Abstract
Pancreatic ductal adenocarcinoma (PDAC) is the most lethal type of cancer and the third leading cause of cancer death with the lowest 5-year survival rate. Heterogeneity, difficulty in diagnosis, and rapid metastatic progression are the causes of high mortality in pancreatic cancer. Recent studies have shown that Protein arginine methyltransferase 5 (PRMT5) is overexpressed in pancreatic cancers, and these patients have a worse prognosis. Recently, PRMT5 as an anti-cancer target has gained considerable interest. In this study, we investigated whether inhibition of PRMT5 activity was synergistic with blockade of TGF-β1 signaling, which plays an important role in the construction of the desmoplastic matrix in pancreatic cancer and induces therapeutic vulnerability. Compared with T1-44, a selective inhibitor of PRMT5 activity, the combination of T1-44 with the TGF-β1 signaling inhibitor Vactosertib significantly reduced tumor size and surrounding tissue invasion and significantly improved long-term survival. RNA sequencing analysis of mouse tumors revealed that the combination of T1-44 and Vactosertib significantly altered the expression of genes involved in cancer progression, such as cell migration, extracellular matrix, and apoptotic processes. In particular, the expression of Btg2, known as a tumor suppressor factor in various cancers, was markedly induced by combination treatment. Ectopic overexpression of Btg2 inhibited the EMT response, blocking cell migration, and promoted cancer cell death. These data demonstrate that the combination therapy of T1-44 with Vactosertib is synergistic for pancreatic cancer, suggesting that this novel combination therapy has value in the treatment strategy of patients with pancreatic cancer.
Similar content being viewed by others
Introduction
Pancreatic cancer is one of the most unconquered cancer types due to its difficulty in diagnosis and high mortality [1, 2]. The 5-year survival rate for pancreatic cancer is 11%, the lowest among all cancers. Pancreatic cancer also ranks as the third leading cause of death among cancer types with no improvement over the past two decades, despite significant advances in cancer treatment [3]. Since then, many therapies including combination chemotherapy have been tried to increase the survival rate of pancreatic cancer [4, 5]. For patients with pancreatic cancer, 5-fluorouracil-based adjuvant chemotherapy (ex. FOLFIRINOX) or gemcitabine-based combination therapy is commonly used and related clinical studies are also performed [6,7,8]. In addition, research on new treatments to overcome aggressive pancreatic cancer, such as epigenetic modification or immune system regulation, is being actively conducted [9,10,11,12].
PRMT5 is a major type II protein arginine methyltransferase that specifically produces symmetric dimethyl arginine (SDMA). PRMT5 is mostly upregulated and has multiple roles in cancer, including histone methylation, transcriptional regulation, signal transduction, DNA damage, and splicing [13,14,15,16,17,18]. PRMT5 as an anti-cancer target has gained significant interest in recent years and high levels of PRMT5 protein are associated with worse survival outcomes across multiple cancer types, including pancreatic ductal adenocarcinoma (PDAC) [19,20,21,22,23,24]. Particularly, inhibition of PRMT5 reduces the tumor growth via Myc pathway and inhibits epithelial-mesenchymal transition through EGFR-β-catenin axis in pancreatic cancer [19, 20]. In this study, we used T1-44, a selective small molecule inhibitor for PRMT5 protein. T1-44 is a similar derivative of EPZ015666, an inhibitor of PRMT5 enzyme activity that can be administered orally [21, 22]. Barczak et al. demonstrated the effect of T1-44 on cancer cell migration and invasion through regulation of the PRMT5-E2F axis and cell motility-related proteins [22].
In a previous study, it was known that histone H3 and H4 methylation by PRMT5 in combination with MEP50 regulates the transcription of genes involved in cancer cell metastasis and EMT response related to TGF-β signaling [23]. We therefore hypothesized that co-inhibiting PRMT5 activity and the TGF-β signaling pathway would be a powerful approach for PDAC treatment. The TGF-β signaling inhibitor, Vactosertib, is being actively studied as a combination therapy for several aggressive cancers [24, 25]. Our previous study demonstrated that the combination of Vactosertib + nal-irinotecan/5-fluorouracil/leucovorin was effective for cell migration and invasion in a preclinical model of PDAC [26]. Based on these preclinical studies, various clinical trials are currently underway in combination with Vactosertib for drug treatment for various cancers such as non-small cell lung cancer, advanced colorectal cancer, multiple myeloma, and pancreatic cancer [27,28,29,30,31].
Here, we proposed a robust combination therapy strategy for aggressive pancreatic cancer that increases the survival rate in preclinical mouse models. We found that the combination of Vactosertib and T1-44 blocked tumorigenesis and invasion, leading to increased survival.
Results
Effectiveness of PRMT5 inhibitor on pancreatic tumor growth
We first evaluated the effect of T1-44, a selective PRMT5 inhibitor, in pancreatic cancer. T1-44 inhibited symmetrical arginine dimethylation in both murine pancreatic cancer cells Panc02 and human pancreatic cancer cells SNU2491 (Fig. 1A, Supplementary Fig. S1A). To determine the ability of T1-44 in cell death and proliferation, an MTT assay was performed with a dose-dependent treatment for 6 days (Fig. 1B, Supplementary Fig. S1B). The cell viability of SNU2491 decreased to less than 50% in the low range of T1-44, and Panc02 also showed a response at more than 1 μM of T1-44. In addition, T1-44 inhibits the ability of cancer cells to form colonies (Fig. 1C, Supplementary Fig. S1C). To confirm the in vivo antitumor activity of T1-44, we subcutaneously injected Panc02 cells into C57BL/6 mice and administered T1-44 50 mg/kg twice a day (BID). T1-44 treatment slightly suppressed tumor growth rate and reduced final tumor volume and weight. (Fig. 1D, E, F). We also tested the same experiments using SNU2491 human pancreatic cancer cell line and immunodeficient mice NRGA (Supplementary Fig. S2A, B, C). In summary, targeting PRMT5 activity through T1-44 treatment results suppression of tumor growth in pancreatic tumor growth.
