Abstract
Ferroptosis, a newly discovered iron-dependent cell death pathway, is characterized by lipid peroxidation and GSH depletion mediated by iron metabolism and is morphologically, biologically and genetically different from other programmed cell deaths. Besides, ferroptosis is usually found accompanied by inflammatory reactions. So far, it has been found participating in the development of many kinds of diseases. Macrophages are a group of immune cells that widely exist in our body for host defense and play an important role in tissue homeostasis by mediating inflammation and regulating iron, lipid and amino acid metabolisms through their unique functions like phagocytosis and efferocytosis, cytokines secretion and ROS production under different polarization. According to these common points in ferroptosis characteristics and macrophages functions, it’s obvious that there must be relationship between macrophages and ferroptosis. Therefore, our review aims at revealing the interaction between macrophages and ferroptosis concerning three metabolisms and integrating the application of certain relationship in curing diseases, mostly cancer. Finally, we also provide inspirations for further studies in therapy for some diseases by targeting certain resident macrophages in distinct tissues to regulate ferroptosis.
Facts
-
Ferroptosis is considered as a newly discovered form characterized by its nonapoptotic and iron-dependent lipid hydroperoxide, concerning iron, lipid and amino acid metabolisms.
-
Ferroptosis has been widely found playing a crucial part in various diseases, including hepatic diseases, neurological diseases, cancer, etc.
-
Macrophages are phagocytic immune cells, widely existing and owning various functions such as phagocytosis and efferocytosis, cytokines secretion and ROS production.
-
Macrophages are proved to participate in mediating metabolisms and initiating immune reactions to maintain balance in our body.
-
Recent studies try to treat cancer by altering macrophages’ polarization which damages tumor microenvironment and induces ferroptosis of cancer cells.
Open questions
-
How do macrophages regulate ferroptosis of other tissue cells specifically?
-
Can we use the interaction between macrophages and ferroptosis in treating diseases other than cancer?
-
What can we do to treat diseases related to ferroptosis by targeting macrophages?
-
Is the use of the relationship between macrophages and ferroptosis more effective than other therapies when treating diseases?
Similar content being viewed by others
Introduction
Ferroptosis is a newly discovered cell programmed death that is found in various diseases, such as renal diseases, hepatic diseases, neurological diseases, etc. [1,2,3,4]. The manifestation of ferroptosis is different from other cell death like apoptosis or necrosis for its iron-dependent lipid peroxidization [5]. Fenton reaction between Fe2+ and Fe3+ contributes a lot to the production of ROS. The transcription and expression of iron metabolic genes such as transferrin (TRF), ferroportin (FPN), FTH and FTL are closely related with occurrence of ferroptosis [6,7,8,9,10,11,12]. In addition to iron metabolism, lipid and amino acid metabolism play a part in ferroptosis as well. In order to deal with the damage caused by ROS, GPX4 and GSH are responsible for regulating ROS level via Nrf2 pathway [13,14,15,16]. As GPX4 or GSH deplete, ROS accumulation in cells can directly lead to lipid peroxidation. Besides, overactivation of ACSL4 and LOXs can also convert PUFAs into lipid peroxide. All processes above make up the causes of ferroptosis [17,18,19,20,21,22,23,24,25,26]. After study the characteristics and consequences of ferroptosis, iron overload and some of the substances released from ferroptotic cells are proved to affect the polarization and recruitment of macrophages [27,28,29,30,31,32,33,34,35,36].
Macrophages, a significant group of immune cells in the body, were firstly discovered for their clearance of foreign substances. They usually exist in two distinct phenotypes, the classically activated (M1 type) macrophages or the alternatively activated (M2 type) macrophages. After further studied, macrophages are classified into different categories according their origination or functions under various circumstances [37,38,39,40]. The inflammatory factors like IL-6, IL-1β and TNF-α that initiate inflammation response are mostly secreted by M1 type macrophages, and they influence the activity of enzymes in iron, lipid and amino acid metabolisms [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. What’s more, hemophagocytosis of macrophages shows their tight relationship with the iron metabolism and production for other tissue cells [27,28,29,30,31, 58]. Besides, ROS produced by macrophages have effects on the state of both tissue cells and macrophages themselves by activating various pathways concerning iron, lipid and amino acid metabolisms [32,33,34,35,36, 59,60,61,62].
Due to the similarity in macrophages’ functions and the manifestations of ferroptotic cells, there must have been interactions between the occurrence of ferroptosis and the state of macrophages. So far, the relationship between ferroptosis and macrophages have been applied to studying the therapy for kinds of diseases, especially cancer [63,64,65,66,67,68,69,70]. Therefore, it’s meaningful to integrate the key points of the two sides in three metabolisms and provide inspirations for further studies.
The overview of ferroptosis
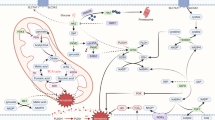
Ferroptosis is considered as a newly discovered form of Regulated Cell Death in 2012 by Dixon that is characterized by its nonapoptotic and iron-dependent lipid hydroperoxide [5]. Researchers have proved that various diseases are related to the ferroptosis, such as cardiovascular diseases, neurological diseases, hepatic toxicity, kidney injury, etc. [1,2,3,4]. Ferroptosis is mainly driven by peroxidation of membrane phospholipids (PLs) in enzymatic and nonenzymatic ways. The overload of iron and the inactivation of GPX4 in ferroptotic cells implies the relationship with the metabolism of iron, lipid and amino acid (Fig. 1).
The process of ferroptosis is associated with three main metabolisms (iron, lipid and amino acid metabolisms). Cells intake iron into endosome in the form of Fe3+ through binding between transferrin and TFR1. Fe3+ can be reduced to Fe2+ by STEAP3, which is then transported into cytosol by DMT1. Most of Fe2+ can be released by FPN, used for enzyme synthesis or stored in ferritin. The rest of Fe2+ may change into Fe3+ and ROS by Fenton Reaction. ROS accumulation will cause ferroptosis for it make PL-PUFA(PE)-OH oxidized into PL-PUFA(PE)-OOH. System Xc-, composed of SLC7A11 and SLC3A2, acts as a transporter responsible for releasing glutamate and intaking cysteine, which can be further transformed in to cysteine that is an important component of GSH. GSH can deoxidize PL-PUFA(PE)-OOH back into PL-PUFA(PE)-OH with the help of GPX4, which protects cells from ferroptosis. PUFAs, especially AA and AdA, are changed into PUFA-CoAs which is modified by LPCAT3 in order to be integrated into cell membrane. Catalyzed by LOXs, PL-PUFA(PE)-OH changes into PL-PUFA(PE)-OOH, leading to ferroptosis.
