Abstract
Background
Luminal breast cancer (BC) is the predominant subtype of breast cancer with a sustained risk of late recurrence and death. Understanding the molecular mechanisms for the oncogenesis of luminal BC would improve the prognosis for this large subset of patients. SPDEF was reported to be dysregulated in breast cancers. However, the biological functions and underlying molecular mechanism of SPDEF in luminal BC remains largely unknown. The aim of the present study was to elucidate the potential roles of SPDEF underlying subtype-specific functions in BC, especially in luminal subtypes.
Methods
The expressions and clinicopathological characteristics of SPDEF in luminal BC patients were evaluated bioinformatically. In vitro and in vivo assays were performed to investigate the oncogenic function and stemness maintenance of SPDEF in luminal BC. Chromatin immunoprecipitation (ChIP) and dual luciferase reporter assays were conducted to determine the transcription regulation of GALNT7 by SPDEF. GALNT7 levels in serum from luminal BC patients were further detected by enzyme-linked immunosorbent assay (ELISA).
Results
SPDEF is markedly upregulated in luminal BC and positively associated with tumor progression and poor prognosis. Furthermore, we confirmed that SPDEF enhanced the proliferation, migration, invasion and stemness of luminal BC cells in vitro as well the tumorigenicity in vivo. Mechanistically, we demonstrated the stimulative effect of SPDEF on the progression and stemness of luminal BC, which is mediated by its directly transcriptional target GALNT7. Clinically, we verified that the GALNT7 can be used as a noninvasive diagnostic marker. Noteworthy, the combined detection of serum GALNT7 and traditional tumor markers can enhance diagnostic accuracy thus is of vital importance in the early diagnosis of luminal BC.
Conclusions
Our study reveals a novel mechanism by which SPDEF transcriptionally activates GALNT7 via directly binding to its promoter to promote cell proliferation, motility and stemness, and led to luminal BC tumorigenesis and poor prognosis.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most common cancer worldwide and the leading cause of cancer-related death among women [1]. The high heterogeneous and diversity of BC brings difficulty to mechanisms research and hinders the development of molecularly targeted drugs, thereby enhancing the mortality of BC patients [2]. BC could be clustered into molecular subtypes, including luminal, human epidermal growth factor receptor 2-positive (HER2+) and triple-negative breast cancer (TNBC) tumors based on the expression of the estrogen receptor (ER), progesterone receptor (PR), and the overexpression of the HER2. Hereinto, the most common subtype is the luminal BC, which represents approximately two-thirds of all breast cancer [3]. Luminal BC is typically ER+ and/or PR+ and is generally associated with a better prognosis [4, 5]. While initial ER and/or PR-dependent treatment can be promising, the long-term efficacy of endocrine therapy is diminished by the recurrence of resistant tumors that have lost dependence on estrogen for growth [6]. Therefore, the survival of luminal BC patients is no better than that of patients with basal-like tumors after 20 years of follow-up [7]. Understanding the molecular mechanisms for the oncogenesis of luminal BC should lead to the identification of new molecules for targeted therapy that would improve the prognosis for this large subset of patients.
SPDEF (SAM pointed domain containing ETS transcription factor), belongs to the ETS (E26 transformation-specific) transcription factor family [8, 9], whose expression is largely restricted to epithelial tissues, including the lung, stomach, colon, and hormone-regulated epithelia such as the prostate, breast, and ovary [10,11,12]. SPDEF plays fundamental roles in mucus secretion, goblet cell differentiation, and tumorigenesis [11]. However, the roles of SPDEF in tumor progression currently remain controversial. The expression and molecular functions of SPDEF vary in different tumors. SPDEF is overexpressed and promotes the tumorigenesis and metastasis of ovarian and gastric cancers [11, 13], whereas it can inhibit prostate and colorectal tumor progression [14, 15]. In particular, the expression, biological role and the clinical significance of SPDEF in BC remains elusive. Several studies have demonstrated that SPDEF expression is lost in invasive BC cancer cell lines, supporting a tumor-suppressive function [16,17,18]. Conversely, SPDEF expression is up-regulated during BC progression, associated with increased tumor aggressiveness, suggesting instead a possible oncogenic role [19, 20]. Thus, it is great important to investigate the potential mechanisms of SPDEF underlying subtype-specific functions in BC.
In this study, we verified the oncogenic role of SPDEF in luminal BC progression and its clinicopathologic and prognostic importance in patients with luminal BC. Moreover, we revealed a novel mechanism by which SPDEF transcriptionally activates GALNT7 via directly binding to its promoter to facilitate proliferation, motility and stemness of luminal BC cells in vitro as well as tumorigenicity in vivo. Finally, we found that GALNT7 exerted a tumorigenic role which served as a promising no-invasion biomarker for combined diagnosis.
Materials and Methods
Gene expression profiling interactive analysis (GEPIA database)
GEPIA [21, 22] (http://gepia2.cancer-pku.cn/#survival) provides website analysis functions, including the gene expression analysis of tumor/normal, differential analysis of cancer types or pathological stages and survival analysis. The expression and relapse-free survival analysis (RFS) analysis of GALNT7 in BC were analyzed by GEPIA.
TCGA database analysis
mRNA expression in human BC subtypes was analyzed by TCGA Research Network (http://cancergenome.nih.gov). The original data from the TCGA database was normalized and analyzed by the edgeR analysis method [23]. To analyze the overall survival of patients with BC subtypes, patient samples were analyzed by Kaplan-Meier analysis.
Immunohistochemistry (IHC)
Tissue specimens were collected from 128 participants, which involved 59 adjacent tissues and 69 luminal BC cases. Paraffin-embedded BC tissues were obtained from the Pathology Department of the Affiliated Hospital of Southwest Medical University. Firstly, paraffin-embedded tissue slides were dewaxed and blocked with normal goat serum (ZSGB-BIO, China) for nonspecific staining treatment at temperature for 30 min, after which they were treated with the primary antibody rabbit anti-SPDEF (1:200) (ABclone, USA) overnight at 4 °C. Subsequently, the slides were treated with biotinylated anti-rabbit IgG secondary antibody and next with horseradish peroxidase-conjugated streptavidin complex (ZSGB-BIO, China). Finally, the slides were incubated with adiaminobenzidine chromogen (DAB) solution and counterstained with hematoxylin to develop the colour reaction. Patient Consent Forms were obtained according to protocols approved by the Institutional Review Board of the Affiliated Hospital of Southwest Medical University. The H-score, or histochemical score was used to conduct quantitative assessment in immunohistochemistry (IHC). The staining intensity is scored on a scale of 0 to 3 (with 0 being no staining, 1+ being weak staining, 2+ being moderate staining, and 3+ being strong staining). The percentage of cells stained at each intensity level is then determined. H-score = (Percentage of cells stained at 1+ intensity) + (2 x Percentage of cells stained at 2+ intensity) + (3 x Percentage of cells stained at 3+ intensity).
