Abstract
Helicobacter pylori (H. pylori) is one of the main causes of gastric cancer. It has been reported that circRNAs play a vital role in the development of multiple types of cancer. However, the role of H. pylori-induced circRNAs in the development of gastric cancer has not been studied. In this study, we found that H. pylori could induce the upregulation of circMAN1A2 in AGS and BGC823 cells independent of CagA. The downregulation of circMAN1A2 could inhibit the proliferation, migration and invasion of gastric cancer cells, and circMAN1A2 could promote the progression of gastric cancer induced by H. pylori by sponging miR-1236-3p to regulate MTA2 expression. Furthermore, circMAN1A2 knockdown inhibited xenograft tumour growth in vivo, and the overexpression of circMAN1A2 was associated with the progression of gastric cancer. Hence, Helicobacter pylori induced circMAN1A2 expression to promote the carcinogenesis of gastric cancer, and circMAN1A2 might be a new potential diagnostic marker and therapeutic target for gastric cancer.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is one of the most common malignant tumours in the world. According to statistics in 2018, the incidence and mortality of gastric cancer in East Asia ranks first in the world [1, 2]. Once they are diagnosed, most patients are in an advanced stage of GC with poor prognosis [3], and thus, it is extremely important to study the pathogenesis of GC. The occurrence of GC is a multifactor and multilevel process. It has been demonstrated that Helicobacter pylori (H. pylori) is the main environmental factor that can induce GC, and H. pylori infection is an important initial factor for the development of GC [4,5,6]. Studies have also shown that H. pylori can give rise to cancerization by regulating the expression of noncoding RNA to induce proliferation and apoptosis of gastric epithelial cells [7,8,9].
With the development of high-throughput sequencing technology, circRNA, which has a highly conserved and stable covalently closed cyclic structure, has aroused interest [10]. CircRNA is abundant and stable in eukaryotic cells, and it can survive under RNase R treatment [11, 12]. CircRNA was originally thought to be a by-product of mRNA production during mis-splicing without biological functions [13, 14]. However, thousands of circRNAs have been found to be involved in the regulation of gene expression and play a significant role in cell differentiation, tissue homeostasis, and disease development [15,16,17]. It is thought to be one of the most important biological functions that circRNAs can act as miRNA sponges to improve the expression level of miRNA target genes by inhibiting the activity of miRNAs [18, 19]. For example, circHIPK3 can sponge multiple miRNAs to regulate cell growth; [20] circIRAK3 acts as a miR-3607 sponge to promote breast cancer metastasis; [21] and circHLA-C can sponge miR150 to regulate the development of lupus nephritis [22]. Recent research reports that circRNAs are involved in the development of GC, but the mechanism of action of circRNAs in the development of GC is not fully understood [19]. H. pylori infection is an important factor in the malignant transformation of gastric mucosa. Studies have also shown that H. pylori can regulate the development of gastric cancer by regulating the expression of a variety of noncoding RNAs or mRNAs [23, 24]. However, there is no report about the role of circRNAs in GC induced by H. pylori.
Therefore, our study analysed the expression profile of circRNAs in gastric cancer cells infected by H. pylori by RNA sequencing. Then, circMAN1A2 with the most significant expression difference was selected according to the sequencing results to study the epigenetic mechanism of H. pylori-induced gastric carcinogenesis. We studied the role of circMAN1A2 in the development of gastric cancer induced by H. pylori and revealed the molecular mechanism by which circMAN1A2 promotes gastric mucosal malignant transformation, providing a basis for the prevention and early diagnosis of GC.
Methods
Cell lines, H. pylori strains and the infection model
GES-1 human gastric epithelial cells and AGS and BGC823 human gastric cancer cell lines and two H. pylori strains were cultured according to the methods described in our previous article [25].
RNA-seq data analysis and identification
Pair readings were collected by the Illumina HiSeq Sequencer. The total number of readings in the obtained sample was used for normalization and log2 conversion, and the number of standardized readings was compared between the samples. The original reading and human reference genome (hg19) were compared via STAR software, and the Ensemble (V70) GTF file was used as a guide. To align high-quality reads with the reference genome/transcriptome, Bowtie2 software was used, and the find_circ software was used to detect and identify circRNAs. CircRNAs showing a fold-change > 2.0 and P value < 0.05 were classified as significantly differentially expressed circRNAs. Hierarchical clustering was performed using Heatmap2 in R software for circulating RNAs with a fold variation greater than 2.0.