Related metabolism pathway
Iron metabolism
Iron is transported from extracellular environment into intracellular space through transferrin receptor 1 located on the cell membrane in the form of ferric ion (Fe3+) [6], which is reduced to ferrous ion (Fe2+) by ferric reductases such as STEAP3 in endosome [7] and then released into cytosol by divalent metal transporter 1 [8], participating in the synthesis of iron-dependent enzymes. The rest part of Fe2+ is generally stored in the labile iron pool or ferritin composed of ferritin heavy chain 1 and ferritin light chain (FTL) [9]. Ferroportin1 (FPN) is the identified iron exporter in cells [10]. The loss of FPN is an important contributor to iron overload and the development of ferroptosis [11]. Toxicity of iron ions comes from the Fenton Reaction between Fe2+ and Fe3+, producing reactive oxygen species (ROS) [12] which can impair lipids, proteins and DNA and cause ferroptosis.
Lipid metabolism
Lipid oxidization and reduction generally maintain a dynamic balance in normal cells, while excessive lipid oxide is found in ferroptotic cells, due to external factors or the disorder in related gene expression. Evidence has proved that ferroptosis is triggered by peroxidation of PLs [17]. Ferroptosis shows obvious lipid peroxidation stress and damage in cell membrane. Polyunsaturated fatty acids (PUFAs) are believed to contribute to the form of lipid peroxide and the occurrence of ferroptosis [18]. Among all kinds of PUFAs. Moreover, studies suggested that arachidonic acid and adrenic acid (AdA) are the significant contributors to the occurrence of ferroptosis. Esterified by Acyl-CoA synthetase long-chain family member-4, PUFAs transform into PUFA-CoA which can be modified by lysophosphatidylcholine acyltransferase-3 in order to be integrated into the cell membrane [19, 20, 25]. The PL-PUFA(PE)-OH are further oxidized into lipid hydroperoxides (PL-PUFA(PE)-OOH) either catalyzed by lipoxygenases (LOXs) [21] or ROS produced by Fenton reaction. Besides, 4-Hydroxynonenal (4-HNE), a major product of lipid peroxidation, is reported to induce the generation of ROS, which will aggravate the lipid peroxidation and ferroptosis, so 4-HNE can be a marker of ferroptosis as well [22,23,24, 26].
Amino acid metabolism
One of the manifestations of ferroptosis is the inactivation of glutathione peroxidase 4 (GPX4) and the depletion of GSH, which is the most important antioxidized pathway to repair the damage in cell membrane caused by lipid hydroperoxides and clean the ROS. GPX4 and System Xc- are two crucial regulators of ferroptosis. System Xc- (cystine/glutamate antiporter) acts as an important antioxidant protein encoded by SLC7A11 and SLC3A2, providing cystine that is the raw material for the synthesis of GSH. Experiments have proved that suppressing expression of System Xc- can make cells vulnerable to the induction of ferroptosis [13, 14]. GSH, a glutathione peroxidase, converts reduced glutathione (GSH) into oxidized glutathione (GSSG) to detoxify the hydroperoxide group (−OOH) of fatty acids [15]. Inhibiting GPX4 directly by Honokiol is able to induce ferroptosis without affecting the function of System Xc- [16].
Background of macrophage
Macrophages are phagocytic immune cells, existing in various tissue and playing an important part in immune system. The evaluation of macrophages’ role is quite sophisticated. Though macrophages ingest harmful particles or microorganisms to maintain host defense, cytokines or ROS they produce, generally used to attack foreign substances, may also damage normal cells and cause chronic diseases.
Origination of macrophage
People have believed that macrophages are all come from monocytes derived from bone marrow until a special group of macrophages is found in certain tissue. According to studies conducted in different ways, these macrophages are proved to have their own origination and characteristics. And therefore, macrophages are mainly divided into two types: 1. resident macrophages that are located in tissues/organs at a relatively stable rate for tissue defense and homeostasis. Evidence embodies that tissue-resident macrophages originate from Tie2+ (also known as Tek) cellular pathway that produces Csf1r+ erythro-myeloid progenitors. 2. recruited or bone marrow derived macrophages which come from adult hematopoietic stem cells (HSCs) [37]. Though resident macrophages can self-renew independent of HSCs, they are similar to recruited macrophages in biological functions and sometimes can still be replaced by recruited macrophages, especially when the tissue is damaged [38].
Functions of macrophage
Phagocytosis and efferocytosis of macrophages
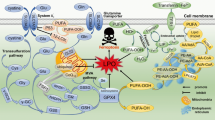
As a critical part in immune system, macrophages help remove the debris and apoptotic cells from the body to maintain homeostasis. Experiments suggested that endocrine-resident macrophages were more efficient in efferocytosis and phagocytosis, both in vitro and in vivo, than exocrine-resident macrophages, which may be one of the factors to induce endocrine macrophages’ polarization [71]. Efferocytosis means the remove of cells experiencing programmed cell death before further necrosis or the release of inflammation-inducing ingredients. Abnormal efferocytosis has something to do with various diseases, containing cardiovascular diseases, autoimmune diseases, brain diseases, etc. [72,73,74]. Interaction between macrophage and apoptotic cell is required for phagocytosis and efferocytosis of macrophages (Fig. 2). Macrophages identify apoptotic cells through the phosphatidylserine (PtdSer) on the surface of dying cells, which is known as the “eat-me” signal [75]. As PtdSer flips over to cell surface, PtdSer receptor cell immunoglobulin mucin receptor 4 (TIM4) and TAM family receptor tyrosine kinase receptors (mainly MerTK and AXL) expressed by macrophages will recognize the signal [76]. TIM4 can bind to PtdSer on apoptotic cells tightly but it fails to engulf them. It needs to cooperate with MerTK which requires growth arrest-specific 6 (GAS6) or protein S 1 (PROS1) to assist binding with PtdSer and initiates rapid engulfment activity [77, 78]. Experiments imply that substances like Angiotensin II impairs macrophages efferocytosis through AT1R/ROS/p38/MAPK/ADAM17 pathway [79]. Besides the role of MerTK in efferocytosis, the level of AXL expression can also greatly influence macrophages’ functions. The transcription factor MafB is proved to enhance the efferocytosis in RAW264.7 macrophages by upregulating the AXL expression, though its detailed mechanism is still unknown [80].
As PtdSer is flipped onto the surface of dying cells, TIM4 on macrophages can identify and bind to PtdSer tightly. However, the binding cannot initiate engulfment. To engulf dying cells, TAM family, mainly MerTK and AXL, is required to bind with GAS6 or PROS1 in order to initiate rapid engulfment activity.