Survival analysis in Kaplan-Meier plotter database
To analyze the survival rates of patients with luminal BC, the prognostic significance of mRNA expression was evaluated using Kaplan-Meier Plotter [24, 25] (http://kmplot.com/analysis), which contained gene expression data and survival information of luminal BC patients.
Cell culture
Normal human mammary cells MCF10A and human breast cancer cell lines, MCF7, T47D and BT-474 cell lines were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). MCF10A were cultured in special medium (Procell, China). MCF7 and T47D cells were maintained in DMEM high glucose medium (Hyclone, USA) supplemented with 10% fetal bovine serum (Gibco, USA). BT-474 cell were maintained in RPMI-1640 (Gibco, USA) supplemented with 20% fetal bovine serum (Gibco, USA), 10 μg/mL Insulin (Beyotime, China) and 2mM L-glutamine (Procell, China). Cells were incubated at 37 °C in an atmosphere of 5% CO2.
RNA extraction and quantitative reverse transcription PCR (RT‐qPCR) analysis
Total RNA was extracted from cells using the Trizol (Takara, Japan) and chloroform. RNA concentration was measured using NanoDrop OneTM (Thermo Scientific, USA). And RNA converted into cDNA using PrimeScript RT reagent Kit (Takara, Japan). Quantitative PCR (qPCR) was conducted using the TB Green™ Premix Ex Taq™ II (Takara, Japan) in the Bio‐Rad CFX96 system according to the manufacturer’s protocol. The sequences of PCR primers are listed in Table S1.
Stable knockdown of genes by lentivirus infection
Stable knockdown of SPDEF and over-expression of GALNT7 in breast cancer cells was achieved with infection of sh-SPDEF (GeneBiogist, China) in the presence of 8 µg/ml polybrene (Genepharma, China) for 24 h. 48 h post the infection, the efficiency of infection was determined by RT-qPCR. Corresponding negative control (NC) cell lines were established by infection of viruses expressing empty vectors.
Western blot
The proteins were extracted by Protein Extraction Kit (NCM Biotech, China). 50 µg protein sample was separated on 10% polyacrylamide gel (NCM Biotech, China) and transferred to PVDF membrane (Bio‐Rad, USA). Blocking the membrane in 5% notfat milk dissolved in TBST for 1-hour at room temperature. Then the membrane was hybridized in primary antibodies overnight at 4 °C on a shaker. The following primary antibodies were used: anti-SPDEF (1:7500 dilution, ABclone, USA), anti-GALNT7 (1:2500 dilution, TianQiShun, China) and anti-GAPDH (1:7500 dilution, Proteintech, USA). The membrane was incubated with the secondary antibodies diluted in TBST. Finally, the ECL system was used for HRP-conjugated secondary antibody (Bio-Rad, USA).
Cell proliferation assay
Cellular proliferation was detected using a CCK-8 colorimetric assay kit (Beyotime, China). Luminal BC cells (2 × 103/well) were seeded in 96-well plates to culture. CCK8 (10 μl) reagent was added to each well and the absorbance of each well at 450 nm was measured every 24 h with the multimode microplate reader (EnSpire, Singapore). Cell proliferation activity was continuously detected for 6 days.
Wound scratch assay
The scratch test was used for cell migration assay. 3 × 105 cells were seeded into a 6-well plate and cultured in medium with 10% FBS. When the cells grew to 80% confluence, the scratch was scored monolayer cells with sterile pipette tip, and continue to incubate with serum-free medium.
Transwell migration and invasion assays
Transwell chambers were used to execute cell invasion assay with Matrigel (60 μl, 1:10 dilution in serum-free medium, Corning, USA) and migration assay without Matrigel. Liminal BC cells (5 × 104/well) were seeded in the upper chamber in 100 μl of serum-free medium, at the same time, 600 μl medium with 10% FBS was added to the lower chamber as a chemoattractant. After incubation at 37 °C for 24 h, the upper surface of the membrane was gently removed with a swab. While the cells invaded the lower surface, the membrane was stained with 0.1% crystal violet. The stained cells in 3 randomly selected fields were counted.
Transient overexpression assay
hGALNT7 pReceiver‐M98 and negative control were purchased from GeneCopoeia (GeneCopoeia, USA). Transient overexpression assay was performed and optimized using Lipo8000TM (Beyotime, China) and MEM medium (Gibco, USA) according to the manufacturer’s protocol.
Gene set enrichment analysis (GSEA)
GSEA software [26, 27] (http://www.gsea-msigdb.org/gsea/index.jsp) and C2 gene sets (curated gene sets) were used to analyze the TCGA Luminal BC dataset.
Clinical characteristics and differential analysis of stemness indices
The stemness indices, mRNAsi (mRNA expression-based stemness index) and mDNAsi (DNA methylation-based stemness index) were uesd to describe the similar features between cancer cells and stem cells and it might be considered as the stemness indices of Cancer Stem Cells (CSCs) [28]. TCGA luminal BC samples were divided into two groups according to mRNA expressions and the mRNAsi and mDNAsi indices of the two groups were evaluated based on R analysis.
Sphere formation assay
Luminal BC cells were plated at 1 × 103 cells/well in low adhesion 6-well plates and were maintained in DMEM/F12 medium (Gibco, USA), supplemented with 2% B27 (Gibco, USA), 20 ng/ml Recombinant Human EGF (Peprotech, USA) and 20 ng/ml Recombinant Human bFGF (Peprotech, USA). After the cells were incubated for 12 days, the size and number of tumor spheres were calculated.
Colony formation assay
MCF7 or T47D cells were digested with trypsin, suspended as a single cell and counted using the Bullboar counting plate. 1 × 103 cells were seeded in 6-well plates and incubated for 1 week at 37 °C. Then, cells were washed with PBS, fixed with 4% Methanol for 30 min (mins) and stained for 10 min with 0.1% crystal violet.
Subcutaneous tumor transplantation assay
Single-cell suspensions at designated concentrations were combined with equal volume of Matrigel Matrix (R&D Systems, USA). Then the mixture was injected subcutaneously into each side of the hind leg of BALB/c nude mice. After tumor cell transplantation, the tumor dimensions were measured every other day and the volume was calculated using V = (length × width^2)/2. When the volume reached about 1 cm [3], the mice were sacrificed and the neoplasm was excised for weighting. All protocols were approved by the Institutional Animal Care and Use Committee of Southwest Medical University and conducted with humane animal care.