Small interfering RNA (siRNA), plasmid and lentivirus transfection
The circMAN1A2 siRNAs and miR-1236-3p were designed and purchased from the Suzhou Ribo Life Science Co., Ltd. (Suzhou, China). According to the sequence across the back-splicing junction site of circMAN1A2, circMAN1A2 siRNAs were designed to specifically knock down the expression of circMAN1A2. The pcDNA3.1-CagA plasmid was kindly provided by Yongliang Zhu (Zhejiang University, China). pcDNA3.1-circMAN1A2-WT and pcDNA3.1-circMAN1A2-Mut were purchased from the Hanbio Biotechnology Co., Ltd. (Shanghai, China). The siRNA and plasmid were transfected with jetRPIME transfection reagent (Polyplus, Illkirch, France). Lentiviruses of sh-NC and sh-circMAN1A2 were purchased from the Shanghai GeneChem Co., Ltd. (Shanghai, China). Cells stably transfected with sh-NC and sh-circMAN1A2 lentiviruses were screened by puromycin. The sequences of siRNAs were as follows:
si-negative control (si-NC): 5ʹ-UUCUUCGAAACGUGUCACGUT-3ʹ;
si-circMAN1A2-1: 5ʹ-AUUUCCUCUCUUGACUUUCTT-3ʹ;
si-circMAN1A2-2: 5ʹ-UUUCUGCUCGAAUUUCCUCTT-3ʹ.
Patients and specimens
Fifty-two Hp− human gastritis specimens and 47 Hp+ human gastritis specimens were collected from patients undergoing gastroscopy. A total of 101 pairs of GC tissues were obtained from patients with GC during surgery. Preoperative plasma was obtained from 49 GC gastric cancer patients. The infection of Helicobacter pylori in gastritis patients was detected via 14C urea breath tests, and gastritis tissues were used to detect H. pylori infection by rapid urease test. All patient tissues and plasma were obtained from Qilu Hospital at Shandong University. Plasma from 46 healthy people was donated by volunteers. Our research was approved by the Ethics Committee of Shandong University School of Medicine (Jinan, China) and conducted in accordance with the Declaration of Helsinki.
RNA preparation and qRT–PCR
TRIzol (Invitrogen, Waltham, MA, USA) was used to extract total RNA from cells and tissues. The purity and quantity of total RNA were then detected by a NanoDrop Lite Spectrophotometer (Thermo Scientific, USA).
Nuclear and cytoplasmic RNAs were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, USA) according to the manufacturer’s protocol.
The RNA was reverse transcribed into cDNA with a Prime Script RT Reagent Kit (Takara Bio Inc, Osaka, Japan). qRT–PCR was conducted using a SYBR Premix Ex Taq System (Takara) and a CFX96 Real-time PCR System (Bio–Rad, USA). All of the primers are listed in Supplementary Table 1.
Western blot
The high efficiency RIPA lysis fluid and the protease inhibitor were prepared into a protein lysate in a ratio of 100:1 to extract the total cell protein. The purity and concentration of total protein were then detected by NanoDrop Lite Spectrophotometer (Thermo Scientific, USA). Proteins were separated by SDS-PAGE and then transferred to polyvinylidene difluoride membranes (Merck Millipore, Germany), blocked in 5% skim milk for 1 h, and then incubated with anti-CagA primary antibody (1:500, Santa, sc-28368, USA), GAPDH (1:5000, Abways, AB0036, China) at 4 °C overnight. Anti-mouse and anti-rabbit horseradish peroxidase-conjugated secondary antibodies were incubated for 1 h at room temperature and exposure using enhanced chemiluminescence (Millipore, USA).
CCK-8, colony formation and transwell assays
CCK-8, colony formation and transwell assays methods are detailed in the methods described in our previous article [25].
RNase R treatment
A RNeasy MinElute Cleanup Kit (Qiagen, USA) was used to purify the resulting RNA. Total RNA (2 μg) was incubated with or without 3 U/μg RNase R at 37 °C for 20 min. RNA expression was detected by qRT–PCR.
Dual-luciferase reporter assay
The pcDNA3.1-circMAN1A2-WT, pcDNA3.1-circMAN1A2-Mut and miR-1236-3p mimics were cotransfected into GC cells. After 48 hours of transfection, the luciferase activities were quantified with a dual-luciferase reporter assay (Promega, USA) detected by Centro XS3 LB960 (Berthold Technologies, Germany).
Animal experiments
Four-week-old female BALB/c nude mice were procured from Beijing Vital River Laboratory Animal Technology (Beijing, China). Nude mice were randomly divided into two groups with 6 mice in each group. BGC823 cells stably transfected with sh-circMAN1A2 and sh-NC lentivirus were resuspended, and 100 µl of cell suspension containing 1 × 106 cells was injected into the subcutaneous axilla of nude mice. The length and width of the tumour were measured with a Vernier caliper once a week and recorded for 4 weeks. After 4 weeks, the tumour was removed and weighed. This research was approved and guided by the Ethics Committee of Shandong University School of Medicine (Jinan, China).