A Dose-dependent inhibition of symmetric arginine di-methylation of SmD3 by T1-44 in Panc02 murine pancreatic cancer cells. B Cell viability of Panc02 by T1-44 measured by MTT assay. Means with SD of OD values were presented relative to control. C Colony formation assay by treatment with various concentrations of T1-44. Colonies were counted after treatment of T1-44 for 2 weeks. D Administration of T1-44 (50 mg/kg, BID) to a subcutaneous tumor model of Panc02 cells. Individual tumor tissue was represented by tumor size for each group (bottom panel). E Graph of tumor volume measured every 3 days. F Tumor weights of vehicle and T1-44 treated groups at endpoint.
Synergetic effect of TGF-β inhibitor and PRMT5 inhibitor combination in tumor progression
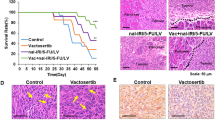
To synchronize the tumor microenvironment with pancreatic cancer patients, we established a syngeneic orthotopic model of Panc02 mouse pancreatic ductal adenocarcinoma cells and C57BL/6 mice, followed by oral administration 50 mg/kg of T1-44 (BID). Unexpectedly, T1-44 could not improve the survival rate of orthotopic tumor model (Supplementary Fig. S3A). We detected aggressive invasion of tumor cells and desmoplastic stromal tissue formation in pancreatic tumor lesions both in vehicle and T1-44 treated groups (Supplementary Fig. S3B). These phenomena are the major features of malignant pancreatic cancer and TGF- β signaling is one of the most crucial pathways for managing them [32, 33]. According to previous studies, Vactosertib, an effective TGF-β signaling inhibitor, incredibly inhibits invasion and desmoplastic stroma when combined with other anti-cancer drugs in pancreatic cancer [26, 34]. Therefore, we decided to treat T1-44 combined with Vactosertib on Panc02-C57BL/6 syngeneic orthotopic pancreas tumor model. When analyzed at the endpoint of the experiment, single administration of Vactosertib or T1-44 did not increase the survival rate of mice, whereas the combination treatment group of T1-44 and Vactosertib showed significantly improved results; 60% of mice survived more than 50 days (Fig. 2A). After 4 weeks of treatment, mice in each group were sacrificed and tumors localized to the pancreas and surrounding tissues were observed. There was no significant difference in the primary tumor size between the vehicle control group and the Vactosertib group, but the tumor size decreased in the T1-44 group alone. In particular, we found very small tumor tissue in the Vactosertib and T1-44 combination treatment group (Fig. 2B). We measured the relative tumor area in each group using section staining of tumor tissue. The average tumor area of the combined treatment group was reduced by more than 90% compared to the vehicle treatment group (Fig. 2C). Taking all the macroscopic results of the in vivo experiments, combination treatment of T1-44 with Vactosertib significantly reduced tumor growth and progression in a mouse pancreatic cancer model.
A Survival rate plot of Panc02 syngeneic mouse model. Four groups (vehicle, Vactosertib, T1-44 mono-treatment and combination treatment) were calculated the percentage of live/total mice number for 47 days. B Representative picture of pancreas primary tumors with normal pancreas and spleen (top). Histological images (H&E) of tumor tissues distinguish between tumor and normal pancreas by blue lines (bottom). C Calculation of relative tumor area using H&E scan of four group. D Heatmap, E GO term analysis, and F Network analysis results of DEGs between Vactosertib+T1-44 and control from RNA sequencing analysis.
To determine which biological pathways were altered by the combination of Vactosertib and T1-44 and led to significant inhibition of cancer progression, we performed RNA sequencing analysis using primary tumor tissues from a mouse pancreatic cancer model. Differentially expressed genes (DEGs) between the co-treatment and vehicle groups were identified and visualized as heatmaps (Fig. 2D). Several cancer-associated genes were varied by Vactosertib and T1-44, in particular genes promoting cancer progression were highly downregulated and tumor suppressor upregulated compared to controls. Gene ontology (GO) terms with DEG were analyzed and rated by significance (Fig. 2E). GO terms related to cancer progression such as cell migration, extracellular matrix, angiogenesis, and apoptotic progression are placed at the top rank. Network analysis of DEGs revealed that the tumor suppressive effect of Vactosertib and T1-44 combination treatment alters gene expression primarily involved in the regulation of apoptotic processes and cell migration (Fig. 2F). The functional categories were highly interacted with cell surface receptor signaling, extracellular matrix, response to tumor cell, and cytokine secretion. In the network, eight genes related to the apoptotic process and cell migration, including Mmp9, Cxcl12, Cdh1, Fap, Itga6, Mdk, Aif1, and Mif, were downregulated with co-treatment, which may play vital roles in inhibition of cancer progression and cell death. Additionally, Prf1 exhibited remarkable up-regulation in Vactosertib and T1-44 co-treatment, probably suggesting the potential of natural killer cell activity [35]. Briefly, combination therapy of Vactosertib with T1-44 was effective in suppressing pancreatic cancer by regulating cancer cell migration and apoptosis processes.