Flow cytometry
The expression of the ALDH1A1 marker was evaluated using flow cytometry, adhering strictly to the manufacturer’s protocol. We utilized the primary anti‐ALDH1A1 antibody at specific working dilutions (0.2 μg of 60171-1-Ig, Clone:1A10A2, sourced from Proteintech, USA). This was followed by the application of the secondary antibody, Fluorescein (FITC)–conjugated Affinipure Goat Anti-Mouse IgG(H + L), at a dilution ranging between 1:20 and 1:100 (also provided by Proteintech, USA).
Drug sensitivity assays
CCK-8 colorimetric assay was used to detect the sensitivity of cells to paclitaxel in vitro. Cells were resuspended in a final concentration of 2 × 103 cells/well, seeded into 96 well plates and subjected to paclitaxel after preincubation for 48 h. After 24 h of incubation withpaclitaxel, CCK-8 reagent (10 µl/well) was added and the plates were further incubated for 2 h. Subsequently, absorbance was determined at 450 nm by microplate reader.
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed to detect the molecular interactions of SPDEF with the promoter of GALNT7 according to the manufacturer’s instruction (Beyotime, China). The primers of ChIP -qPCR listed in Table S2.
Luciferase reporter
JASPAR software (http://jaspar.genereg.net/) was used to predict the presumptive binding sites of SPDEF that identified the GALNT7 promoter region. The first three matching sequences were selected to verification. Luciferase reporter vectors containing the wild-type (WT) or mutant sequences (Mut) towards the SPDEF binding of the GALNT7 promoter region were constructed (Tsingke, China). The pGL3-basic vector was included as the control. For luciferase activity assays, luminal BC cells were seeded in 24-well plates and cultured 24 h before transfection. Then cells were co-transfected with pGL3-basic or WT/Mut vector, and SPDEF over-expression plasmid/empty vector by EndoFectinTM-Max transfection reagent (GeneCopoeia, USA). The activity of Renilla plasmid was measured using the Dual-Luciferase Reporter Assay Kit (GeneCopoeia, USA).
Enzyme-linked immunosorbent assay (ELISA)
Serum samples were collected from 64 participants, including 33 healthy control and 31 luminal BC patients without therapy, respectively. The study was carried out in accordance with the Institutional Review Board of the Affiliated Hospital of Southwest Medical University, and signed consent forms were obtained from each participant. GALNT7 in human serum were detected by ELISA kit (Ruixin Biological Technology Co., Ltd, Quanzhou, China). CEA and CA125 were quantified by Cobas e602 Electrochemiluminescence System (Roche, Switzerland).
Statistical analysis
All data were analyzed by to the Shapiro-Wilk and Kolmogorov-Smirnov Test to check for normality, as well as the Levene’s test to verify the homogeneity of variances. Subsequent difference analyses were conducted by the t-test, chi-square test, Welch’s t-test, Wilcoxon or Mann-Whitney tests as appropriate using GraphPad Prism 7 and R, and presented as mean ± SEM. The P values less than 0.05 were considered statistically significant, *P < 0.05, **P < 0.01, ***P < 0.001. Correlation analysis was calculated with the Pearson correlation coefficient formula. Each experiment was replicated 3 times to ensure the reliability and reproducibility of the results.
Results
SPDEF is markedly upregulated in luminal BC and associated with tumor progression and poor prognosis
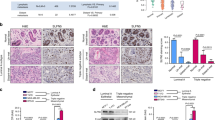
Association between SPDEF and clinicopathological characteristics were performed to access the influence of SPDEF in breast cancer patients. We initially analyzed SPDEF mRNA expression in the publicly available datasets (GEPIA and TCGA). As showing in Fig. 1A, B, SPDEF mRNA expression was notably upregulated in luminal and HER2 + BC tissues, while significantly decreased in triple-negative BC (TNBC) compared to normal individuals. Importantly, SPDEF mRNA expression level was much higher in luminal BC patients with advanced stage (stage III ~ IV) disease compared with those with early stage (stage I ~ II) disease (Fig. 1C). However, SPDEF was not associated with tumor progression in the other two breast cancer subtypes (HER2 + BC and TNBC). Thus, we additionally compared the transcription levels of SPDEF among groups of luminal BC patients, according to different clinicopathological characteristics (Table 1). The results uncovered that there was no significant difference in the SPDEF expression level was observed in luminal BC cases with regard to different age, lymphoid nodal status and distant metastasis status. But remarkably, high SPDEF expression was positively associated with stage (P = 0.022) and tumor invasion (P = 0.031).
A–B SPDEF expression level in different subtypes of BC samples compared to noncancerous samples by GEPIA (A) and TCGA database (B). C Differential expressions of SPDEF in early, late and unknown tumor stage according to BC subtypes from TCGA database. D Representative immunohistochemical staining of SPDEF in luminal BC tissues, scale bar = 60 μm (upper panels), scale bar = 30 μm (lower panels). E–G Kaplan-Meier estimates of the OS (E), RFS (F), and DMFS (G) of patients with luminal BC according to SPDEF levels by TCGA and KM Plotter database.
Further, to confirm the findings of bioinformatics analysis, we detected the protein expression of SPDEF in paraffin embedded tissues by immunohistochemistry (IHC) staining. As expected, luminal BC tissues were found to express higher levels of SPDEF protein than adjacent normal tissues and almost localized both in nucleus and cytoplasm (Fig. 1D and Table S3). Subsequently, to assess the prognostic potential of SPDEF in luminal BC, we found that high SPDEF expression was a significant indicator of poor overall survival (OS), relapse-free survival (RFS), and distance metastasis free survival (DMFS) (Fig. 1E-G). Taken together, these results suggest that the substantial increase of SPDEF expression contributes to the progression of luminal BC, leading to poor prognosis.