Cytokines secretion and polarization of macrophages
Macrophages are characterized by their diversity and plasticity for their rapid response to the stimuli in certain environment and convert into a specific functional phenotype, which is a process called polarization. Due to the different gene expression in different functional profiles, the secretion of cytokines is not the same in diverse states of macrophages (Fig. 3). Therefore, according to the difference in cytokine secretion and metabolic adaptions, macrophages are divided into 2 types, M1 type (classically activated or pro-inflammatory macrophages) and M2 type (alternatively activated or anti-inflammatory macrophages).
M1: M1 macrophages are recognized by their functions of killing bacteria, cleaning cancer cells and presenting antigen information to Th1 lymphocytes. M1 cells can be classically activated by IFN-γ, TNFα or LPS, and release the pro-inflammatory factors like TNF-α, IL-1α, IL-1β, IL-6, IL-12 and IL-23.
M2: M2 type macrophages can be further classified into four groups mainly according to their different stimuli, cell expression markers, secreted mediators and functions.
M2a: M2a macrophages generally aim at anti-inflammatory activity, responsible for promoting wound healing (tissue repairing) and resolution of inflammation. Induced by cytokines IL-4 or IL-13, M2a macrophages appear and mainly secret anti-inflammatory factors IL-10, TGF-β.
M2b: M2b macrophages are formed through the stimulation by immune complexes, TLR ligands or IL-1β. By producing cytokines like TNF-α, IL-1β, IL-6 and IL-10, these macrophages are mainly responsible for Th2 activation and regulation of immune reactions.
M2c: Macrophages stimulated by Glucocorticoids, IL-10 or TGF-β can be differentiated into M2c type. They mainly produce cytokines such as IL-10, TGF-β which are anti-inflammatory cytokines. Besides, these cells own the function of phagocytosis of apoptotic cells.
M2d (TAM): M2d type macrophages are quite different from other types for they exist in tumor microenvironment. These macrophages are also called tumor-associated macrophages (TAM) and they can help enhance angiogenesis and tumor growth. The stimuli of TAM are mainly TLR antagonists and they can produce cytokines like IL-10 and vascular endothelial growth factor, thus facilitating the development and metastasis of tumor [39, 40].
Production of reactive oxygen species (ROS)
Virus can activate Toll like receptor 7 (TLR7) on macrophages and increase the ROS level via the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), composed of various cytosolic and membrane subunits [81]. NOX is a major enzymatic source for the production of cellular ROS under various pathologic conditions that involve synthesis of superoxide. Among all, NOX2 mediated by NF-kB pathway contributes most to the NADPH-dependent generation of ROS [82]. Besides, NOX1 and NOX4 are also found being related to the production of ROS [83]. Generally, ROS can be produced by M1 macrophages via NOX2 pathway to help kill the pathogens in the immunologic process. Advanced glycation end products (AGEs), produced by some nonenzymatic glycation reactions, can also induce the generation of ROS in macrophages and their polarization toward M1 type via RAGE/ROS/TLR4/STAT1 pathway [84]. Moreover, miR-148a-3p promoted M1 polarization of macrophages upon Notch activation and its overexpression reinforce ability to engulf and kill bacteria through excessive production of ROS. ROS is highly produced by M1 macrophages and associated with M1 polarization (Fig. 4). Further studies show that the generation of ROS is mainly due to PTEN/AKT pathway with the activation of NF-κB signaling [85]. In addition to H2O2 generated from NOX, another source of ROS in macrophages is identified as γ-glutamyltransferase (GGT), which is found in exposure to crocidolite and neuroinflammation. The oxidative injury and clinical signs in neuroinflammation can be suppressed by targeting GGT activity [86, 87]. Compared with M1 macrophages, M2 macrophages have lower levels of ROS and hydrogen peroxide, which implies M1 types’ pro-inflammatory and M2 types’ anti-inflammatory functions [88] (Fig. 4). Proper ROS assists immune protection, but excessive production of ROS will impair cell membrane and DNA, leading to cell death. Overproduction of ROS under inflammatory condition can polarize macrophages toward M1 type and decreasing ROS via NAC can partially reverses the M1/M2 polarization.
After polarization of macrophages into M1 type, ROS is highly produced through two different ways, NOX and GGT, thus leading to pro-inflammation property. Besides, high level of ROS can also promote macrophages’ polarization toward M1. In contrary, M2 macrophages have low ROS and anti-inflammation property.
Interaction between ferroptosis and macrophages
As is integrated above, the properties of ferroptosis and functions of macrophages have a lot in common, indicating the relationship between ferroptosis and macrophages. Firstly, iron accumulation in ferroptosis and rich iron storage in macrophages, especially in M1 type, may have something to do with each other. Secondly, in ferroptotic cells, various kinds of inflammatory cytokines are tested in experiments and the levels of them are witnessed obvious escalation. As is known to all, one of the most important characteristics of M1 macrophages is to release inflammatory factors and initiate inflammation, suggesting their interaction. Last but not least, the main cause of ferroptosis is the ROS from Fenton Reaction or depletion of GPX4 and the catalysis of lipoxidase. Studies have proved that macrophages can produce ROS to kill foreign substances like bacteria and microorganisms and the cytokines from macrophages can regulate the activity of LOX in cells, which means macrophages can participate in iron and lipid metabolism to induce ferroptosis. All these similarities have implied the link between ferroptosis and macrophages, which greatly arouse researchers’ interests.
The effect of ferroptosis on macrophages
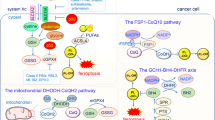
Iron metabolism affects macrophages’ polarization
Iron is a significant element that participate in activities like cell proliferation, metabolism and differentiation. Most of the iron needed for physiological processes is from the recycling iron in RBCs by macrophages through complex pathways. Iron can determine macrophages’ fate and function, especially cell development and differentiation. Macrophages generally store iron by binding it to ferritin (Ft). According to different stages of macrophages polarization, the expression of Fe-related genes will shift. Compared with M2 macrophages, M1 type expresses higher Hamp and FTH/FTL, but lower FPN and IRP1/2, indicating more iron storage [89]. Iron overload induces M1 polarization (Fig. 5). Studies prove that iron overload can increase the level of M1 markers such as IL-6, TNF-α and IL-1β and decrease M2 makers like TGM2, in another word, promoting polarization to M1 macrophages [90]. In addition to inducing inflammatory factors, iron overload is found to aggravate the development of atherosclerosis by enhancing glycolysis in order to prompt M1 phenotype [91]. Besides, ROS production and p53 acetylation caused by iron overload also contribute to M1 polarization [92]. However, iron overload doesn’t always cause M1 polarization. Research in 2020 reveals that under the circumstances of chronic iron overload, THP-1 monocyte-derived macrophages tend to exhibit signs of M2 type and downregulate the markers of M1 macrophages [93].