Inhibition of cancer cell motility and invasion with combination treatment in mouse pancreatic tumor model
We wonder why the survival rate of mice with orthotopic injection of Panc02 cells into the pancreas was significantly increased only in the Vactosertib and T1-44 combination treatment groups compared to the vehicle or single treatment groups. RNA sequencing data of pancreatic tumor tissue showed that the expression of genes related to cell migration or extracellular matrix was greatly altered by the combination treatment of Vactosertib and T1-44. Therefore, we histologically analyzed the pancreatic primary tumor and surrounding tissues to determine the effect of this combination on cancer cell invasion into surrounding tissues. When a primary tumor forms in the pancreas, cancer cells invade normal pancreatic tissue. Treatment with T1-44 reduced invasiveness to surrounding tissues, but some of the borders were destroyed by tumor cells. On the other hand, the Vactosertib alone or co-treated group showed a clear boundary between the tumor and normal tissue without an invaded morphology (Fig. 3A). Treatment with either Vactosertib or T1-44 also transformed the cancer cell morphology, mesenchymal (spindle) type, into an epithelial (round) cell type (Fig. 3B). Expression of E-cadherin, a representative marker of mesenchymal-epithelial transition (MET), explained the change by Vactosertib or T1-44 alone treatment in each group of tumor tissues, and suppressed EMT-induced cell invasion, and the combined treatment maximized the effect (Fig. 3C). Moreover, Sirius red staining and α-SMA immunohistochemical staining revealed tissue fibrosis enhancing cancer metastasis, and chemical resistance was significantly reduced in the Vactosertib and T1-44 combination treatment group (Fig. 3D, E). Pancreatic cancer cell migration in vitro was reduced by the combination treatment of Vactosertib and T1-44 and TGF-β-induced migration was also blocked by the combination treatment (Fig. 3F, Supplementary Fig. S4). Consistently, the mRNA and protein levels of TGF-β target genes, including EMT marker genes, regulated by TGF-β treatment, were significantly modulated when combination treatment compared with treatment with Vactosertib alone or T1-44 alone (Fig. 3G, H; Supplementary Fig. S5). Thus, these findings suggest that the combination treatment of Vactosertib with T1-44 significantly reduces cancer cell invasion and migration by inhibiting EMT both in vivo and in vitro.
A Histological analysis of pancreas tumor and normal tissues. Border lines between tumor and normal tissue were drawn by blue lines. Broken part of lines represents invading structure of tumor. B H&E images showing cell morphologies of each group. Spindle type (vehicle) and round type (Vactosertib, T1-44, and Vac+T1-44). C Immunofluorescence staining results of E-cadherin in tumor tissues. D Sirius Red staining indicating collagen deposition (strong red color) in tumor surrounding tissues. E α-SMA immunohistochemistry-stained tumor tissues (brown). F Panc02 cell migration assay using trans-well system. TGF-β1 (5 ng/ml), Vactosertib (Vac) (500 ng/ml), and T1-44 (5 μg/ml) were primarily treated for 48 h. G mRNA levels of EMT markers using quantitative PCR data. H Protein levels of EMT markers using western blotting.
Regulation of apoptotic processes by Vactosertib and PRMT5 inhibitor co-treatment in the syngeneic mouse model of pancreatic cancer
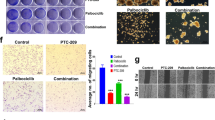
Another notable GO term for DEG in RNA sequencing is the regulation of apoptotic processes. Tumor tissue staining of TUNEL indicates that combination treatment of Vactosertib with T1-44 increased the number of positive apoptotic cells in mouse primary tissues of pancreatic cancer (Fig. 4A). In addition, cleaved form of Capase3 indicating signaling pathway of apoptosis was highest expressed in tumor of co-treated group (Fig. 4B). In vitro data from mouse and human pancreatic cancer cells also support that the combination treatment of Vactosertib and T1-44 positively regulated the apoptotic process, neither as a control nor as monotherapy. Cleaved PARP and cleaved Caspase3, representative markers of apoptosis, were significantly enhanced in the combination treatment group (Fig. 4C). Also, Annexin V staining results showed that the number of apoptotic cells was particularly abundant in the combination treatment group compared to the control group (Fig. 4D). In addition, cell colony formation and growth were decreased in the combination treatment group (Fig. 4E), suggesting that apoptosis plays a major role in effective pancreatic tumor reduction by combination therapy of Vactosertib and PRMT5.
A TUNEL assay of primary tumor tissues from Panc02 mouse experiment. Green fluorescence dots indicate apoptotic positive cells. B Immunohistochemistry staining of cleaved-caspase3 C. Western blotting data of PARP and Caspase3. Cleaved forms of two protein indicate apoptosis signaling. D Flow cytometry of Annexin V-7AAD staining. E Colony forming assay of Panc02 and SNU2491 cell lines under treatment of Vactosertib and T1-44.
Elevated expression of tumor suppressor Btg2 by combination treatment of Vactosertib and T1-44
Analysis of RNA sequencing data suggests several candidates for co-therapeutic target genes of Vactosertib and T1-44. Among the candidates, we were interested in several genes involved in tumor progression or suppression and validated those genes using RNA from pancreatic tumor tissue and cancer cells. Expressions of Btg2 and Dll4, previously studied as tumor suppressors, were upregulated in the Vactosertib and T1-44 combination treatment group (Fig. 5A–C; Supplementary Fig. S6). On the other hand, we identified repressed genes such as Mdk, Fap, Mmp9, Itga6, and Cxcl12, known as mediators of tumor growth, migration, invasion, and fibrosis (Supplementary Fig. S7-S9). Similarly, mRNA levels of candidate genes were similarly altered in Panc02 mouse cells and SNU2491 human cells under combination treatment (Fig. 5C, Supplementary Fig. S9).
A FPKM value of Btg2 from RNA sequencing. B qPCR validation using mRNA from combination treated-tumor tissues and C co-treated Panc02 and SNU2491 cell lines. D Relative mRNA expression of BTG2 E Cell migration assay, F Protein markers of EMT response, G apoptosis markers, H flow cytometry analysis of Annexin V, and colony formation assay with overexpressed stable cell lines.