SPDEF facilitates the proliferation, migration and invasion of luminal BC cells in vitro
To further characterize the biological functions of SPDEF in luminal BC cells, we firstly found that luminal BC cells, including MCF7, T47D and BT-474, had elevated SPDEF expression in comparison with MCF10A cells using RT-qPCR (Fig. 2A and Figure S1A) and western blot (Fig. 2B, C and Figure S1B, C). We then observed whether SPDEF knockdown could affect biological behaviors of luminal BC cells in which SPDEF expression was stably inhibited by RNA interference (sh-NC and sh-SPDEF groups). Effective inhibition of SPDEF expression was confirmed by RT-qPCR (Fig. 2D and Figure S1D) and western blot (Fig. 2E, F and Figure S1E, F), respectively. CCK-8 assay was used for detecting the cell viability of MCF7, T47D and BT-474 cells. Figure 2G and Figure S1G displayed that proliferation of luminal BC cells was significantly slowed down following transfection with sh-SPDEF. Subsequently, migration and invasion capacities were assessed via wound scratch and transwell assays. The data showed that the migration distances of MCF7 and T47D cells were decreased following transfection with shRNA targeting SPDEF (Fig. 2H, I). Furthermore, transwell assay demonstrated that SPDEF knockdown distinctly inhibited migration and invasion ability of luminal BC cells (Fig. 2J-M and Figure S1H-I). We further verified the remarkably increased mRNA (Figure S2A) and protein expression (Figure S2B, C) of SPDEF in MCF7 and BT-474 cells with SPDEF overexpression. Most importantly, overexpression of SPDEF significantly enhanced luminal cell proliferation (Figure S2D), invasive, and migratory (Figure S2E-J), confirming the oncogenic function of SPDEF in luminal BC.
A–C. SPDEF abundance in MCF10A, MCF7 and T47D measured by RT-qPCR (A), protein abundance (B) and quantitative analysis (C) of SPDEF in MCF10A, MCF7 and T47D, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference control. D mRNA abundance of SPDEF in sh-NC and sh-SPDEF of MCF7 and T47D measured by RT-qPCR, sh-NC was the negative control, sh-SPDEF was the knockdown of SPDEF. E–F Protein abundance (E) and quantitative analysis (F) of SPDEF in sh-NC and sh-SPDEF measured by Western-blotting. G Proliferation activities of sh-NC and sh-SPDEF measured by the CCK-8 assay. H–I. Scratch wound healing assay results (H) and quantitative analysis (I). Images of all groups at 0 h, 24 h and 48 h time intervals post injury. J-M. Representative images of migrated and invaded cells and quantification assay of sh-NC and sh-SPDEF in MCF7 (J–K) and T47D cells (L–M), scale bar = 60 μm. **P < 0.01, ***P < 0.001.
SPDEF promotes cancer stem cell-like features and tumorigenicity in luminal BC
SPDEF was proved to act as a tumor-promoter in hepatocellular and lung cancers by maintaining the self-renewal of CSCs [29, 30]. Aimed to excavate the influence of SPDEF on stem cell-like properties, we firstly confirmed the expression of stem markers to verify the relationship between the expression level of SPDEF and stemness in luminal BC cases from TCGA database. The results revealed that the expression of embryonic stem cell markers (SOX2, KRT18, CD24, NANOG) were abundantly expressed in the high SPDEF group compared to that in the low group (Fig. 3A). Moreover, GSEA analysis showed that high SPDEF expression was positively associated with curated gene sets of stem cell in luminal BC samples from TCGA database (Fig. 3B). Further, we provide novel stemness indices for assessing the degree of oncogenic dedifferentiation in BC [28]. The stemness indices, mRNAsi and mDNAsi were remarkably elevated in high SPDEF expression group than that in low group (Fig. 3C). Notably, the overexpression of SPDEF was also positively correlated with above stemness indices (Fig. 3D). For mRNAsi and mDNAsi, by using the OCLR algorithm, the stemness index based on the gene expression profiles of 507(mRNAsi)/379(mRNAsi) luminal BC patients was calculated and then ranked from low to high to explore the associations between mRNAsi/mDNAsi and clinical features (Fig. 3E, F). As shown in Figure S3 patients in advanced stages had significantly higher mRNAsi and mDNAsi scores than those in early stage. Patients with poor clinical outcome demonstrated significantly higher mRNAsi scores, while patients aged ≤50 years had significantly higher mDNAsi scores than older patients. However, the stemness index scores both with mRNAsi and mDNAsi of metastatic status did not differ significantly among the groups.
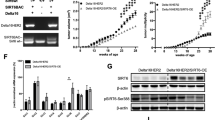
A The differential expression of stem cell markers in high and low SPDEF expression groups. B GSEA assessment of the enrichment score profile of stemness gene set in the SPDEF high and low groups. C TCGA database analysis of mRNAsi and mDNAsi differences in SPDEF high and low groups. D Correlation of SPDEF expression and mRNAsi and mDNAsi in luminal BC. E–F An overview of the association between SPDEF and clinical features and mRNAsi (E) /mDNAsi (F) in luminal BC. G Expression of stem genes in sh-NC and sh-SPDEF of MCF7 and T47D cells by RT-qPCR. H–I The morphology of spheroid formation (H) and quantitative analysis (I) in sh-NC and sh-SPDEF of MCF7 and T47D cells, scale bar = 120 μm. J–K The soft agar colony formation assay (J) and quantitative analysis (K) in sh-NC and sh-SPDEF of MCF7 and T47D cells. L Tumor formation in nude mice following injection of control cells and genetically modified luminal BC cells (sh-NC and sh-SPDEF), respectively. M–N Tumor growth curves of luminal BC cells (sh-NC and sh-SPDEF) in nude mice. (M) Tumor volume. (N) Tumor weight. *P < 0.05, **P < 0.01, ***P < 0.001.
To further elucidate the pivotal role of SPDEF in the stemness of luminal BC cells experimentally, the expressions of stemness genes were assessed between sh-NC and sh-SPDEF groups using RT-qPCR. The data indicated that NANOG, MYC, SOX2, OCT4, BMI1, NES, and CD44 was remarkably reduced in sh-SPDEF cells compared to that in sh-NC cells (Fig. 3G). Next, we examined spheroid formation efficiency by the spheroid formation assay. SPDEF knockdown altered the size of spheroids and cell morphology (Fig. 3H), and greatly reduced the number of spheroids (Fig. 3I). Similarly, soft agar colony formation assay suggests that the number of clonogenic spheroids in the sh-SPDEF cells group was significantly lower than that in sh-NC cells group (Fig. 3J, K). In addition, the expression of ALDH1A1, the most widely used cell surface marker for isolating breast tumor-initiating cells, known as cancer stem cells, was also higher in MCF7 and BT-474 cells with over-expressed SPDEF compared to control group (Figure S4A, B). Drug sensitivity assays further revealed a higher paclitaxel (TAX) resistance in MCF7 and BT-474 cells with over-expressed SPDEF compared to control group. (Figure S4C, D). In vivo, 106 MCF7-sh-NC and 106 MCF7-sh-SPDEF cells were transplanted subcutaneously into the hinder leg of nude mice. Macroscopic observation of the subcutaneously injected nude mice revealed that the size of tumor nodes was smaller in the sh-SPDEF infected mice than in the sh-NC nude mice (Fig. 3L). Meanwhile, the growth rate of tumor volume (Fig. 3M) and the average weight of harvested nodules (Fig. 3N) were also inhibited in SPDEF knockdown tumors compared to control group. Notably, the stemness phenotype was further validated using an extreme gradient tumor formation assay in which MCF7 cells with up-regulated SPDEF were most efficient in tumor initiation compared with the control group, as evident by the sizes of the tumors and the growth kinetics of tumors (Figure S4E-G). Based on the above observations, we conclude that SPDEF promotes luminal cells self-renewal ability and clonogenic activity in vitro and in vivo.