Statistical analysis
Statistical analysis was conducted using SPSS v25.0 (IBM Corp.) and Graphpad 8.0. All data were presented as the mean ± SD of at least three biological replicates. ANOVA with an appropriate post-hoc correction was used for comparisons among multiple groups. Student’s t test was generally used to analyze the differences between two groups, but when the variances differed, the Mann–Whitney U test was used. P < 0.05 (two-tailed) was considered to indicate a statistically significant difference.
Date deposition
The circRNA sequencing data used in this study has been uploaded to the Gene Expression Omnibus (GEO) with the accession code GSE183628.
Results
Differentially expressed circRNAs in AGS cells infected with Hp26695 vs. control
To explore whether H. pylori infection could cause circRNA changes in GC, we treated AGS with PBS and H. pylori to perform RNA-seq analysis. A total of 18 308 different circRNA candidates were obtained in the experiment, of which 10 454 of these circRNAs contained at least two unique back-junction readings (Fig. 1a). Of these 18,308 circRNA candidates, only 1628 (8.9%) were exon-loop RNAs produced by the exon of the mononuclear protein-coding gene (Fig. 1b). The majority of circRNAs produced by the exon of the mononuclear protein-coding gene were less than 1500 nucleotides in length and had a median length of 500 (Fig. 1c).
a The number of reverse splicing events of circRNAs was identified in PBS-treated and Hp-infected AGS cells: 10,454 common circRNAs have two or more reverse splicing events. b The origin and distribution of circRNAs: exon circRNA (9%), intron circRNA (20%), intergenic region circRNA (21%), overlapping region circRNA (22%) and antisense circRNA (28%). c Length distribution of circRNAs (n = 18 308). d Heat map of the expression levels of circRNAs in AGS cells treated with PBS and Hp26695. (n = 6). Green to red represents circRNA expression from low to high, respectively. e Volcano plot of differentially expressed circRNAs: red dots indicate differentially expressed circRNAs; the left panel shows downregulated circRNAs, and the right panel shows upregulated circRNAs. (fold change > 2.0, P < 0.05, FDR < 0.05). f Scatter plots identified changes in circRNA expression (dots above the diagonal line indicate upregulated circRNAs, and dots below the diagonal line indicate downregulated circRNAs). g Chromosomal locus distribution of differentially expressed circRNAs. h The number of differentially expressed circRNAs.
Compared with the control, 101 significantly differentially expressed circRNAs were identified in the AGS cells infected with H. pylori, including 84 upregulated circRNAs and 17 downregulated circRNAs (Fig. 1d–f). The 101 differentially expressed circRNAs were located on all human chromosomes, including 22 autosomes, the X chromosome, and the mitochondria (Fig. 1g). Among the 84 upregulated circRNAs, 12 circRNAs were identified previously and listed in the published circRNA database or articles, and 72 circRNAs might be potential novel circRNAs that were identified for the first time. Among the 17 downregulated circRNAs, 4 circRNAs were identified previously, and 13 circRNAs might be potential novel circRNAs (Fig. 1h).
H. pylori could upregulate the expression of circMAN1A2 in gastric cancer cells
Five upregulated circRNAs and one downregulated circRNA were selected based on the difference in gene expression, and the expression levels of these circRNAs were verified in AGS cells infected with H. pylori. The changes in circRNA expression detected by qRT–PCR were consistent with the RNA-seq data (Fig. 2a). Since the expression difference of circMAN1A2 was the most significant, we chose circMAN1A2 for further verification. We also identified that the expression of circMAN1A2 was much higher in Hp+ chronic gastritis tissues than in Hp− chronic gastritis tissues (Fig. 2b). Then, we infected AGS and BGC823 cells with Hp26695 at a specific time and concentration. The results showed that Hp26695 could significantly upregulate the expression of circMAN1A2 when it infected AGS and BGC823 cells for 12 and 24 h, or at the concentration of MOI = 100 and 150 (Fig. 2c, d).
