin VP. Efficient nanocarriers of siRNA therapeutics for cancer treatment. Transl Res. 2019;214:62–91. https://doi.org/10.1016/j.trsl.2019.07.006.
Crintea A, Dutu AG, Samasca G, Florian IA, Lupan I, Craciun AM. The nanosystems involved in treating lung cancer. Life. 2021;11:682. https://doi.org/10.3390/life11070682.
Mukherjee A, Paul M, Mukherjee S. Recent progress in the theranostics application of nanomedicine in lung cancer. Cancers. 2019;11:597. https://doi.org/10.3390/cancers11050597.
Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharm Ther. 2018;188:1–11. https://doi.org/10.1016/j.pharmthera.2018.02.013.
Wong CH, Chen YC. Clinical significance of exosomes as potential biomarkers in cancer. World J Clin Cases. 2019;7:171–90. https://doi.org/10.12998/wjcc.v7.i2.171.
Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteom Clin Appl. 2015;9:358–67. https://doi.org/10.1002/prca.201400114.
Kim KU, Kim WH, Jeong CH, Yi DY, Min H. More than nutrition: therapeutic potential of breast milk-derived exosomes in cancer. Int J Mol Sci. 2020;21:7327. https://doi.org/10.3390/ijms21197327.
Morad G, Carman CV, Hagedorn EJ, Perlin JR, Zon LI, Mustafaoglu N, et al. Tumor-derived extracellular vesicles breach the intact blood-brain barrier via transcytosis. ACS Nano. 2019;13:13853–65. https://doi.org/10.1021/acsnano.9b04397.
Le BT, Raguraman P, Kosbar TR, Fletcher S, Wilton SD, Veedu RN. Antisense oligonucleotides targeting angiogenic factors as potential cancer therapeutics. Mol Ther Nucleic Acids. 2019;14:142–57. https://doi.org/10.1016/j.omtn.2018.11.007.
Nardella C, Clohessy JG, Alimonti A, Pandolfi PP. Pro-senescence therapy for cancer treatment. Nat Rev Cancer. 2011;11:503–11. https://doi.org/10.1038/nrc3057.
Di Fusco D, Dinallo V, Marafini I, Figliuzzi MM, Romano B, Monteleone G. Antisense oligonucleotide: basic concepts and therapeutic application in inflammatory bowel disease. Front Pharmacol. 2019;10:305.
Wang K, Li L, Fu L, Yuan Y, Dai H, Zhu T, et al. Integrated bioinformatics analysis the function of RNA binding proteins (RBPs) and their prognostic value in breast cancer. Front Pharm. 2019;10:140. https://doi.org/10.3389/fphar.2019.00140.
Li W, Gao LN, Song PP, et al. Development and validation of a RNA binding protein-associated prognostic model for lung adenocarcinoma. Aging 2020;12:3558–73. https://doi.org/10.18632/aging.10282. Erratum in: Aging. 2021;13(5):7708.
Mitchell SF, Parker R. Principles and properties of eukaryotic mRNPs. Mol Cell. 2014;54:547–58. https://doi.org/10.1016/j.molcel.2014.04.033.
Tu HC, Schwitalla S, Qian Z, LaPier GS, Yermalovich A, Ku YC, et al. LIN28 cooperates with WNT signaling to drive invasive intestinal and colorectal adenocarcinoma in mice and humans. Genes Dev. 2015;29:1074–86. https://doi.org/10.1101/gad.256693.114.
Huang YH, Peng W, Furuuchi N, Gerhart J, Rhodes K, Mukherjee N, et al. Delivery of therapeutics targeting the mRNA-binding protein HuR using 3DNA nanocarriers suppresses ovarian tumor growth. Cancer Res. 2016;76:1549–59. https://doi.org/10.1158/0008-5472.CAN-15-2073. Erratum in: Cancer Res. 2016; 76:3437.
Roos M, Pradère U, Ngondo RP, Behera A, Allegrini S, Civenni G, et al. A small-molecule inhibitor of Lin28. ACS Chem Biol. 2016;11:2773–81. https://doi.org/10.1021/acschembio.6b00232.
Wang Y, Cheong CG, Hall TM, Wang Z. Engineering splicing factors with designed specificities. Nat Methods. 2009;6:825–30. https://doi.org/10.1038/nmeth.1379.
Kaczmarek JC, Kowalski PS, Anderson DG. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017;9:60. https://doi.org/10.1186/s13073-017-0450-0.
Wang X, Chen G, Zhang Y, Ghareeb WM, Yu Q, Zhu H, et al. The impact of circumferential tumour location on the clinical outcome of rectal cancer patients managed with neoadjuvant chemoradiotherapy followed by total mesorectal excision. Eur J Surg Oncol. 2020;46:1118–23. https://doi.org/10.1016/j.ejso.2020.02.034.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31371386); and the Natural Science Foundation of Henan Province (No.162300410042).
Author information
Authors and Affiliations
Contributions
FAK conducted research and drafted the original manuscript. FAK, and SJ provided assistance in the process of revising the manuscript and figures and tables construction. FAK and SJ contributed to the conceptual framework. SJ, WJ, and NF supervised the study and revised the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and have been approved for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Consent For Publication All authors consent to publication.
Edited by Dr.George Calin
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, F.A., Fang, N., Zhang, W. et al. The multifaceted role of Fragile X-Related Protein 1 (FXR1) in cellular processes: an updated review on cancer and clinical applications. Cell Death Dis 15, 72 (2024). https://doi.org/10.1038/s41419-023-06413-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-023-06413-8