SPDEF was involved in cancer stem cell-like characteristics and tumorigenesis by regulating GALNT7 expression in luminal BC
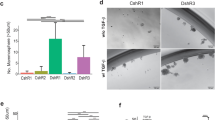
Since SPDEF is a transcription co-activator, it may promote liminal BC cells cancer stem cell-like properties and in vivo cancer biology characteristics by transcriptional activation of genes that are regulators of CSCs. Therefore, we further investigated to seek out possible targets of SPDEF that may regulate CSC-like properties in luminal BC cells. We identified six genes (GALNT7, SIDT1, TRIM3, ERGIC1, NCBP2L, and SMIM14) closely related to SPDEF from TCGA database (Fig. 4A). The histogram demonstrated that the most significant different expression of mRNA was GALNT7 in six genes (Fig. 4B). We next examined the expression of GALNT7 after SPDEF knockdown by RT-qPCR and Western blot. Down-regulation of GALNT7 expression upon SPDEF inhibited was observed in MCF7 and T47D cells (Fig. 4C, D). Thus, we hypothesized that GALNT7 could be a bona fide transcriptional target of SPDEF. To confirm that GALNT7 is indeed the downstream target that mediating the tumor-promoting and stemness maintenance property of SPDEF, the expression of GALNT7 was restored in SPDEF stably inhibited sh-SPDEF cells by overexpressing of GALNT7 (Fig. 4E, F). The restoration of GALNT7 expression in luminal BC cells reversed the ability of proliferation, migration and invasion, measured by CCK-8 assay (Fig. 4G), transwell assay (Fig. 4H-K) and scratch assay (Fig. 4L, M) in vitro. Further, we found the expressions of embryonic stem cell markers were significantly recovered after overexpression of GALNT7 in sh-SPDEF group in luminal BC (Fig. 4N). Meanwhile, the restoration of GALNT7 expression reverted sh-SPDEF cells back to clonogenic activity and self‐renewal ability in via serial spheroid formation assay (Fig. 4O, P) and clonogenic assay (Fig. 4Q, R). Most importantly, overexpression of GALNT7 in sh-SPDEF cells can significantly restored tumorigenicity, confirming the regulation mechanism and its function in vivo (Fig. 4S-U). Conversely, down-regulation of GALNT7 knock down after over-expressed SPDEF was observed in MCF7 and BT474 cells using RT-qPCR and Western-blotting (Figure S5A-C). In vitro, GALNT7 inhibited reversed the enhanced proliferation (Figure S5D), migration (Figure S5E, F), invasion capabilities (Figure S5G-J) and even the cancer stem-like cell features due to SPDEF overexpression in luminal BC (Figure S6).
A Pearson test was performed to determine the correlation between SPDEF and six candidate genes in luminal BC. B The expression level of six candidate genes in luminal BC compared to non-cancerous samples by TCGA database. C GALNT7 abundance in sh-NC and sh-SPDEF of MCF7 and T47D cells measured by RT-qPCR. D Protein abundance of GALNT7 in sh-NC and sh-SPDEF measured by Western-blotting. E GALNT7 abundance in shNC+OE-Vector, sh-SPDEF + OE-vector and sh-SPDEF + OE-GALNT7 cells measured by RT-qPCR. sh-NC was the negative control sh-RNA, sh-SPDEF was the knockdown of SPDEF, OE-vector was the negative control for the gene over-expression, OE-GALNT7 was the over-expression of GALNT7. F Protein abundance of GALNT7 in shNC+OE-Vector, sh-SPDEF + OE-vector and sh-SPDEF + OE-GALNT7 cells measured by Western-blotting. G Proliferation activities of shNC+OE-Vector, sh-SPDEF + OE-vector and sh-SPDEF + OE-GALNT7 measured by the CCK-8 assay. H-K. Representative images of migrated and invaded cells and quantification assay of shNC+OE-Vector, sh-SPDEF + OE-vector and sh-SPDEF + OE-GALNT7 in MCF7 (H–I) and T47D cells (J–K), scale bar = 60 μm. L–M Scratch wound healing assay results (L) and quantitative analysis (M). Images of all groups at 0 h and 48 h time intervals post injury, scale bar = 120 μm. N. Expression of stem genes in shNC+OE-Vector, sh-SPDEF + OE-vector and sh-SPDEF + OE-GALNT7 of MCF7 and T47D cells by RT-qPCR. O-P. The morphology of spheroid formation (O) and quantitative analysis (P) in shNC+OE-Vector, sh-SPDEF + OE-vector and sh-SPDEF + OE-GALNT7 of MCF7 and T47D cells, scale bar = 120 μm. Q-R. The soft agar colony formation assay (Q) and quantitative analysis (R) in shNC+OE-Vector, sh-SPDEF + OE-vector and sh-SPDEF + OE-GALNT7 of MCF7 and T47D cells. S-U. Subcutaneous tumors were harvested after sh-SPDEF cells infected with OE-GALNT7 in BALB/c nude mice. S tumor volume, T tumor growth curve, U tumor weight. *P < 0.05, **P < 0.01, ***P < 0.001.
SPDEF transcriptionally activates GALNT7 via promoter binding
To address whether SPDEF could directly interact with the GALNT7 prompter, we utilized the motif of SPDEF and promotor sequence of GALNT7 to predict transcriptional factor binding site by bioinformatics in JASPAR database (Fig. 5A). We analyzed the region ~2200 bp upstream of the transcription start site (TSS) for GALNT7 and identified three putative transcription factor-binding sites (Fig. 5B). Subsequently, we carried out chromatin immunoprecipitation (ChIP) assay to confirm the bioinformatics analysis. ChIP primers for the potential three sites were designed to amplify promoter regions containing putative binding sites, and the distal region primers were used as a negative control (Table S2). We conducted ChIP assay by incubating luminal BC cells nuclear extracts in the presence of anti-SPDEF, anti‐RNA Polymerase II antibody (as a positive control) or IgG (as a negative control). As shown in Fig. 5C and Figure S7A, SPDEF localized in +1918 ~ +1931 region of GALNT7 promoter was responsible for the majority of its transcriptional activation activity compared with the IgG control in luminal BC cells by ChIP-qPCR. The DNA gel electrophoresis also exhibited the similar results (Fig. 5D and Figure S7B).