Iron accumulation in macrophages promotes M1 polarization in three main ways. M1 markers such as IL-6, TNF-α and IL-1β increase and M2 markers like TGM2 decrease. Iron overload can enhance glycolysis and cause M1 type. Besides, the rise of ROS level and p53 acetylation can also lead to M1 macrophages.
Ferroptotic cells initiate macrophages’ recruitment
Ferroptosis is identified in many kinds of diseases, and the clearance of the ferroptotic cells is executed by macrophages. As is mentioned above, macrophages’ phagocytosis plays an important part in immune system. Ferroptotic cells activate macrophages’ functions and recruitment (Fig. 6). Damage-associated molecular pattern molecules (DAMPs) are endogenous danger signals that can recruit and activate macrophages, alerting the immune defense. In the occurrence of ferroptosis, studies suggest that ferroptotic cells can release a DAMP called HMGB1, which depends on autophagy manner. Advanced glycosylation end-product specific receptor is required for HMGB1 to mediate inflammation in macrophages [94]. In terms of dealing with ferroptosis, TLR2 on macrophages first interact with oxidized PL, 1-steaoryl-2-15-HpETE-sn-glycero-3-phosphatidylethanolamine (SAPE-OOH) on the surface of ferroptotic cells, which help improve the efficiency for macrophages to engulf ferroptotic cells. What’s more, anti-HMGB1 neutralizing antibodies or the depletion of AGRE relieves the inflammatory response in macrophages, implying that limit the expression of HMGB1 can be a method to deal with the inflammation in macrophages [95]. Besides HMGB1, ferroptotic cells triggered inflammation and recruitment of macrophages through the activation of molecule inflammatory pathways. The expression of various inflammation-related genes is induced in ferroptosis, especially CCL2 and CCL7 which assist recruitment and chemotaxis of macrophages [96,97,98].
In dealing with ferroptotic cells, TLR2 on macrophages identifies and binds to SAPE-OOH on the surface of ferroptotic cells to enhance engulfment. HMGB1 released by dying cells can interact with AGRE on macrophages in order to mediate inflammation reactions in macrophages. Some molecules like CCL2 or CCL7 can initiate the recruitment and chemotaxis of macrophages for intensity of immune reactions.
The role of macrophages in ferroptosis
Phagocytosis and iron accumulation
Under normal circumstances, macrophages play an important part in the recycling of iron through the engulfment of red blood cells (RBCs). As the number of RBCs suddenly rises or the RBCs are seriously damaged, increased erythrophagocytosis will happen [58] (Fig. 7). For one thing, a large number of RBCs will be eaten and digested with the help of Nramp1 in macrophages [27]. Iron overload in macrophages from RBCs is haem-iron. Haem in macrophages is first catabolized by haem oxygenases, generating iron, carbon monoxide and biliverdin [28, 29]. When it comes to resident macrophages, such as Kupffer Cells (KCs) in liver, they degrade hemoglobin into hemes and increase the iron in cells [30]. The iron accumulates in the macrophages, accompanied by the release of ROS and the generation of lipid peroxidation via Fenton Reaction, leading to ferroptosis of macrophages. For another, the iron accumulation in macrophages, especially resident macrophages, can affect other cells in certain tissue. Experiments prove that ferroportin1 in hepatocytes and macrophages are responsible for the iron mobilization between cells and bloodstream in order to store iron for the whole body [31]. Body iron content universally remain at stable levels via the hepcidin/ferroportin regulatory system. If the balance is broken by the accumulation of iron in macrophages, the unregulated iron export will cause tissue and even systematic iron overload, which creates opportunities for the occurrence of ferroptosis in tissue cells.
Macrophages engulf RBCs and digest them into hemoglobin, which further break down into heme. Heme can be decomposed into biliverdin, carbon monoxide and iron. Iron generated from heme can either promote ROS production and lipid peroxidation or released into the environment through FPN, thus raising the iron level in both macrophages and the tissue environment.
Cytokines and ferroptosis regulation
Macrophages universally execute their functions and regulate biochemical reactions through the secretion of certain cytokines. So far, some cytokines mostly produced by macrophages are reported to help induce or inhibit the process of ferroptosis via different ways.
Typically, IL-6, a marker of M1 macrophages, is found promoting lipid peroxidation and disrupting iron homeostasis in bronchial epithelial cells, thus leading to ferroptosis [41, 42]. IL-6 affect iron levels in cells by controlling the transcription of hepcidin which is a short cysteine-rich peptide hormone that can influence intestinal absorption and the release of iron in macrophages Fe stores. The transcription of hepcidin is mediated by IL-6 via the activation of JAK-STAT3 pathway [43, 44], and the expression of hepcidin is found to be activated by BMP/SMAD pathway [45]. Hepcidin first binds to ferroportin (FPN) and cause internalization of hepcidin-FPN that is soon degraded into lysosomes [46]. As a result, promoting the production of hepcidin will obviously reduce the expression of FPN, thus aggravating iron deposition. Furthermore, IL-6 can even induce the polarization of macrophages toward M1 or M2 under different circumstances [40], which is related to the occurrence of ferroptosis as well.
TNF-α is another symbolic inflammatory factor released from M1 macrophages. Its functions in lipid metabolic and inflammation are worth studying in the process of ferroptosis. First, TNF-α is able to upregulate ACSL3, a key enzyme in the synthesis of acyl-CoA, which means TNF-α promotes the lipid accumulation in cells, creating the conditions for the occurrence of inflammation response and ferroptosis [57].
As for IL-1β, studies show that it can increase the expression of FPN in glial cells through the activation of p38-MAPK pathway, which may cause the excessive iron efflux and deposition in nerve cells and the environment [47]. Besides, IL-1β secreted by macrophages is proved to upregulate the transcription of hepcidin via enhancing the expression of CCAAT enhancer-binding protein (C/EBP) [48, 49] and the expression of hepcidin with the help of phosphorylated c-Jun N-terminal kinase and its substrates c-Jun and JunB [50], thus leading to FPN degradation and iron overload. In some aspects, IL-1β and IL-6 can cause the same effect in the activation of hepcidin but through different signaling ways [51]. In fact, IL-1β is also reported to be relative to the lipid homeostasis in certain tissue, especially kidney. IL-1β urge glomerular mesangial cells (HMCs) to express lectin-like Ox-LDL receptor 1 (LOX-1) and uptake more oxidized low-density lipoprotein (Ox-LDL) [52, 53]. Therefore, the lipid accumulation caused by excessive IL-1β may easily arouse the inflammation reaction in cells and tissue.
iNOS is another typical inflammatory factor in our body. Though it is considered to promote inflammation, experiments found that it had protective functions in ferroptosis. The activation of NOX and iNOS can cause the production of ROS and RNS, resulting in the depletion of GSH and GPX and aggravating lipid peroxidation [54]. Inhibition of iNOS can reverse the ferroptosis caused by overexpression of iNOS [55]. However, recently some studies found that there is something different with the inflammatory cytokine iNOS. A study found that inhibition of iNOS by using Nω-nitro-L-arginine will make the matter worse in beta-cell death. It led to a massive lipid peroxidation in exposure to pro-inflammatory cytokines, indicating that nitric oxide help prevent the induction of ferroptosis [56].