Among the candidate lists of target genes, we focused on the Btg2 gene, which has been studied as a tumor suppressor whose expression is suppressed in several types of cancer. In the pancreatic adenocarcinoma TCGA data, BTG2 expression is significantly suppressed in tumor tissues compared to normal tissues (Supplementary Fig. S10A). Further, BTG2 expression and disease-free survival rate of pancreatic cancer is positively correlated (Supplementary Fig. S10B). We generated cell lines stably overexpressing BTG2 in Panc02 and SNU2491 (Fig. 5D). Ectopic expression of BTG2 suppressed cell migration and EMT-related markers in both cell lines (Fig. 5E, F). Moreover, positive regulation of the apoptotic process, represented by enhanced cleaved PARP, cleaved Capase3 expression (Fig. 5G) and annexin V-stained cell number, was also seen in BTG2 overexpressed cells (Fig. 5H). Cell colony formation was also reduced by overexpression of BTG2 (Fig. 5I). Collectively, various genes involved in cancer progression were regulated by the combination treatment of Vactosertib and T1-44, and in particular, BTG2 is a key regulator of EMT-induced inhibition of cell migration and increased tumor cell death.
Discussion
Genetic mutations are the most well-known hallmarks of pancreatic cancer, and epigenetic processes are also important for cancer development and progression [36, 37]. The type II methyltransferase PRMT5 has diverse effects on cancer biological functions, such as histone methylation and inhibition or promotion of tumor-associated signaling pathways. Histone arginine methylation by PRMT5 attenuates oncogenic signaling pathways, thereby regulating transcriptional gene expression [38,39,40]. The arginine residue of E2F1, a key molecule in cancer cell growth, is the target of methylation by PRMT5 [41]. In lymphoma cells, PRMT5 activates the WNT/β-catenin and AKT/GSK3β signaling pathways and regulates target gene expression [42]. In addition, the tumorigenicity and aerobic glycolysis of pancreatic cancer is increased by PRMT5, mediating epigenetic silencing of E3 ligase FBW7 with upregulation of c-Myc [43]. A selective PRMT5 inhibitor, EPZ015666, has antitumor effect via reducing proliferative role of cancer cells and tumor growth in mantle cell lymphoma (MCL) xenograft model [21]. In this study, we attempted to investigate whether the combination treatment of T1-44, an inhibitor of PRMT5 methyltransferase activity, and Vactosertib, a TGF-β signaling inhibitor, was more effective in inhibiting cancer growth and metastasis and increasing survival rate than T1-44 or Vactosertib alone using human and mouse pancreatic cancer cells. Our in vitro and in vivo experiments demonstrated that combination treatment of T1-44 with Vactosertib significantly reduced tumor growth and invasion and increased survival in a mouse pancreatic cancer model. EMT marker gene expression regulation and apoptosis induction were more effective when T1-44 and Vactosertib were treated together than T1-44 alone.
The heterogeneity and frequent alteration of components of the tumor microenvironment are considered important factors of pancreatic cancer malignancy and chemoresistance [44,45,46]. Cancer stromal tissue consists of the extracellular matrix (ECM), which creates a physical barrier and reduces sensitivity to drugs [47, 48]. In addition, cancer-associated fibroblasts (CAFs) in the tissues surrounding the PDAC tumor promote cancer progression, such as tumorigenesis and metastasis [49,50,51]. TGF-β signaling is important for the differentiation and metabolism of CAFs and mediates crosstalk between tumor cells and stromal cells [52,53,54]. The collagen deposition and expression of α-SMA were highly exited around the tumor tissues from Panc02 mouse model and lessened by Vactosertib and T1-44 combination treatment. Moreover, TGF-β signaling is the key factor involved cancer invasion and metastasis, regulating EMT response and tumor-related transcript factors [55,56,57].
A previous study has shown that PRMT5 is essential for expression of marker genes for metastasis and the EMT and is required for the epigenetic mechanism of the TGF-β response [23]. Interestingly, TGF-β treatment in A549 cells dramatically increased the relative abundance of PRMT5 protein and altered EMT protein markers with loss of E-cadherin and increased expression of Vimentin and Snail, suggesting the possibility that PRMT5 mediates the expression regulation of the EMT marker gene of TGF-β. This study showed that treatment of A549 lung adenocarcinoma cells with a specific PRMT5 inhibitor, GSK591, suppressed the expression of TGF-β1-induced EMT protein markers such as Snail and Vimentin, treatment of T1-44 alone did not suppress the expression of PRMT5. However, in our study the expression of these marker genes was suppressed only in the case of co-treatment of T1-44 with Vactosertib in pancreatic cancer cells. Whether these differences are due to cancer type, cell lines, or different PRMT5 inhibitors remains to be investigated in the future. Nevertheless, inhibition of the TGF-β signaling pathway in cancer therapy is a promising therapeutic target to prevent cancer invasion [58, 59].
BTG2, a member of the antiproliferative BTG/Tob family, is known as a tumor suppressor in various cancer types [60, 61]. Loss of BTG2 promotes tumor growth and reduces survival in patients with triple-negative breast cancer [62]. BTG2 can inhibit tumor growth and progression in breast cancer by suppressing the tumor microenvironments by regulating the mTORc2-AKT1-NFAT1-PHLPP2 signaling cascade [63]. In non-small cell lung cancer, BTG2 is a promising target for increasing radiation sensitivity with increased apoptosis and may be an early prognostic gene [64]. In transcriptomic analysis of mouse pancreatic tumor tissue, we identified BTG2 as a tumor suppressor gene whose expression was significantly induced by treatment with both T1-44 and Vactosertib in a mouse pancreatic tumor model. We discovered that the overexpression of BTG2 could suppress cell migration, colony formation, and EMT response in Panc02 and SNU2491 pancreatic cancer cells.