A Motif of SPDEF binding sites. B Binding sites of SPDEF in the promoter region of GALNT7. C ChIP-qPCR analysis of the interaction of SPDEF with GALNT7 promoter. D Agarose gel electrophoresis confirmed the binding site of SPDEF to the GALNT7 promoter. GAPDH was used as a positive control. E Schematic diagram depicting the dual luciferase reporter assay for amplification of the regions. F–H SPDEF promoted GALNT7 transcription through activating its promoter. (F) mutant 3 site. (G) mutant 1 site. H mutant 2 site. **P < 0.01, ***P < 0.001.
To further investigate whether GALNT7 is transcriptionally induced by SPDEF, substitution mutations of the top three individual sites with the highest predicted scores were prepared for dual luciferase reporter assay (Fig. 5E). Abrogation of the mutant 3 site repressed luciferase activity in the luminal BC cells (Fig. 5F and Figure S7C). However, the two other mutant sites didn’t show significant decrease in transcriptional activity as compared with the vector control (Fig. 5G, H and Figure S7D, E). Taken together, these results indicated that SPDEF could directly bind to the predicted sites in the GALNT7 promoter region +1918 ~ +1931 and were crucial for the transcriptional activation of GALNT7 expression.
GALNT7 promotes stemness characteristics and is a potential diagnostic biomarker of luminal BC
To determine the clinical relevance of GALNT7 in BC, we analyzed the transcription levels of GALNT7 by using GEPIA and TCGA database. As shown in Fig. 6A, B, GALNT7 was remarkably overexpressed due to increased mRNA in luminal and HER2 + BC compared to normal individuals (Fig. 6A, B). Kaplan–Meier plots analysis revealed that luminal BC patients with high GALNT7 expression levels had significantly worse overall survival and relapse-free survival in comparison with patients with low GALNT7 expression level (Fig. 6C). Moreover, we investigated the relationship between elevated GALNT7 and cancer stem cell-like properties in luminal BC. GSEA analysis showed that high GALNT7 expression was positively associated with gene sets of stem cell (Fig. 6D). The mRNAsi was remarkably elevated in high GALNT7 expression group than that in low group (Fig. 6E). Furthermore, GALNT7 expression was positively correlated with mRNAsi scores in luminal BC patients (Fig. 6F), and the expression of stemeness markers (SOX2, NANOG, KLF4, BMI1) was significantly increased in the high GALNT7 group compared to that in the low group (Fig. 6G). To further verify the clinical values of GALNT7, we examined the expressions in luminal BC serum by ELISA and quantized on a standard curve. GALNT7 was upregulated in the serum of luminal BC patients compared with that in healthy individuals (Fig. 6H and Table S4). The area under the ROC curve (AUC) was 0.846, which also demonstrates that GALNT7 has higher clinical value as a non-invasive biomarker in luminal BC (Fig. 6I). Meanwhile, the combined detection of GALNT7, CEA and CA125 can improve the diagnosis efficiency of luminal BC with both better sensitivity (85.7%) and specificity (82.2%) (Fig. 6J). These results clearly indicate that GALNT7 modulates cancer stem cell properties and confers well-diagnostic value in luminal BC.
A, B GALNT7 expression level in different subtypes of BC samples compared to non-cancerous samples by GEPIA (A) and TCGA database (B). C Kaplan-Meier estimates of the OS and RFS of patients with luminal BC according to GALNT7 levels. D GSEA assessment of the enrichment score profile of stemness gene set in the GALNT7 high and low groups. E TCGA database analysis of mRNAsi difference in GALNT7 high and low groups. F Correlation of GALNT7 expression and mRNAsi in luminal BC. G The differential expression of stem cell markers in high and low GALNT7 expression groups. H Serum levels of GALNT7 in luminal BC patients and healthy people. I ROC analysis for the GALNT7 in luminal BC patient serum. J. ROC curves for diagnostic models of the combination of GALNT7, CEA and CA125. *P < 0.05, ***P < 0.001.
Discussion
Emerging evidence have demonstrated that the crucial role of SPDEF in the pathogenesis and progression of BC [31, 32]. However, the definition of SPDEF in the dichotomy of cancer-regulatory genes in BC has been controversial [33, 34]. Few studies focus on the mechanisms underlying the pro-oncogenic or tumor suppressive activity of SPDEF depending on different BC subtypes. Prior to this study, we have made a preliminary attempt to elucidate that SPDEF may play a diversity role in the expression levels, clinicopathologic importance, biological function and prognostic evaluation depending on different BC subtyping [35, 36]. However, the oncogenic function and downstream mechanisms of SPDEF in luminal BC are still unclear. Here, we reported the identification of SPDEF which represents a new mechanism of driving cancer stem cell-like properties and tumorigenicity of luminal BC that is mediated by GALNT7.
In the present study, the potent oncogene function of SPDEF in luminal BC has been elucidated by bioinformatics analysis and experimental evidence (Figs. 1, 2 and Figures S1–S3). Prior to this study, the cancer-promoting activity and clinical relevance of SPDEF in luminal BC has not been well explored and established. Only limited reports showed a correlation between SPDEF overexpression and malignant progression in luminal BC. In ER+ BC, SPDEF inversely regulated by ER and GATA3 is essential for tumorigenesis and is also required in models of endocrine-resistance [6]. In another report, high SPDEF expression enhances the tumorigenic growth of luminal BC cells and correlates with poor overall survival for BC patients with ER+ tumors [19]. Consistent with above observations, we revealed the highly expressed SPDEF was positively associated with the progression and poor prognosis in luminal BC. The first notable finding of our study is to provide convincing evidences supporting a rather definitive role of SPDEF in facilitating the proliferation, migration and invasion of luminal BC cells in vitro (Fig. 2 and Figures S1, S2) as well as promoting luminal BC oncogenesis and progression in vivo (Figs. 1 and 3L-N, Table 1). However, further in-depth mechanistic characterization and clinical research will answer whether SPDEF is a novel target for treatment and prognosis in luminal BC.