a After Hp26695-infected AGS cells were infected at an MOI = 100:1 for 12 h, the expression of circRNAs was detected by qRT–PCR. The control group used PBS for the same amount of time. b qRT–PCR analysis of circMAN1A2 expression in Hp+ gastritis tissues (n = 47) and Hp− gastritis tissues (n = 52). (Mann–Whitney U). c After Hp26695 infected AGS and BGC823 cells at a MOI = 100:1 for different times (0, 3, 6, 12 and 24 h), the relative expression of circMAN1A2 was assessed by qRT–PCR. d. After Hp26695 infected AGS and BGC823 cells with different MOIs (0, 50, 100, and 150) for 12 h, the expression of circMAN1A2 was detected by qRT–PCR. e Western blot was used to detect the expression of CagA protein in AGS and BGC823 cells after Hp26695-CagA+ and Hp26695-CagA- infection for 24 h. f After Hp26695-CagA- infected AGS and BGC823 cells at a MOI = 100:1 for different times (0, 3, 6, 12 and 24 h), the expression of circMAN1A2 was detected by qRT–PCR. g CagA protein in AGS and BGC823 cells transfected with the PcDNA3.1-CagA plasmid and PcDNA3.1 plasmid was analysed by Western blot. h qRT–PCR detected the expression of circMAN1A2 in AGS and BGC823 cells transfected with the PcDNA3.1-CagA plasmid and PcDNA3.1 plasmid. i After Hp26695 and heat-killed Hp26695 infected AGS and BGC823 cells at a MOI = 100:1 for 12 h, the expression of circMAN1A2 was assessed by qRT–PCR. The control group used PBS. *P < 0.05, **P < 0.01, ***P < 0.001.
CagA is one of the most important virulence factors of H. pylori, and it can affect multiple pathways in cells. Therefore, we speculated whether the upregulation of circMAN1A2 depended on CagA. We detected the expression of CagA protein in AGS and BGC823 cells after Hp26695-CagA+ and Hp26695-CagA− infection for 24 h (Fig. 2e). However, we found that circMAN1A2 was also overexpressed in AGS and BGC823 cells infected with Hp26695 with CagA depletion for 12 and 24 h. (Fig. 2f). Then we detected the expression of cagA plasmid in gastric cancer cells and found that the CagA plasmid could not upregulate the expression of circMAN1A2 (Fig. 2g, h). Meanwhile, heat-killed Hp26695 did not upregulate the expression of circMAN1A2 (Fig. 2i), indicating that the upregulation of circMAN1A2 induced by H. pylori did not depend on CagA but might depend on the interaction between living bacteria and cells.
CircMAN1A2 could promote the proliferation, migration and invasion of gastric cancer cells
Then, we selected AGS and BGC823 cells with higher expression levels of circMAN1A2 for functional verification in vitro (Fig. 3a). We first knocked down the expression of circMAN1A2 in AGS and BGC823 cells with siRNA (Fig. 3b). The CCK-8 and colony formation results demonstrated that the downregulation of circMAN1A2 could inhibit the proliferation of GC cells (Fig. 3c, d). Transwell assays without or with Matrigel showed that the downregulation of circMAN1A2 could inhibit the migration and invasion of GC cells (Fig. 3e, f).
a qRT–PCR analysis of circMAN1A2 in the human immortalized gastric epithelial cell line GES-1 and gastric cancer cell lines MGC803, AGS, BGC823, SGC7901 and HGC27. b The interference efficiency of circMAN1A2 siRNAs was detected by qRT–PCR in AGS cells and BGC823 cells. c The proliferation ability of AGS and BGC823 cells transfected with circMAN1A2 siRNAs was detected by CCK-8 assay at 5 time points (24, 48, 72, 96 and 120 h). d The colony formation test was used to detect the clonogenic ability of AGS and BGC823 cells transfected with circMAN1A2 siRNAs. e The migration ability of AGS and BGC823 cells transfected with circMAN1A2 siRNAs was detected by Transwell assays without Matrigel. f The invasion ability of AGS and BGC823 cells transfected with circMAN1A2 siRNAs was measured by Transwell assays with Matrigel. *P < 0.05, **P < 0.01, ***P < 0.001.
CircMAN1A2 could act as a miR-1236-3p sponge in GC cells
To study how circMAN1A2 regulates the proliferation, invasion and migration of GC cells, we first confirmed the circular structure of circMAN1A2 by RNase R tolerance test (Fig. 4a). NE-PER nuclear and cytoplasmic extraction test showed that circMAN1A2 was mainly expressed in the cytoplasm (Fig. 4b). This suggested that circMAN1A2 might be involved in the function of gastric cancer cells by absorbing miRNA through sponging action. Therefore, we used RNAhybrid and miRanda software to predict the miRNAs that might bind to circMAN1A2 and found that miR-1236-3p had a stronger binding ability to circMAN1A2 (Fig. 4c). Then, we designed a luciferase reporter assay to further confirm this hypothesis. The luciferase signal decreased when Luc-circMAN1A2-WT was cotransfected with miR-1236-3p mimics into AGS cells. When Luc-circMAN1A2-Mut was cotransfected with miR-1236-3p mimics into AGS and BGC823 cells, no change in the luciferase signal was observed. This finding suggested that circMAN1A2 could bind to miR-1236-3p by the miRNA sponging effect (Fig. 4d).