ROS release and ferroptosis
ROS generated from macrophages first affect their recruitment and polarization. In fact, the role of ROS in polarization is quite complex. Under different conditions, ROS may have reversed effect on macrophages phenotype. In most cases, such as Periodontitis, diabetes, etc, ROS produced in macrophages will induce macrophages polarize toward M1 type, thus increasing the release of a series of inflammatory factors and aggravating inflammation, which provide opportunities and environment for ferroptosis [32, 33]. However, in non-small cell lung cancer, ROS from NADPH oxidase 4 is responsible for both the inflammation and M2 polarization and recruitment of tumor-associated macrophages (TAMs) via ROS/PI3K [34]. Therefore, repolarization of TAMs toward M1 to induce ferroptosis becomes the target of therapy. In addition to NADPH oxidase, mitochondrial ROS (mtROS) can be generated from the activation of TLR pathway that interacts with tumor necrosis factor receptor-associated factor 6 (TRAF6) engaging the ubiquitination of evolutionarily conserved signaling intermediate in Toll pathways. mtROS is also reported to help execute macrophages’ biological functions [35].
ROS induces ferroptosis mainly through lipid hydroperoxides or the depletion of antioxidants such as GSH or GPX4 in amino acid metabolism, which has been mentioned above in detail. For macrophages are the center of iron and lipid metabolism, related proteins in macrophages are quite active and they are linked with the generation and depletion of ROS to maintain the balance.
When it comes to iron metabolism, increased level of mtROS, usually accompanied by the decline of the expression of iron-sulfur cluster assembly scaffold protein ISCU, the antioxidant enzymes SOD1 and SOD2 and other related mitochondrial respiration components, thus affecting iron metabolism and oxidation reactions [36]. In brain, NO• acts on cytosolic aconitase (c-aconitase) and changes it into iron regulatory protein 1 (IRP1), which damages iron homeostasis through IRP1’s binding with to iron response elements in mRNAs of iron-related proteins [59].
However, ROS in cells is recently found to have reversed effects on lipid metabolism in the occurrence of ferroptosis. After discovering that NO•-affecting 15-hydroperoxy-eicosa-tetra-enoyl-phosphatidylethanolamine produced by 15-lipoxygenase (15-LOX) modulate the ferroptosis endurance in M1 macrophages [60], researchers further found that ROS like O2 and NO• uses the same entry pores and channels connecting to 15-LOX-2 catalytic site for competition [61]. Besides, since NO• is small enough to go through the membrane, NO• produced by macrophages can even suppress PA stimulated ferroptosis in distant epithelial cells [62].
Cancer treatment by targeting macrophages
Experiments have proved that M2-like tumor- associated macrophages infiltration is the promotor of the development of cancer. Cancer cells in liver are able to interact with M2 macrophages via Wnt/β-catenin signaling and trigger M2 polarization in macrophages, reinforcing tumor activities [63, 64]. In terms of the key role of macrophages in ferroptosis, researchers start to find new ways to treat cancer by targeting macrophages and inducing ferroptosis. Newly published articles concerning tumor therapies on the basis of macrophages are mainly aims at the repolarization, changing M2 type TAM to M1 type. Engineered magnetosomes delivered to the tumor tissue are reported to induce macrophages’ polarization from M2 toward M1 and release more Fe ion, thus producing excessive hydrogen peroxide and impelling ferroptosis [65]. Other reports indicate that zero-valent-iron nanoparticle (ZVI-NP) can activate repolarization and downregulate the population of regulatory T cells to initiate the anti-tumor immune activity [66]. As a ferroptosis-promoting agent, Herein switches mitochondrial oxidative phosphorylation to glycolysis in TAM cells and induces ferroptosis stress, resisting anti-inflammatory reactions and promoting pro-inflammatory signaling pathways [67]. In addition to inhibit primary tumor, this agent can provoke immune response against cancer through binding with an immune checkpoint blockade. Stimulated phagocytes and enhanced tumor antigens uptake assist immunotherapy with few abnormalities [68]. In lung adenocarcinoma patients, RRM2 inhibition promotes M1 polarization and suppresses M2 polarization, which can be reversed by ferroptosis inhibitors [69].
However, some researchers prove that treating cancer by inducing ferroptosis may have shortcomings. They discover that in pancreatic ductal adenocarcinoma, ferroptosis can result in experimental pancreatitis and assist Kras-driven pancreatic tumorigenesis, which should be taken into account before applying ferroptosis for treatment [70].
Prospect
So far, ferroptosis has been found to have an impact on the development of a variety of diseases, thus targeting the regulation of ferroptosis process may be a new therapy. However, most of the experiments mainly aim at inducing or inhibiting ferroptosis in tissue cells directly. Few articles use the relationship between macrophages and ferroptosis to deal with diseases. Therefore, this review is expected to provide inspirations for future studies.
In terms of liver damage, targeting ferroptosis is found to have an effect on relieving or aggravating the symptoms, indicating the role of iron, lipid and amino acid metabolisms. Non-alcoholic fatty liver disease (NAFLD), like non-alcoholic steatohepatitis (NASH), can be promoted by iron accumulation, lipid peroxidation, GPX4 depletion and inflammatory initiation, which are main processes of ferroptosis [99,100,101]. After given ferroptosis inhibitors, such as liproxstatin-1 or ferrostatin-1, NASH is greatly alleviated [102, 103]. KCs, the residents macrophages in liver, are found to have enhanced phagocytic dysfunction and closely related to iron homeostasis, thus influencing ferroptosis in the development of NAFLD [104,105,106,107]. Besides, KCs participate in the intake and clearance of lipid through M1 type polarization with the help of invariant natural killer T cells in NASH, indicating the role of KCs in lipid metabolism [108,109,110]. As the studies above imply, the regulation of KCs can influence the development of ferroptosis especially in iron and lipid metabolisms, providing a new possible therapy for NAFLD.