In conclusion, this study demonstrates that the combination treatment of T1-44, a PRMT5 inhibitor, and Vactosertib, a TGF-β signaling inhibitor, showed impressive synergistic results in improving survival, inhibiting cell invasion and EMT, and stimulating the apoptosis pathway in pancreatic cancer. This combination therapy could be one of the promising clinical therapies for patients with metastatic pancreatic cancer.
Material and methods
Cell culture and reagents
C57BL/6 mouse-derived pancreatic cancer cell line Panc02 was provided from Medpacto Inc. (Seoul, Korea). The human pancreatic adenocarcinoma cell lines, SNU2491 and PANC-1, were obtained from Korean cell line bank (Seoul, Korea). The cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) or RPMI 1640 contained with 10% FBS and 100 U/ml penicillin and 100 μg/ml streptomycin (WELGENE, Korea).
Selective PRMT5 inhibitor (T1-44) was provided by Argonaut Therapeutics Ltd, Oxford, UK. Vactosertib was obtained from Medpacto Inc. T1-44 and Vactosertib were solubilized in DMSO for in vitro experiments and in artificial gastric fluid (2 g/L NaCl, 3.2 g/L pepsin, 0.06 M HCl) for in vivo experiments. Antibodies against Symmetric Di-Methyl Arginine Motif (SDMA, Cat. No. 13222), Snail (Cat. No. 3879), PARP (Cat. No. 9532), and Caspase-3 (Cat. No. 9662) were obtained from Cell Signaling Technology (Boston, USA). Antibodies for E-cadherin (Cat. No. 610181), N-cadherin (Cat. 610921), and Fibronectin (Cat. No. 610077) were purchased from BD Biosciences. Anti-HA (sc-7392) and anti-Slug (sc-166476) were obtained from Santa Cruz Biotechnology (Texas, USA).
Cell viability assay
A total of 2 × 103 of cancer cells were seeded on 96well plates and treated T1-44 for 72 h. We used 0.5 mg/ml of MTT solution (Sigma, Cat. No. M5655) for colorimetric assay of cell viability. We dissolved the cells in 100 µl DMSO and measured the 540 nm OD values of each well. All data were analysed by the mean of triplicate with SD.
Colony formation assay
A total of 500 of Panc02 and SNU2491 were seeded on 6well plates and treated T1-44 or Vactosertib for 2 weeks. We changed the medium with chemicals every 2–3 days. After 2 weeks, colonies were fixed and stained with 2% methylene blue in 50% ethanol.
Trans-well system for migration and invasion assays
Cancer cells were pre-treated with Vactosertib and T1-44 on 60 mm dishes for 48 h. Then, 5 × 104 of cells were moved to trans-wells (Flacon, Cat. No. 353097) for migration assay. After 48 h, migrated cells were fixed with 70% ethanol and stained with 0.05% crystal violet.
Western blotting
Vactosertib or T1-44 treated cells were collected and washed by PBS twice. The cell pellets were lysed with RIPA buffer (50 mM Tris-HCL, 10 mM NaCl, 1% NP-40, 0.5 sodium deoxycholate, 0.1% sodium dodecyl sulfate, and a protease inhibitor cocktail) on ice for 30 min. Lysed proteins were separated by SDS-PAGE gel and transferred to PVDF membranes (Millipore, Cat. No. IPVH00010). The membranes were incubated with primary antibodies for overnight. The blots were detected with AI600 system (GE Healthcare Life Sciences, UK). The full blots of cropped western blot images were presented in supplementary figures.
RNA isolation, cDNA synthesis and qRT-PCR
RNA from tumor tissues and cancer cells were isolated with easy-BLUE Total RNA extraction kit (Promega, Cat. No. 17061). For reverse transcription, we used M-MLV reverse transcriptase (Promega, CAT. NO. M1705) with 2 μg of mRNA. Synthesized cDNA was used for quantitative RT-PCR with QuantStudio5 Real-Time PCR instrument (Applied Biosystems, Massachusetts, USA) and TOPreal qPCR 2xPreMIX (Enzynomics, Cat. No. RT500M). All the expressions were normalized with Gapdh.
Animal study
For subcutaneous syngeneic and xenograft model, 6-week-old male C57BL/6 (SLC, Japan) and NRGA (JA bio. Korea) mice were used. A total of 5 × 106 of Panc02 and 2 × 107 of SNU2491 were mixed with VitroGel® Hydrogel Matrix (The well bioscience, USA), injected to under skin of right flank of mice. After the tumor size were reached to 100mm3, we started to inject 50 mg/kg of T1-44 orally as twice in a day (BID). We followed up the size of tumors and body weight of mice every 3 days. For orthotopic model of pancreas cancer, 3 × 106 Panc02 cells mixed with Matrigel (Corning, Cat. No. 35631) were injected into the tail of pancreas duct of C57BL/6. The mice were randomized to four groups and orally injected vehicle (gastric fluid), 25 mg/kg Vactosertib (5 days in a week), 50 mg/kg T1-44 (BID), and their combination. All animal experiments were performed following the guidelines and regulations of animal ethics and approved by Woojung Bio Animal facility (Suwon, Korea).
RNA sequencing and data analysis
To perform RNA sequencing (RNA-Seq), cDNA libraries were prepared from 1 μg total RNA of each sample with three replicates using the TruSeq Stranded mRNA Sample Prep Kit (Illumina, Inc., San Diego, CA, USA), according to the manufacturer’s instructions. After qPCR validation, libraries were subjected to paired-end sequencing with a 100 bp read length using an Illumina HiSeq 4000 platform, yielding an average of 37.1 million reads per library.