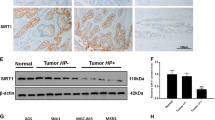
a After RNase R treatment, the tolerance of circMAN1A2 to RNase R was detected by qRT–PCR. b qRT–PCR was used to detect the expression of circMAN1A2 in the nucleus and cytoplasm. GAPDH was used as the cytoplasmic internal reference, and U2 was used as the nuclear internal reference. c Schematic diagram of the binding site of circMAN1A2 and miR-1236-3p and the binding sequence of circMAN1A2 and miR-1236–3p. d Luc-circMAN1A2-WT and Luc-circMAN1A2-Mut cotransfected AGS and BGC823 cells with miR-1236-3p mimics, respectively. The luciferase activity was detected by the dual-luciferase assay in AGS and BGC823 cells. e The expression of miR-1236-3p and MTA2 was detected by qRT–PCR after knockdown of circMAN1A2 in AGS and BGC823 cells. f The expression of MTA2 in normal adjacent tissues (n = 14) and gastric cancer tissues (n = 14) was detected by qRT–PCR. (Mann–Whitney U). g Spearman’s correlation coefficient analysis was used to assess the correlation between circMAN1A2 and MTA2 levels in GC tissues (n = 20). h. After Hp26695 infected AGS and BGC823 cells at a MOI = 100:1 for different times (0, 12 and 24 h), the relative expression of MTA2 was assessed by qRT–PCR. *P < 0.05, **P < 0.01, ***P < 0.001.
The expression of miR-1236-3p was reduced in GC tissues, and its expression was related to lymph node metastasis, differentiation and the clinical stage of GC [26, 27]. The overexpression of miR-1236-3p could repress the proliferation, migration and invasion of GC cells by inhibiting MTA2, and the EMT and PI3K/AKT pathways could be suppressed by miR-1236-3p in GC cells [28,29,30]. We found that after knockdown of circMAN1A2, the expression of miR-1236-3p was upregulated, while the expression of MAT2 mRNA was decreased in GC cells (Fig. 4e). Then, we found that the expression of MTA2 was overexpressed in GC tissues and was positively correlated with circMAN1A2 in GC tissues (Fig. 4f, g). Moreover, the mRNA expression level of MTA2 was upregulated when induced by H. pylori in AGS and BGC823 cells (Fig. 4h). These results further indicated that circMAN1A2 could regulate the development of GC through the miR-1236–3p/MTA2 axis.
H. pylori enhanced GC cell migration by upregulating the expression of circMAN1A2 in vitro
We found that circMAN1A2 could promote the proliferation and migration of GC cells and H. pylori could promote the progression of gastric cancer. To verify whether the effect of H. pylori on gastric cancer depends on circMAN1A2, we infected AGS cells with Hp26695. Twelve hours later, si-circMAN1A2–1 was transfected into the infected cells. The relative expression of circMAN1A2 was detected with qRT–PCR (Fig. 5a). Through Transwell assays without Matrigel, we found that H. pylori enhanced the migration ability of AGS cells, which was weakened by circMAN1A2 siRNAs (Fig. 5b). This result showed that H. pylori could enhance GC cell migration by upregulating the expression of circMAN1A2 in vitro.
a The expression of circMAN1A2 after H. pylori infection and transfection of circMAN1A2 siRNAs in AGS cells was detected by qRT–PCR. b The migration ability of AGS cells after H. pylori infection and transfection of circMAN1A2 siRNAs was detected by Transwell assays without Matrigel. c The expression of circMAN1A2 and miR-1236-3p after transfection of circMAN1A2 siRNAs and miR-1236-3p inhibitor in AGS cells was detected by qRT–PCR. d The migration ability of AGS cells after transfection of circMAN1A2 siRNAs and miR-1236-3p inhibitor was detected by Transwell assays without Matrigel. *P < 0.05, **P < 0.01, ***P < 0.001.
CircMAN1A2 could promote the migration of AGS by regulating miR-1236-3p
According to the above experimental results, circMAN1A2 could act as a miR-1236-3p sponge in GC cells. Therefore, we designed a rescue experiment to determine whether the regulation of circMAN1A2 on gastric cancer progression depended on miR-1236-3p. We interfered with both circMAN1A2 and miR-1236-3p, and the expression of circMAN1A2 and miR-1236-3p was examined by qRT–PCR (Fig. 5c). The results of Transwell experiments without Matrigel showed that circMAN1A2 siRNAs could inhibit the migration ability of AGS cells, which was weakened by miR-1236-3p inhibitor (Fig. 5d). This indicated that circMAN1A2 could promote the migration of AGS by binding to miR-1236-3p.