The second notable finding of this study is that we provided robust evidence for a regulatory role of SPDEF in the maintenance of luminal BC cancer stem cell-like properties. Specifically, we identified and molecularly concluded that SPDEF promotes luminal cells self-renewal ability and clonogenic activity in vitro (Fig. 3A-K and Figure S4A-D) and in vivo (Fig. 3L–N and Figure S4E-G). Accumulating evidence suggests that breast cancer progression might be driven by cancer stem cells that are more metastatic and refractory to conventional chemotherapeutics [37, 38]. Our knowledge of SPDEF on stemness properties comes mostly from studies involving the differentiation of goblet cell, Paneth cell, and normal mammary luminal cells [6, 39, 40]. A recent study reported that SPDEF could bind to the miR-448 promoter to downregulate DOT1L, whereby promoting self-renewal of hepatocellular carcinoma stem cells [30]. Nevertheless, the role of SPDEF in regulating cancer stem-like properties in luminal BC has not been established prior to the present study. This study demonstrated for the first time that SPDEF may promote cancer stem-like features and tumorigenicity in luminal BC, which will add new perspectives to the previously proposed oncogenic activity of SPDEF.
The third novelty is that we demonstrated the stimulative effect of SPDEF on progression and stemness of luminal BC, which is mediated by its direct transcriptional target GALNT7 (Figs. 4, 5 and Figures S5, S6). SPDEF plays critical roles in many biological processes by directly activating downstream target gene transcription. To explore the potential molecular mechanism of action of SPDEF in luminal BC, bioinformatics analysis and experiments were conducted. We confirmed that SPDEF expression was positively correlated with GALNT7 expression in luminal BC patients (Fig. 4A, B). Meanwhile, the direct targeting of GALNT7 by SPDEF was confirmed by restoration of GALNT7 in SPDEF silenced luminal BC cells which successfully reversed the cancer stem cell-like phenotype and oncogenic function in vitro and in vivo (Fig. 4C-U), and the other direction is also true (Figures S5, S6). Furthermore, we showed that SPDEF could directly bind to the GALNT7 promotor to activate its expression by ChIP analysis and dual luciferase reporter assay (Fig. 5 and Figure S7). GALNT7 has been reported as a member of the acetylgalactosaminyltransferase family which materializes a certain biological effect by regulating the interaction between tumor cells and the extracellular environment [41]. Few recent studies have shown the crucial role of GALNT7 in tumorigenesis of colorectal cancer [42], cervical cancer [43], and prostate cancer [44, 45]. Prior to the present study, the role of GALNT7 in regulating stemness and development of luminal BC was not elucidated. To our knowledge, this report is the first evidence that SPDEF regulated GALNT7 to induce cancer cell traits in the luminal BC.
The fourth notable observation derived from this study is the high clinical relevance of GALNT7 in luminal BC, which raises the potential of exploring the diagnosis and prognosis (Fig. 6). In our bioinformatics analysis, the difference expression and prognostic value of GALNT7 was remarkably consistent with that of SPDEF in various BC subtyping (Fig. 6A–C). Furthermore, we provided evidence that GALNT7 may play a similarly important role in regulating the cancer stem cell-like properties in luminal BC (Fig. 6D–G). Importantly, we found that the combined determination of serum GALNT7 and traditional tumor markers (CEA and CA125) could enhance diagnostic accuracy thus is of vital importance in luminal BC (Fig. 6H–J). Therefore, the screening for biomarkers associated with cancer stem cell signatures provided novel insights into the selection of tumor diagnostic biomarkers.
Although we found that SPDEF might enhance cancer stem cell-like phenotype and tumorigenesis of luminal BC by activating GALNT7 transcription, there are still some limitations to the current study. Firstly, IHC and serum sample analysis were conducted in a relatively small number of patients. A larger cohort should be used to further explore the relationship between SPDEF and/or GALNT7 expressions and clinical features. Secondly, the mechanism by which GALNT7 regulates stemness has not been explored. Further investigation is needed to elucidate this mechanism.
Taken together, this study revealed a novel role for SPDEF in luminal BC. We mainly demonstrated that SPDEF transcriptionally activates GALNT7 via directly binding to its promoter to drive cancer stem cell-like properties and tumorigenicity in luminal BC. Our findings provide new insights into the mechanism of liminal BC tumorigenesis and a promising biomarker and therapeutic target for patients with liminal BC.
Data availability
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Bousoik E, Nabiee R, Amirrad F, Nichols A, Witt R, Mahdipoor P, et al. Heterogeneity and Plasticity of Human Breast Cancer Cells in Response to Molecularly-Targeted Drugs. Front Oncol. 2019;9:1070.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47.
Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA. 2019;321:288–300.
Burguin A, Diorio C, Durocher F. Breast Cancer Treatments: Updates and New Challenges. J Pers Med. 2021;11:808.
Buchwalter G, Hickey MM, Cromer A, Selfors LM, Gunawardane RN, Frishman J, et al. PDEF promotes luminal differentiation and acts as a survival factor for ER-positive breast cancer cells. Cancer Cell. 2013;23:753–67.
Haque R, Ahmed SA, Inzhakova G, Shi J, Avila C, Polikoff J, et al. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomark Prev. 2012;21:1848–55.
Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–37.
Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34.
Oettgen P, Finger E, Sun Z, Akbarali Y, Thamrongsak U, Boltax J, et al. PDEF, a novel prostate epithelium-specific ets transcription factor, interacts with the androgen receptor and activates prostate-specific antigen gene expression. J Biol Chem. 2000;275:1216–25.
Rodabaugh KJ, Mhawech-Fauceglia P, Groth J, Lele S, Sood AK. Prostate-derived Ets factor is overexpressed in serous epithelial ovarian tumors. Int J Gynecol Pathol. 2007;26:10–15.
Moussa O, Turner DP, Feldman RJ, Sementchenko VI, McCarragher BD, Desouki MM, et al. PDEF is a negative regulator of colon cancer cell growth and migration. J Cell Biochem. 2009;108:1389–98.
Wu J, Qin W, Wang Y, Sadik A, Liu J, Wang Y, et al. SPDEF is overexpressed in gastric cancer and triggers cell proliferation by forming a positive regulation loop with FoxM1. J Cell Biochem. 2018;119:9042–54.
Noah TK, Lo YH, Price A, Chen G, King E, Washington MK, et al. SPDEF functions as a colorectal tumor suppressor by inhibiting beta-catenin activity. Gastroenterology. 2013;144:1012–23 e1016.
Steffan JJ, Koul HK. Prostate derived ETS factor (PDEF): a putative tumor metastasis suppressor. Cancer Lett. 2011;310:109–17.
Turner DP, Moussa O, Sauane M, Fisher PB, Watson DK. Prostate-derived ETS factor is a mediator of metastatic potential through the inhibition of migration and invasion in breast cancer. Cancer Res. 2007;67:1618–25.