The quality of raw reads was assessed with FastQC (version 0.11.9); the quality scores were greater than Q30. Clean reads, that quality scores were ≥Q30, were processed were aligned to the mouse reference genome GRCm38.p4 mm10 using TopHat2 [65] (version 2.1.1) with a set of gene model annotation. Gene expression quantification was performed using Cufflinks [66] (version 2.2.1), and fragments per kilobase of transcript per million reads mapped (FPKM) was calculated as the expression value. Differential expression (DE) in other treatments, including Vactosertib, T1-44, and Vac+T1-44, compared to vehicle was analyzed by using DEGseq [67] (version 1.50.0) with a cutoff set at P < 0.01 and ≥ |1.5 | -fold change. Expression pattern of differentially expressed genes (DEGs) selected was visualized as heatmap by using MeV (http://mev.tm4.org), and expressions of genes were shown as Z-score for FPKM. Gene ontology (GO) enrichment analysis for DEG datasets was performed by DAVID [68] with a cutoff of P < 0.001. Interaction for genes categorized to the enriched GO terms was searched using STRING database [69] (https://string-db.org/) with default confidence score (≥0.4) and further analyzed using Cytoscape (www.cytoscape.org) on the basis of the degree of connectivity of the nodes.
H&E staining and immunohistochemistry of tumor tissues
Primary and surrounding tissues of pancreatic tumor were fixed in 10% formalin and embedded in paraffin. Tissue slides were stained with haematoxylin and eosin and Sirius red (collagen deposition). For immunohistochemistry staining, anti-α-SMA (Novus, Cat. No. NBP1-30894) and cleaved-Caspase3 (Cell Signaling Tech. Cat. No. 9661) were used.
TUNEL assay
To detect the apoptotic tumor cells in tumor tissues, DeadEnd™ Fluorometric TUNEL System (Promega, Cat. No. G3250) was used with manufacturer’s protocol. Briefly, the tumor tissue slides were deparaffinized and rehydrated with ethanol. The tissues were permeabilized and covered with equilibration buffer and rTdT buffer. SSC buffer was used for stopping the reaction and nuclei were stained with VECTASHIELD® Antifade Mounting Medium with DAPI (Vector lab, Cat. No. H-1200).
Annexin V staining assay
Flow cytometry for annexin V staining was performed using EzWay Annexin V-FITC Apoptosis Detection Kit (KOMA Biotech, Cat. No. K29100). A total of 3 × 105 cells were stained with Annexin V-FITC and 7AAD following the protocol. Labelled cells were detected by BD FACS Canto II and analysed data using Flowjo 10.8 software (BD Biosciences).
TCGA data
The box plot expression and disease-free survival rate analysis in TCGA data were obtained from PAAD (pancreatic adenocarcinoma) dataset in GEPIA2 database [70].
Statistics
All the data were analyzed statistically using two-tailed and unpaired Student’s t-test. Data were presented as the mean with SD by GraphPad Prism 8 software. Significance of data was determined by P-values, which were labelled by * <0.05, ** <0.01, *** <0.001, and **** ≤0.0001.
Data availability
The raw data for RNA sequencing generated in this study have been deposited in the NCBI SRA database under accession code SRR22996981—SRR22996992 (https://trace.ncbi.nlm.nih.gov/Traces/sra/sra.cgi?view=run_browser&run=SRR_number [i.e., SRR number: SRR22996981]).
References
Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet 2020;395:2008–20.
Zhang L, Sanagapalli S, Stoita A. Challenges in diagnosis of pancreatic cancer. World J Gastroenterol. 2018;24:2047–60.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Miller AL, Garcia PL, Yoon KJ. Developing effective combination therapy for pancreatic cancer: an overview. Pharm Res. 2020;155:104740.
McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846–61.
Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801–10.
Janssen QP, Buettner S, Suker M, Beumer BR, Addeo P, Bachellier P, et al. Neoadjuvant FOLFIRINOX in patients with borderline resectable pancreatic cancer: a systematic review and patient-level meta-analysis. J Natl Cancer Inst. 2019;111:782–94.
Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–406.
Bear AS, Vonderheide RH, O’Hara MH. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell. 2020;38:788–802.
Liu XY, Guo CH, Xi ZY, Xu XQ, Zhao QY, Li LS, et al. Histone methylation in pancreatic cancer and its clinical implications. World J Gastroenterol. 2021;27:6004–24.
Paradise BD, Barham W, Fernandez-Zapico ME. Targeting epigenetic aberrations in pancreatic cancer, a new path to improve patient outcomes? Cancers (Basel). 2018;10:128.
Regel I, Mayerle J, Mahajan UM. Current strategies and future perspectives for precision medicine in pancreatic cancer. Cancers (Basel). 2020;12:1024.
Yang Y, Bedford MT. Protein arginine methyltransferases and cancer. Nat Rev Cancer. 2013;13:37–50.
Karkhanis V, Hu YJ, Baiocchi RA, Imbalzano AN, Sif S. Versatility of PRMT5-induced methylation in growth control and development. Trends Biochem Sci. 2011;36:633–41.
Kim H, Ronai ZA. PRMT5 function and targeting in cancer. Cell Stress. 2020;4:199–215.
Fabbrizio E, El Messaoudi S, Polanowska J, Paul C, Cook JR, Lee JH, et al. Negative regulation of transcription by the type II arginine methyltransferase PRMT5. EMBO Rep. 2002;3:641–5.
Roworth AP, Carr SM, Liu G, Barczak W, Miller RL, Munro S, et al. Arginine methylation expands the regulatory mechanisms and extends the genomic landscape under E2F control. Sci Adv. 2019;5:eaaw4640.
Kalev P, Hyer ML, Gross S, Konteatis Z, Chen CC, Fletcher M, et al. MAT2A inhibition blocks the growth of MTAP-deleted cancer cells by reducing PRMT5-dependent mRNA splicing and inducing DNA damage. Cancer Cell. 2021;39:209–24.e11.