Abundant evidence has proved the link between ferroptosis and neurological diseases, like Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and other brain injury [111,112,113,114,115], accompanied by the occurrence of neuroinflammation. A number of macrophages in nerve tissue, which are called Hortega cells or microglia cells, are involved in the regulation of neuroinflammation. Since typical manifestations of ferroptosis and inflammation, such as iron accumulation and GPX4 depletion, are found in these diseases, researchers pay attention to ferroptosis and microglia cells in therapy for injury. After RSL3 treatment, a ferroptosis inducer, the inflammatory reactions of microglia are greatly triggered, and different types of microglia exhibit various sensitivity to ferroptosis, which is consistent with results in other researches. However, RSL3 can attenuate lipopolysaccharides-induced inflammation by targeting Nrf2 expression [116]. Some of ferroptosis inhibitors, including liproxstatin-1 and an arylthiazine backbone (ADA-409-052), are reported to protect against neurological diseases and neuroinflammation through mediating the state of microglia cells [117, 118]. Thus, targeting microglia to regulate ferroptosis in neurological disorders or brain injury is worth further studying.
In other tissues, relationship between resident macrophages and diseases or ferroptosis and diseases are respectively proved. However, the relationship between these resident macrophages and ferroptosis is still unclear. For example, ferroptosis and macrophages are respectively found participating in heart diseases, like ischemia/reperfusion, heart failure, drug induced cardiotoxicity and so on, but few research focus on the interaction between ferroptosis and heart macrophages [119,120,121,122,123,124]. If the direct relation between ferroptosis and resident macrophages can be veiled in future experiments, it will probably contribute to new therapies.
References
Yang K, Song H, Yin D. PDSS2 inhibits the ferroptosis of vascular endothelial cells in atherosclerosis by activating Nrf2. J Cardiovasc Pharm. 2021;77:767–76.
Shibu MA, Bharath M, Velmurugan BK. Regulating inflammation associated ferroptosis—a treatment strategy for Parkinson disease. Curr Med Chem. 2021;28:6895–914.
Zhang Z, Guo M, Shen M, Kong D, Zhang F, Shao J, et al. The BRD7-P53-SLC25A28 axis regulates ferroptosis in hepatic stellate cells. Redox Biol. 2020;36:101619.
Ma D, Li C, Jiang P, Jiang Y, Wang J, Zhang D. Inhibition of ferroptosis attenuates acute kidney injury in rats with severe acute pancreatitis. Dig Dis Sci. 2021;66:483–92.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Sakajiri T, Yamamura T, Kikuchi T, Yajima H. Computational structure models of apo and diferric transferrin-transferrin receptor complexes. Protein J. 2009;28:407–14.
Sendamarai AK, Ohgami RS, Fleming MD, Lawrence CM. Structure of the membrane proximal oxidoreductase domain of human Steap3, the dominant ferrireductase of the erythroid transferrin cycle. Proc Natl Acad Sci USA. 2008;105:7410–5.
Cegarra L, Colins A, Gerdtzen ZP, Nunez MT, Salgado JC. Mathematical modeling of the relocation of the divalent metal transporter DMT1 in the intestinal iron absorption process. PLoS ONE. 2019;14:e218123.
Plays M, Muller S, Rodriguez R. Chemistry and biology of ferritin. Metallomics. 2021;13:mfab021.
Bao WD, Zhou XT, Zhou LT, Wang F, Yin X, Lu Y, et al. Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model. Aging Cell. 2020;19:e13235.
Bao WD, Pang P, Zhou XT, Hu F, Xiong W, Chen K, et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021;28:1548–62.
He YJ, Liu XY, Xing L, Wan X, Chang X, Jiang HL. Fenton reaction-independent ferroptosis therapy via glutathione and iron redox couple sequentially triggered lipid peroxide generator. Biomaterials. 2020;241:119911.
Wang L, Liu Y, Du T, Yang H, Lei L, Guo M, et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc(.). Cell Death Differ. 2020;27:662–75.
Yang J, Zhou Y, Xie S, Wang J, Li Z, Chen L, et al. Metformin induces ferroptosis by inhibiting UFMylation of SLC7A11 in breast cancer. J Exp Clin Cancer Res. 2021;40:206.
Cozza G, Rossetto M, Bosello-Travain V, Maiorino M, Roveri A, Toppo S, et al. Glutathione peroxidase 4-catalyzed reduction of lipid hydroperoxides in membranes: the polar head of membrane phospholipids binds the enzyme and addresses the fatty acid hydroperoxide group toward the redox center. Free Radic Biol Med. 2017;112:1–11.
Guo C, Liu P, Deng G, Han Y, Chen Y, Cai C, et al. Honokiol induces ferroptosis in colon cancer cells by regulating GPX4 activity. Am J Cancer Res. 2021;11:3039–54.
Koleini N, Nickel BE, Edel AL, Fandrich RR, Ravandi A, Kardami E. Oxidized phospholipids in doxorubicin-induced cardiotoxicity. Chem Biol Interact. 2019;303:35–9.
Dierge E, Debock E, Guilbaud C, Corbet C, Mignolet E, Mignard L, et al. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. 2021;33:1701–15.
Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–43.
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–8.
Shah R, Shchepinov MS, Pratt DA. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent Sci. 2018;4:387–96.
Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–43.
Toyokuni S, Luo XP, Tanaka T, Uchida K, Hiai H, Lehotay DC. Induction of a wide range of C(2-12) aldehydes and C(7-12) acyloins in the kidney of Wistar rats after treatment with a renal carcinogen, ferric nitrilotriacetate. Free Radic Biol Med. 1997;22:1019–27.
Liu H, Gambino FJ, Algenio CS, Wu C, Gao Y, Bouchard CS, et al. Inflammation and oxidative stress induced by lipid peroxidation metabolite 4-hydroxynonenal in human corneal epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2020;258:1717–25.
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90.
Toyokuni S, Uchida K, Okamoto K, Hattori-Nakakuki Y, Hiai H, Stadtman ER. Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc Natl Acad Sci USA. 1994;91:2616–20.
Soe-Lin S, Sheftel AD, Wasyluk B, Ponka P. Nramp1 equips macrophages for efficient iron recycling. Exp Hematol. 2008;36:929–37.
Cazzola M, Della PM, Malcovati L. Clinical relevance of anemia and transfusion iron overload in myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2008;1:166–75.
Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88:7–15.
Lu K, Dong S, Xia T, Mao L. Kupffer cells degrade (14)C-labeled few-layer graphene to (14)CO2 in liver through erythrophagocytosis. ACS Nano. 2021;15:396–409.
Zhang Z, Zhang F, Guo X, An P, Tao Y, Wang F. Ferroportin1 in hepatocytes and macrophages is required for the efficient mobilization of body iron stores in mice. Hepatology. 2012 ;56:961–71.
Yuan Y, Chen Y, Peng T, Li L, Zhu W, Liu F, et al. Mitochondrial ROS-induced lysosomal dysfunction impairs autophagic flux and contributes to M1 macrophage polarization in a diabetic condition. Clin Sci. 2019;133:1759–77.
Zhang B, Yang Y, Yi J, Zhao Z, Ye R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J Periodontal Res. 2021;56:991–1005.