Downregulation of circMAN1A2 inhibits the proliferation of gastric cancer cells in vivo
Previous studies showed that downregulating the expression of circMAN1A2 can significantly inhibit the proliferation of gastric cancer cells in vitro. Therefore, we established a xenograft mouse model to detect the effect of circMAN1A2 on the growth of gastric cancer in vivo. We knocked down the expression of circMAN1A2 in BGC823 cells with sh-circMAN1A2 lentivirus (Fig. 6a). Compared with the control group, the downregulation of circMAN1A2 expression significantly inhibited tumour growth (Fig. 6b). Moreover, the weight and volume of the tumours in the sh-circMAN1A2 group were significantly less than that in the control group (Fig. 6c, d). These results suggest that downregulation of circMAN1A2 can inhibit the proliferation of gastric cancer cells in vivo.
a qRT–PCR analysis of circMAN1A2 expression in BGC823 cells transfected with sh-circMAN1A2 and sh-NC. b The tumours of the xenograft mouse model were removed and imaged. c, d The tumour volume and weight were measured and analysed. e The expression of circMAN1A2 in normal adjacent tissues (n = 101) and gastric cancer tissues (n = 101) was detected by qRT–PCR. f qRT–PCR analysis of circMAN1A2 expression in gastric cancer tissues in different TNM stages. g Relative expression of circMAN1A2 assessed by qRT–PCR in the plasma of gastric cancer patients (n = 49) and healthy donors (n = 46). *P < 0.05, **P < 0.01, ***P < 0.001.
CircMAN1A2 was overexpressed in GC tissues and the preoperative plasma of GC patients
To further study the correlation between circMAN1A2 and pathological parameters in patients with GC, we detected the expression of circMAN1A2 in GC tissues. Detailed clinical pathologic information of these patients with GC was presented in Table 1. The results showed that circMAN1A2 was significantly overexpressed in GC tissues compared with adjacent tissues (Fig. 6e). In addition, the expression level of circMAN1A2 was even higher in the advanced stage of GC tissues than in the early stage, indicating that the high expression of circMAN1A2 might contribute to the progression of GC (Fig. 6f). Furthermore, we identified that the expression of circMAN1A2 was much higher in the preoperative plasma of GC patients than in healthy plasma (Fig. 6g). This finding suggested that circMAN1A2 might be used as a novel diagnostic marker for GC.
Discussion
H. pylori is the main cause of chronic active gastritis and peptic ulcers, and persistent infection with H. pylori is closely related to gastric mucosal lymph tissue lymphoma and gastric cancer [31, 32]. Recent studies have found that circRNAs can regulate the occurrence and development of tumours, and they are expected to become a basic theoretical target for cancer prevention, diagnosis and treatment [16]. To explore the role of circRNAs in the development and progression of gastric cancer mediated by H. pylori infection, circRNAs in AGS gastric cancer cells infected with H. pylori were detected by circRNA sequencing technology. CircMAN1A2 was found to be upregulated significantly after H. pylori infection. Infection with H. pylori upregulated the expression of circMAN1A2 at the tissue and cell levels. CagA is one of the important carcinogenic virulence factors in H. pylori and is involved in the malignant transformation of gastric mucosa. However, the results showed that the abnormal expression of circMAN1A2 regulated by H. pylori was independent of CagA, and the interaction between living bacteria and cells was one of the necessary conditions to induce the aberrant expression of circMAN1A2. The specific molecular mechanism of H. pylori affecting circMAN1A2 expression needs further experimental exploration.
Downregulation of circMAN1A2 expression significantly inhibited the migration, invasion and proliferation of GC cells in vitro and circMAN1A2 knockdown inhibited xenograft tumour growth in vivo. Recently, circMAN1A2 was reported to be upregulated in nasopharyngeal cancer, oral cancer, thyroid cancer, ovarian cancer and lung cancer, indicating that circMAN1A2 might be a biomarker for malignant tumours [33].
Although studies have found that circRNAs have a variety of biological functions, such as miRNA sponging effects, binding with RNA binding proteins, and regulation of transcription and translation, the sponge adsorption function of circRNAs is currently the most widely and deeply studied mechanism [34,35,36]. For example, circSETD3 (Hsa_circ_0000567), as a microRNA-421 sponge, inhibits hepatocellular carcinoma growth [37]; meanwhile, hsa_circ_0072309 can absorb miR-492 and inhibit the proliferation and invasion of breast cancer cells [38]. RNAhybrid and miRanda analysis indicated that miR-1236-3p had a stronger binding ability to circMAN1A2. The luciferase reporter assay proved that circMAN1A2 could indeed bind to miR-1236-3p. Studies have confirmed that miR-1236-3p can inhibit the invasion and proliferation of gastric cancer cells by inhibiting the expression of MTA2 [28,29,30]. Subsequently, we found that MTA2 was upregulated in GC tissues and that the expression of circMAN1A2 and MTA2 was positively correlated in GC tissues. Therefore, circMAN1A2 could promote the progression of GC caused by H. pylori by regulating the miR-1236-3p/MTA2 axis.