Orben F, Lankes K, Schneeweis C, Hassan Z, Jakubowsky H, Krauss L, et al. Epigenetic drug screening defines a PRMT5 inhibitor-sensitive pancreatic cancer subtype. JCI Insight. 2022;7:e151353.
Ge L, Wang H, Xu X, Zhou Z, He J, Peng W, et al. PRMT5 promotes epithelial-mesenchymal transition via EGFR-beta-catenin axis in pancreatic cancer cells. J Cell Mol Med. 2020;24:1969–79.
Chan-Penebre E, Kuplast KG, Majer CR, Boriack-Sjodin PA, Wigle TJ, Johnston LD, et al. A selective inhibitor of PRMT5 with in vivo and in vitro potency in MCL models. Nat Chem Biol. 2015;11:432–7.
Barczak W, Jin L, Carr SM, Munro S, Ward S, Kanapin A, et al. PRMT5 promotes cancer cell migration and invasion through the E2F pathway. Cell Death Dis. 2020;11:572.
Chen H, Lorton B, Gupta V, Shechter D. A TGFbeta-PRMT5-MEP50 axis regulates cancer cell invasion through histone H3 and H4 arginine methylation coupled transcriptional activation and repression. Oncogene 2017;36:373–86.
Jung SY, Yug JS, Clarke JM, Bauer TM, Keedy VL, Hwang S, et al. Population pharmacokinetics of vactosertib, a new TGF-beta receptor type Iota inhibitor, in patients with advanced solid tumors. Cancer Chemother Pharm. 2020;85:173–83.
Jung SY, Hwang S, Clarke JM, Bauer TM, Keedy VL, Lee H, et al. Pharmacokinetic characteristics of vactosertib, a new activin receptor-like kinase 5 inhibitor, in patients with advanced solid tumors in a first-in-human phase 1 study. Invest N Drugs. 2020;38:812–20.
Hong E, Park S, Ooshima A, Hong CP, Park J, Heo JS, et al. Inhibition of TGF-beta signalling in combination with nal-IRI plus 5-Fluorouracil/Leucovorin suppresses invasion and prolongs survival in pancreatic tumour mouse models. Sci Rep. 2020;10:2935.
Kim TW, Lee KW, Ahn JB, Lee J, Ryu J, Oh B, et al. Efficacy and safety of vactosertib and pembrolizumab combination in patients with previously treated microsatellite stable metastatic colorectal cancer. J Clin Oncol. 2021;39:3573.
Choi J, Park J, Cho I, Sheen Y. Co-treatment with vactosertib, a novel, orally bioavailable activin receptor-like kinase 5 inhibitor, suppresses radiotherapy-induced epithelial-to-mesenchymal transition, cancer cell stemness, and lung metastasis of breast cancer. Radio Oncol. 2022;56:185–97.
Cho BC, Lee KH, Han J-Y, Yong Shim B, Kim HR, Pyo K-H, et al. 363 Vactosertib and durvalumab as second or later line treatment for PD-L1 positive non-small cell lung cancer: interim result. J Immunother Cancer. 2020;8:A222–A.
Malek E, Hwang S, Caimi PF, Metheny LL, Tomlinson BK, Cooper BW, et al. Phase Ib trial of vactosertib in combination with pomalidomide in relapsed multiple myeloma: a corticosteroid-free approach by targeting TGF-β signaling pathway. J Clin Oncol. 2021;39:8039.
Park JO, Kim ST, Hong JY, Kim S-J, Park YS. Phase 1b study of vactosertib in combination with nal-IRI plus 5FU/LV in patients with metastatic pancreatic ductal adenocarcinoma who have failed first-line gemcitabine/nab-paclitaxel. J Clin Oncol. 2022;40:TPS632–TPS.
Keleg S, Buchler P, Ludwig R, Buchler MW, Friess H. Invasion and metastasis in pancreatic cancer. Mol Cancer. 2003;2:14.
Shen W, Tao G-Q, Zhang Y, Cai B, Sun J, Tian Z-Q. TGF-β in pancreatic cancer initiation and progression: two sides of the same coin. Cell Biosci. 2017;7:39.
Zhao X, Yang X, Wang X, Zhao X, Zhang Y, Liu S, et al. Penetration cascade of size switchable nanosystem in desmoplastic stroma for improved pancreatic cancer therapy. ACS Nano. 2021;15:14149–61.
Brennan AJ, Chia J, Trapani JA, Voskoboinik I. Perforin deficiency and susceptibility to cancer. Cell Death Differ. 2010;17:607–15.
Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet 2004;363:1049–57.
Sato N, Goggins M. The role of epigenetic alterations in pancreatic cancer. J Hepatobiliary Pancreat Surg. 2006;13:286–95.
Zhao Q, Rank G, Tan YT, Li H, Moritz RL, Simpson RJ, et al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat Struct Mol Biol. 2009;16:304–11.
Chiang K, Zielinska AE, Shaaban AM, Sanchez-Bailon MP, Jarrold J, Clarke TL, et al. PRMT5 is a critical regulator of breast cancer stem cell function via histone methylation and FOXP1 expression. Cell Rep. 2017;21:3498–513.
Tarighat SS, Santhanam R, Frankhouser D, Radomska HS, Lai H, Anghelina M, et al. The dual epigenetic role of PRMT5 in acute myeloid leukemia: gene activation and repression via histone arginine methylation. Leukemia 2016;30:789–99.
Cho EC, Zheng S, Munro S, Liu G, Carr SM, Moehlenbrink J, et al. Arginine methylation controls growth regulation by E2F-1. EMBO J. 2012;31:1785–97.
Chung J, Karkhanis V, Baiocchi RA, Sif S. Protein arginine methyltransferase 5 (PRMT5) promotes survival of lymphoma cells via activation of WNT/β-catenin and AKT/GSK3β proliferative signaling. J Biol Chem. 2019;294:7692–710.