Zhang J, Li H, Wu Q, Chen Y, Deng Y, Yang Z, et al. Tumoral NOX4 recruits M2 tumor-associated macrophages via ROS/PI3K signaling-dependent various cytokine production to promote NSCLC growth. Redox Biol. 2019;22:101116.
West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–80.
Aerbajinai W, Ghosh MC, Liu J, Kumkhaek C, Zhu J, Chin K, et al. Glia maturation factor-gamma regulates murine macrophage iron metabolism and M2 polarization through mitochondrial ROS. Blood Adv. 2019;3:1211–25.
Gomez PE, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–51.
Beattie L, Sawtell A, Mann J, Frame T, Teal B, de Labastida RF, et al. Bone marrow-derived and resident liver macrophages display unique transcriptomic signatures but similar biological functions. J Hepatol. 2016;65:758–68.
Munoz J, Akhavan NS, Mullins AP, Arjmandi BH. Macrophage polarization and osteoporosis: a review. Nutrients. 2020;12:2999.
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40.
Han F, Li S, Yang Y, Bai Z. Interleukin-6 promotes ferroptosis in bronchial epithelial cells by inducing reactive oxygen species-dependent lipid peroxidation and disrupting iron homeostasis. Bioengineered. 2021;12:5279–88.
Wang CY, Babitt JL. Hepcidin regulation in the anemia of inflammation. Curr Opin Hematol. 2016;23:189–97.
Ren F, Yang Y, Wu K, Zhao T, Shi Y, Song M, et al. The effects of dandelion polysaccharides on iron metabolism by regulating hepcidin via JAK/STAT signaling pathway. Oxid Med Cell Longev. 2021;2021:7184760.
Li B, Gong J, Sheng S, Lu M, Guo S, Zhao X, et al. Increased hepcidin in hemorrhagic plaques correlates with iron-stimulated IL-6/STAT3 pathway activation in macrophages. Biochem Biophys Res Commun. 2019;515:394–400.
Varga E, Pap R, Janosa G, Sipos K, Pandur E. IL-6 regulates hepcidin expression via the BMP/SMAD pathway by altering BMP6, TMPRSS6 and TfR2 expressions at normal and inflammatory conditions in BV2 microglia. Neurochem Res. 2021;46:1224–38.
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3.
Persichini T, Maio N, di Patti MC, Rizzo G, Toscano S, Colasanti M, et al. Interleukin-1beta induces ceruloplasmin and ferroportin-1 gene expression via MAP kinases and C/EBPbeta, AP-1, and NF-kappaB activation. Neurosci Lett. 2010;484:133–8.
Shanmugam NK, Chen K, Cherayil BJ. Commensal bacteria-induced interleukin 1beta (IL-1beta) secreted by macrophages up-regulates hepcidin expression in hepatocytes by activating the bone morphogenetic protein signaling pathway. J Biol Chem. 2015;290:30637–47.
Kanamori Y, Murakami M, Sugiyama M, Hashimoto O, Matsui T, Funaba M. Interleukin-1beta (IL-1beta) transcriptionally activates hepcidin by inducing CCAAT enhancer-binding protein delta (C/EBPdelta) expression in hepatocytes. J Biol Chem. 2017;292:10275–87.
Kanamori Y, Murakami M, Matsui T, Funaba M. JNK facilitates IL-1beta-induced hepcidin transcription via JunB activation. Cytokine. 2018;111:295–302.
Kanamori Y, Murakami M, Sugiyama M, Hashimoto O, Matsui T, Funaba M. Hepcidin and IL-1beta. Vitam Horm. 2019;110:143–56.
Liu H, Li Y, Lin N, Dong X, Li W, Deng Y, et al. Interleukin-1beta promotes Ox-LDL uptake by human glomerular mesangial cells via LOX-1. Int J Med Sci. 2020;17:1056–61.
Liu H, Deng Y, Wu L, Li Y, Lin N, Li W, et al. Interleukin-1beta regulates lipid homeostasis in human glomerular mesangial cells. J Nutr Health Aging. 2020;24:246–50.
Wen Y, Chen H, Zhang L, Wu M, Zhang F, Yang D, et al. Glycyrrhetinic acid induces oxidative/nitrative stress and drives ferroptosis through activating NADPH oxidases and iNOS, and depriving glutathione in triple-negative breast cancer cells. Free Radic Biol Med. 2021;173:41–51.
Jiang L, Zheng H, Lyu Q, Hayashi S, Sato K, Sekido Y, et al. Lysosomal nitric oxide determines transition from autophagy to ferroptosis after exposure to plasma-activated Ringer’s lactate. Redox Biol. 2021;43:101989.
Krummel B, Plotz T, Jorns A, Lenzen S, Mehmeti I. The central role of glutathione peroxidase 4 in the regulation of ferroptosis and its implications for pro-inflammatory cytokine-mediated beta-cell death. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166114.
Jung HS, Shimizu-Albergine M, Shen X, Kramer F, Shao D, Vivekanandan-Giri A, et al. TNF-alpha induces acyl-CoA synthetase 3 to promote lipid droplet formation in human endothelial cells. J Lipid Res. 2020;61:33–44.
Youssef LA, Rebbaa A, Pampou S, Weisberg SP, Stockwell BR, Hod EA, et al. Increased erythrophagocytosis induces ferroptosis in red pulp macrophages in a mouse model of transfusion. Blood. 2018;131:2581–93.
Urrutia PJ, Borquez DA, Nunez MT. Inflaming the brain with iron. Antioxidants. 2021;10:61.
Kapralov AA, Yang Q, Dar HH, Tyurina YY, Anthonymuthu TS, Kim R, et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol. 2020;16:278–90.
Mikulska-Ruminska K, Anthonymuthu TS, Levkina A, Shrivastava IH, Kapralov AA, Bayir H, et al. NO() represses the oxygenation of arachidonoyl PE by 15LOX/PEBP1: mechanism and role in ferroptosis. Int J Mol Sci. 2021;22:5253.
Dar HH, Anthonymuthu TS, Ponomareva LA, Souryavong AB, Shurin GV, Kapralov AO, et al. A new thiol-independent mechanism of epithelial host defense against Pseudomonas aeruginosa: iNOS/NO(*) sabotage of theft-ferroptosis. Redox Biol. 2021;45:102045.
Mao J, Wang D, Wang Z, Tian W, Li X, Duan J, et al. Combretastatin A-1 phosphate, a microtubule inhibitor, acts on both hepatocellular carcinoma cells and tumor-associated macrophages by inhibiting the Wnt/beta-catenin pathway. Cancer Lett. 2016;380:134–43.
Sarode P, Zheng X, Giotopoulou GA, Weigert A, Kuenne C, Gunther S, et al. Reprogramming of tumor-associated macrophages by targeting beta-catenin/FOSL2/ARID5A signaling: a potential treatment of lung cancer. Sci Adv. 2020;6:z6105.