To further confirm our findings, rescue experiments were performed. The results showed that the oncogenic effect of H. pylori in gastric cancer depended on circMAN1A2, and the antitumour effect of circMAN1A2 interference was weaken by miR-1236-3p inhibition in gastric cancer. Furthermore, the expression of circMAN1A2 was much higher in the preoperative plasma of GC patients than in healthy plasma. This finding suggested that circMAN1A2 might be used as a novel diagnostic marker for gastric cancer.
In summary, our study found that H. pylori could upregulate the expression of circMAN1A2 and affect the occurrence and development of gastric cancer by regulating the circMAN1A2-miR-1236-3p-MTA2 axis. H. pylori-circMAN1A2-miR-1236-3p-MTA2, a new signalling cascade, provides novel research ideas for clarifying the oncogenic mechanism of H. pylori and provides an experimental basis for exploring the molecular mechanism by which circMAN1A2 promotes the occurrence and development of gastric cancer. Our study also provides a theoretical basis for circMAN1A2 to be a diagnostic marker and intervention target for gastric cancer treatment.
Data availability
All data are available in this manuscript and supplementary files.
References
Fidler MM, Bray F. Global cancer inequalities. Front Oncol. 2018;8:293.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2018;68:394–424.
Song M, Rabkin CS, Camargo MC. Gastric cancer: an evolving disease. Curr Treat Options Gastroenterol. 2018;16:561–9.
Koga S, Takebayashi M, Kaibara N, Nishidoi H, Kimura O, Kawasumi H, et al. Pathological characteristics of gastric cancer that develop hematogenous recurrence, with special reference to the site of recurrence. J Surgical Oncol. 1987;36:239–42.
Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surgical Oncol. 2013;107:230–6.
Rieder G, Hofmann JA, Hatz RA, Stolte M, Enders GA. Up-regulation of inducible nitric oxide synthase in Helicobacter pylori-associated gastritis may represent an increased risk factor to develop gastric carcinoma of the intestinal type. Int J Med Microbiol. 2003;293:403–12.
Li N, Feng Y, Hu Y, He C, Xie C, Ouyang Y, et al. Helicobacter pylori CagA promotes epithelial mesenchymal transition in gastric carcinogenesis via triggering oncogenic YAP pathway. J Exp Clin Cancer Res. 2018;37:280.
Lim JH, Kim SG, Choi JM, Yang HJ, Kim JS, Jung HC. Is associated with expression through promoter methylation in gastric carcinogenesis. Gut Liver. 2018;12:58–66.
Shao L, Chen Z, Soutto M, Zhu S, Lu H, Judith RG, et al. Helicobacter pylori-induced miR-135b-5p promotes cisplatin resistance in gastric cancer. FASEB J. 2019;33:264–74.
Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci USA. 1976;73:3852–6.
Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453–61.
Suzuki H, Zuo Y, Wang J, Zhang MQ, Malhotra A, Mayeda A. Characterization of RNase R-digested cellular RNA source that consists of lariat and circular RNAs from pre-mRNA splicing. Nucleic Acids Res. 2006;34:e63.
Cocquerelle C, Mascrez B, Hétuin D, Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J. 1993;7:155–60.
Qu S, Yang X, Li X, Wang J, Li H. Circular RNA: a new star of noncoding RNAs. Cancer Lett. 2015;365:141–8.
Yao T, Chen Q, Fu L, Guo J. Circular RNAs: biogenesis, properties, roles, and their relationships with liver diseases. Hepatol Res. 2017;47:497–504.
Zhang HD, Jiang LH, Sun DW, Hou JC, Ji ZL. CircRNA: a novel type of biomarker for cancer. Breast Cancer. 2018;25:1–7.
Wang C, Tan S, Liu WR, Lei Q, Qiao W, Wu Y, et al. RNA-Seq profiling of circular RNA in human lung adenocarcinoma and squamous cell carcinoma. Mol Cancer. 2019;18:134.
Hentze MW, Preiss T. Circular RNAs: splicing’s enigma variations. EMBO J. 2013;32:923–5.
Zhang J, Liu H, Hou L, Wang G, Zhang R, Huang YX, et al. Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424-5p and regulating LATS1 expression. Mol Cancer. 2017;16:151.
Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215.
Wu J, Jiang Z, Chen C, Hu Q, Fu Z, Chen J, et al. CircIRAK3 sponges miR-3607 to facilitate breast cancer metastasis. Cancer Lett. 2018;430:179–92.