Qin Y, Hu Q, Xu J, Ji S, Dai W, Liu W, et al. PRMT5 enhances tumorigenicity and glycolysis in pancreatic cancer via the FBW7/cMyc axis. Cell Commun Signal. 2019;17:30.
Helms E, Onate MK, Sherman MH. Fibroblast heterogeneity in the pancreatic tumor microenvironment. Cancer Disco. 2020;10:648–56.
Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–76.
Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer — clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17:527–40.
Henke E, Nandigama R, Ergun S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci. 2019;6:160.
Kesh K, Gupta VK, Durden B, Garrido V, Mateo-Victoriano B, Lavania SP, et al. Therapy resistance, cancer stem cells and ECM in cancer: the matrix reloaded. Cancers (Basel). 2020;12:3067.
Pereira BA, Vennin C, Papanicolaou M, Chambers CR, Herrmann D, Morton JP, et al. CAF subpopulations: a new reservoir of stromal targets in pancreatic cancer. Trends Cancer. 2019;5:724–41.
Norton J, Foster D, Chinta M, Titan A, Longaker M. Pancreatic cancer associated fibroblasts (CAF): under-explored target for pancreatic cancer treatment. Cancers (Basel). 2020;12:1347.
Geng X, Chen H, Zhao L, Hu J, Yang W, Li G, et al. Cancer-associated fibroblast (CAF) heterogeneity and targeting therapy of CAFs in pancreatic cancer. Front Cell Dev Biol. 2021;9:655152.
Calon A, Tauriello DV, Batlle E. TGF-beta in CAF-mediated tumor growth and metastasis. Semin Cancer Biol. 2014;25:15–22.
Papageorgis P, Stylianopoulos T. Role of TGFβ in regulation of the tumor microenvironment and drug delivery (Review). Int J Oncol. 2015;46:933–43.
Yoon H, Tang C-M, Banerjee S, Delgado AL, Yebra M, Davis J, et al. TGF-β1-mediated transition of resident fibroblasts to cancer-associated fibroblasts promotes cancer metastasis in gastrointestinal stromal tumor. Oncogenesis 2021;10:13.
Padua D, Massagué J. Roles of TGFβ in metastasis. Cell Res. 2009;19:89–102.
Shen W, Tao GQ, Zhang Y, Cai B, Sun J, Tian ZQ. TGF-beta in pancreatic cancer initiation and progression: two sides of the same coin. Cell Biosci. 2017;7:39.
Rajagopal MU, Bansal S, Kaur P, Jain SK, Altadil T, Hinzman CP, et al. TGFbeta drives metabolic perturbations during epithelial mesenchymal transition in pancreatic cancer: TGFbeta induced EMT in PDAC. Cancers (Basel). 2021;13:6204.
Huang C-Y, Chung C-L, Hu T-H, Chen J-J, Liu P-F, Chen C-L. Recent progress in TGF-β inhibitors for cancer therapy. Biomed Pharmacother. 2021;134:111046.
Haque S, Morris JC. Transforming growth factor-β: a therapeutic target for cancer. Hum Vaccines Immunotherapeutics. 2017;13:1741–50.
Mao B, Zhang Z, Wang G. BTG2: a rising star of tumor suppressors (Review). Int J Oncol. 2015;46:459–64.
Yuniati L, Scheijen B, van der Meer LT, van Leeuwen FN. Tumor suppressors BTG1 and BTG2: beyond growth control. J Cell Physiol. 2019;234:5379–89.
Devanand P, Sundaramoorthy S, Ryu MS, Jayabalan AK, Ohn T, Lim IK. Translational downregulation of Twist1 expression by antiproliferative gene, B-cell translocation gene 2, in the triple negative breast cancer cells. Cell Death Dis. 2019;10:410.
Sundaramoorthy S, Devanand P, Ryu MS, Song KY, Noh DY, Lim IK. TIS21(/BTG2) inhibits breast cancer growth and progression by differential regulation of mTORc1 and mTORc2-AKT1-NFAT1-PHLPP2 signaling axis. J Cancer Res Clin Oncol. 2018;144:1445–62.
Zhu C, Zhang S, Xue A, Feng G, Fan S. Elevated BTG2 improves the radiosensitivity of non-small cell lung cancer (NSCLC) through apoptosis. Thorac Cancer. 2022;13:1441–8.
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36.
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–5.
Wang L, Feng Z, Wang X, Wang X, Zhang X. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010;26:136–8.
Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57.
Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49:D605–D12.
Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556–W60.
Acknowledgements
This work was supported by a grant from the National R&D Program for Cancer Control (HA17C0037), a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (HI14C2640), funded by the Ministry of Health & Welfare, Republic of Korea.
Author information
Authors and Affiliations
Contributions
E.H., S.J.K., S.P., W.B., S.M., N.B.L.T., S.H.P., and J.O.P. designed the experiments. E.H., J.S.H., and H.A. performed the experiments. A.O. performed histological analysis, and C.H. performed RNA-sequencing analysis. E.H. and S.J.K drafted the manuscript, and S.P., J.P., N.B.L.T., J.O.P., and S.J.K. revised the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
S.J.K. is a shareholder of Medpacto Inc. E.H., W.B., S.P., J.S.H., A.O., S.M., C.P.H., J.P., H.A., J.O.P., S.H.P, N.B.L.T. declare that they have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Boris Zhivotovsky
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hong, E., Barczak, W., Park, S. et al. Combination treatment of T1-44, a PRMT5 inhibitor with Vactosertib, an inhibitor of TGF-β signaling, inhibits invasion and prolongs survival in a mouse model of pancreatic tumors. Cell Death Dis 14, 93 (2023). https://doi.org/10.1038/s41419-023-05630-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-023-05630-5