Feldman RJ, Sementchenko VI, Gayed M, Fraig MM, Watson DK. Pdef expression in human breast cancer is correlated with invasive potential and altered gene expression. Cancer Res. 2003;63:4626–31.
Ghadersohi A, Pan D, Fayazi Z, Hicks DG, Winston JS, Li F. Prostate-derived Ets transcription factor (PDEF) downregulates survivin expression and inhibits breast cancer cell growth in vitro and xenograft tumor formation in vivo. Breast Cancer Res Treat. 2007;102:19–30.
Sood AK, Wang J, Mhawech-Fauceglia P, Jana B, Liang P, Geradts J. Sam-pointed domain containing Ets transcription factor in luminal breast cancer pathogenesis. Cancer Epidemiol Biomark Prev. 2009;18:1899–903.
Cao L, Li C, Xu C, Xiang G, Liu F, Liu X, et al. Clinical significance of PDEF factor expression and its relation to androgen receptor in ER(-) breast cancer. Histopathology. 2018;73:819–31.
Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556–W560.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
Lanczky A, Gyorffy B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J Med Internet Res. 2021;23:e27633.
Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–31.
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN, et al. Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell. 2018;173:338–54 e315.
Cai S, Li N, Bai X, Liu L, Banerjee A, Lavudi K, et al. ERK inactivation enhances stemness of NSCLC cells via promoting Slug-mediated epithelial-to-mesenchymal transition. Theranostics. 2022;12:7051–66.
Guo JC, Yang YJ, Guo M, Zhang JQ, Zheng JF, Liu Z. Involvement of CDK11B-mediated SPDEF ubiquitination and SPDEF-mediated microRNA-448 activation in the oncogenicity and self-renewal of hepatocellular carcinoma stem cells. Cancer Gene Ther. 2021;28:1136–49.
Cao L, Xu C, Xiang G, Liu F, Liu X, Li C, et al. AR-PDEF pathway promotes tumour proliferation and upregulates MYC-mediated gene transcription by promoting MAD1 degradation in ER-negative breast cancer. Mol Cancer. 2018;17:136.
Cao L, Xiang G, Liu F, Xu C, Liu J, Meng Q, et al. A high AR:ERalpha or PDEF:ERalpha ratio predicts a sub-optimal response to tamoxifen therapy in ERalpha-positive breast cancer. Cancer Chemother Pharm. 2019;84:609–20.
Gunawardane RN, Sgroi DC, Wrobel CN, Koh E, Daley GQ, Brugge JS. Novel role for PDEF in epithelial cell migration and invasion. Cancer Res. 2005;65:11572–80.
Schaefer JS, Sabherwal Y, Shi HY, Sriraman V, Richards J, Minella A, et al. Transcriptional regulation of p21/CIP1 cell cycle inhibitor by PDEF controls cell proliferation and mammary tumor progression. J Biol Chem. 2010;285:11258–69.
Ye T, Li J, Feng J, Guo J, Wan X, Xie D, et al. The subtype-specific molecular function of SPDEF in breast cancer and insights into prognostic significance. J Cell Mol Med. 2021;25:7307–20.
Ye T, Feng J, Wan X, Xie D, Liu J. Double Agent: SPDEF Gene with Both Oncogenic and Tumor-Suppressor Functions in Breast Cancer. Cancer Manag Res. 2020;12:3891–902.
Bai X, Ni J, Beretov J, Graham P, Li Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev. 2018;69:152–63.
Dittmer J. Breast cancer stem cells: Features, key drivers and treatment options. Semin Cancer Biol. 2018;53:59–74.
Noah TK, Kazanjian A, Whitsett J, Shroyer NF. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp Cell Res. 2010;316:452–65.
Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, et al. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–45 e1331-1333.
Kato K, Hansen L, Clausen H. Polypeptide N-acetylgalactosaminyltransferase-Associated Phenotypes in Mammals. Molecules. 2021;26:5504.
Li Y, Zeng C, Hu J, Pan Y, Shan Y, Liu B, et al. Long non-coding RNA-SNHG7 acts as a target of miR-34a to increase GALNT7 level and regulate PI3K/Akt/mTOR pathway in colorectal cancer progression. J Hematol Oncol. 2018;11:89.
Cao Q, Wang N, Ren L, Tian J, Yang S, Cheng H. miR-125a-5p post-transcriptionally suppresses GALNT7 to inhibit proliferation and invasion in cervical cancer cells via the EGFR/PI3K/AKT pathway. Cancer Cell Int. 2020;20:117.
Munkley J. Glycosylation is a global target for androgen control in prostate cancer cells. Endocr Relat Cancer. 2017;24:R49–R64.
Munkley J, Vodak D, Livermore KE, James K, Wilson BT, Knight B, et al. Glycosylation is an Androgen-Regulated Process Essential for Prostate Cancer Cell Viability. EBioMedicine. 2016;8:103–16.
Acknowledgements
We would like to thank the members in the research group who are not listed in the authors. This work was supported by the Cooperative Scientific Research Project of ‘Chunhui Plan’ of Ministry of Education, China (Grant No.HZKY20220575), the Science and technology strategic cooperation project of Luzhou People’s Government and Southwest Medical University, China (Grant No.2021LZXNYD-J08), the Sichuan Science and Technology Program for key Research and Development, China (Grant No. 2021YFS0226) and Doctoral Research Initiation Fund of Affiliated Hospital of Southwest Medical University, China (Grant No. 19077).
Author information
Authors and Affiliations
Contributions
JL, XW and DX substantially contributed to acquisition of data, analysis and interpretation of data and drafting of the manuscript. TY contributed to the conception and design, interpretation of the data, revising it critically for important intellectual content, final approval of the version to be published and acquisition of funding. HY, QP, YL, YC and JX contributed to acquisition of data, analysis and interpretation of the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
Animal studies were conducted in accordance with an approved protocol and with the institutional animal welfare guidelines of the Institutional Animal Care and Use Committee of Southwest Medical University. Patient consent forms were obtained according to protocols approved by the Institutional Review Board of the Affiliated Hospital of Southwest Medical University, the study was carried out in accordance with the Institutional Review Board of the Affiliated Hospital of Southwest Medical University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Massimiliano Agostini
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Wan, X., Xie, D. et al. SPDEF enhances cancer stem cell-like properties and tumorigenesis through directly promoting GALNT7 transcription in luminal breast cancer. Cell Death Dis 14, 569 (2023). https://doi.org/10.1038/s41419-023-06098-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-023-06098-z