Luan J, Jiao C, Kong W, Fu J, Qu W, Chen Y, et al. circHLA-C plays an important role in lupus nephritis by sponging miR-150. Mol Ther Nucleic Acids. 2018;10:245–53.
Liu L, Shuai T, Li B, Zhu L, Li X. Long non‑coding RNA lnc‑GNAT1‑1 inhibits gastric cancer cell proliferation and invasion through the Wnt/β‑catenin pathway in Helicobacter pylori infection. Mol Med Rep. 2018;18:4009–15.
Zhu S, Soutto M, Chen Z, Peng DF, Romero-Gallo J, Krishna US, et al. Helicobacter pylori-induced cell death is counteracted by NF-κB-mediated transcription of DARPP-32. Gut. 2017;66:761–2.
Ma F, Li X, Ren J, Guo R, Li W. Downregulation of eukaryotic translation initiation factor 3b inhibited proliferation and metastasis of gastric cancer. Cell Death Dis. 2019;10:623.
Zhu XP, Wang XL, Ma J, Fang YF, Zhang HJ, Zhang C, et al. Down-regulation of miR-1236-3p is correlated with clinical progression and unfavorable prognosis in gastric cancer. Eur Rev Med Pharmacol Sci. 2018;22:5914–9.
An JX, Ma ZS, Ma MH, Shao S, Cao FL, Dai DQ. MiR-1236-3p serves as a new diagnostic and prognostic biomarker for gastric cancer. Cancer Biomark. 2019;25:127–32.
An JX, Ma MH, Zhang CD, Shao S, Zhou NM, Dai DQ. miR-1236-3p inhibits invasion and metastasis in gastric cancer by targeting MTA2. Cancer Cell Int. 2018;18:66.
Zhou C, Ji J, Cai Q, Min S, Zhang J. MTA2 promotes gastric cancer cells invasion and is transcriptionally regulated by Sp1. Mol Cancer. 2013;12:102.
Zhou C, Ji J, Cai Q, Shi M, Chen X, Yu Y, et al. MTA2 enhances colony formation and tumor growth of gastric cancer cells through IL-11. BMC Cancer. 2015;15:343.
Sugiyama T, Asaka M. Helicobacter pylori as a new paradigm in the pathogenesis of upper GI diseases. Nihon rinsho Jpn J Clin Med. 1999;57:5–10.
Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–78.
Fan CM, Wang JP, Tang YY, Zhao J, He SY, Xiong F, et al. CircMAN1A2 could serve as a novel serum biomarker for malignant tumors. Cancer Sci. 2019;110:2180–8.
Ouyang Y, Li Y, Huang Y, Li X, Zhu Y, Long Y, et al. CircRNA circPDSS1 promotes the gastric cancer progression by sponging miR-186-5p and modulating NEK2. J Cell Physiol. 2019;234:10458–69.
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8.
Meng S, Zhou H, Feng Z, Xu Z, Ying T, Li P, et al. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16:94.
Xu L, Feng X, Hao X, Wang P, Zhang Y, Zheng X, et al. CircSETD3 (Hsa_circ_0000567) acts as a sponge for microRNA-421 inhibiting hepatocellular carcinoma growth. J Exp Clin Cancer Res. 2019;38:98.
Yan L, Zheng M, Wang H. Circular RNA hsa_circ_0072309 inhibits proliferation and invasion of breast cancer cells via targeting miR-492. Cancer Manag Res. 2019;11:1033–41.
Acknowledgements
We thanked the support of the National Natural Science Foundation of China [No. 82072244, 81772143, 81372680, 81402108, 81871621 and 81671981] and the Key Research and Development Foundation of Shandong Province [No. 2018GSF118093].
Author information
Authors and Affiliations
Contributions
RG, XC and XL performed experiments, data analysis and article writing. WZ, MC, ZS conducted literature search and data sorting. ZL, YS, JJ provided technical support, and WL provided project ideas and experimental design. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Hans-Uwe Simon
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, R., Cui, X., Li, X. et al. CircMAN1A2 is upregulated by Helicobacter pylori and promotes development of gastric cancer. Cell Death Dis 13, 409 (2022). https://doi.org/10.1038/s41419-022-04811-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-022-04811-y
This article is cited by
-
Emerging roles of circular RNAs in tumorigenesis, progression, and treatment of gastric cancer
Journal of Translational Medicine (2024)
-
CircRNA_15430 reduced by Helicobacter pylori infection and suppressed gastric cancer progression via miR-382-5p/ZCCHC14 axis
Biology Direct (2023)
-
Roles of circRNAs in regulating the tumor microenvironment
Medical Oncology (2023)
-
The possible role of Helicobacter pylori in liver diseases
Archives of Microbiology (2023)