Zhang F, Li F, Lu GH, Nie W, Zhang L, Lv Y, et al. Engineering magnetosomes for ferroptosis/immunomodulation synergism in cancer. ACS Nano. 2019;13:5662–73.
Hsieh CH, Hsieh HC, Shih FS, Wang PW, Yang LX, Shieh DB, et al. An innovative NRF2 nano-modulator induces lung cancer ferroptosis and elicits an immunostimulatory tumor microenvironment. Theranostics. 2021;11:7072–91.
Gu Z, Liu T, Liu C, Yang Y, Tang J, Song H, et al. Ferroptosis-strengthened metabolic and inflammatory regulation of tumor-associated macrophages provokes potent tumoricidal activities. Nano Lett. 2021;21:6471–9.
Li Z, Rong L. Cascade reaction-mediated efficient ferroptosis synergizes with immunomodulation for high-performance cancer therapy. Biomater Sci. 2020;8:6272–85.
Tang B, Xu W, Wang Y, Zhu J, Wang H, Tu J, et al. Identification of critical ferroptosis regulators in lung adenocarcinoma that RRM2 facilitates tumor immune infiltration by inhibiting ferroptotic death. Clin Immunol. 2021;232:108872.
Liu J, Dai E, Kang R, Kroemer G, Tang D. The dark side of ferroptosis in pancreatic cancer. Oncoimmunology. 2021;10:1868691.
Parv K, Westerlund N, Merchant K, Komijani M, Lindsay RS. Christoffersson G. phagocytosis and efferocytosis by resident macrophages in the mouse pancreas. Front Endocrinol. 2021;12:606175.
Kasikara C, Schilperoort M, Gerlach B, Xue C, Wang X, Zheng Z, et al. Deficiency of macrophage PHACTR1 impairs efferocytosis and promotes atherosclerotic plaque necrosis. J Clin Invest. 2021;131:e145275.
Lescoat A, Ballerie A, Lelong M, Augagneur Y, Morzadec C, Jouneau S, et al. Crystalline silica impairs efferocytosis abilities of human and mouse macrophages: implication for silica-associated systemic sclerosis. Front Immunol. 2020;11:219.
Cai W, Dai X, Chen J, Zhao J, Xu M, Zhang L, et al. STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight. 2019;4:e131355.
Lemke G. How macrophages deal with death. Nat Rev Immunol. 2019;19:539–49.
Nishi C, Yanagihashi Y, Segawa K, Nagata S. MERTK tyrosine kinase receptor together with TIM4 phosphatidylserine receptor mediates distinct signal transduction pathways for efferocytosis and cell proliferation. J Biol Chem. 2019;294:7221–30.
Nishi C, Toda S, Segawa K, Nagata S. Tim4- and MerTK-mediated engulfment of apoptotic cells by mouse resident peritoneal macrophages. Mol Cell Biol. 2014;34:1512–20.
Moon B, Lee J, Lee SA, Min C, Moon H, Kim D, et al. Mertk interacts with Tim-4 to enhance Tim-4-mediated efferocytosis. Cells. 2020;9:1625.
Zhang Y, Wang Y, Zhou D, Zhang LS, Deng FX, Shu S, et al. Angiotensin II deteriorates advanced atherosclerosis by promoting MerTK cleavage and impairing efferocytosis through the AT1R/ROS/p38 MAPK/ADAM17 pathway. Am J Physiol Cell Physiol. 2019;317:C776–87.
Sato M, Shibata Y, Inoue S, Igarashi A, Tokairin Y, Yamauchi K, et al. MafB enhances efferocytosis in RAW264.7 macrophages by regulating Axl expression. Immunobiology. 2018;223:94–100.
To EE, Broughton BR, Hendricks KS, Vlahos R, Selemidis S. Influenza A virus and TLR7 activation potentiate NOX2 oxidase-dependent ROS production in macrophages. Free Radic Res. 2014;48:940–7.
Kim MJ, Nepal S, Lee ES, Jeong TC, Kim SH, Park PH. Ethanol increases matrix metalloproteinase-12 expression via NADPH oxidase-dependent ROS production in macrophages. Toxicol Appl Pharm. 2013;273:77–89.
Liu L, Wu X, Xu H, Yu L, Zhang X, Li L, et al. Myocardin-related transcription factor A (MRTF-A) contributes to acute kidney injury by regulating macrophage ROS production. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3109–21.
Liu Z, Ma Y, Cui Q, Xu J, Tang Z, Wang Y, et al. Toll-like receptor 4 plays a key role in advanced glycation end products-induced M1 macrophage polarization. Biochem Biophys Res Commun. 2020;531:602–8.
Huang F, Zhao JL, Wang L, Gao CC, Liang SQ, An DJ, et al. miR-148a-3p mediates notch signaling to promote the differentiation and M1 activation of macrophages. Front Immunol. 2017;8:1327.
Corti A, Bonetti J, Dominici S, Piaggi S, Fierabracci V, Foddis R, et al. Induction of gamma-glutamyltransferase activity and consequent pro-oxidant reactions in human macrophages exposed to crocidolite asbestos. Toxicol Sci. 2020;177:476–82.
Mendiola AS, Ryu JK, Bardehle S, Meyer-Franke A, Ang KK, Wilson C, et al. Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat Immunol. 2020;21:513–24.
Griess B, Mir S, Datta K, Teoh-Fitzgerald M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic Biol Med. 2020;147:48–60.
Marques L, Negre-Salvayre A, Costa L, Canonne-Hergaux F. Iron gene expression profile in atherogenic Mox macrophages. Biochim Biophys Acta. 2016;1862:1137–46.
Handa P, Thomas S, Morgan-Stevenson V, Maliken BD, Gochanour E, Boukhar S, et al. Iron alters macrophage polarization status and leads to steatohepatitis and fibrogenesis. J Leukoc Biol. 2019;105:1015–26.
Hu X, Cai X, Ma R, Fu W, Zhang C, Du X. Iron-load exacerbates the severity of atherosclerosis via inducing inflammation and enhancing the glycolysis in macrophages. J Cell Physiol. 2019;234:18792–800.
Zhou Y, Que KT, Zhang Z, Yi ZJ, Zhao PX, You Y, et al. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018;7:4012–22.
Kao JK, Wang SC, Ho LW, Huang SW, Lee CH, Lee MS, et al. M2-like polarization of THP-1 monocyte-derived macrophages under chronic iron overload. Ann Hematol. 2020;99:431–41.
Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510:278–83.
Luo X, Gong HB, Gao HY, Wu YP, Sun WY, Li ZQ, et al. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ. 2021;28:1971–89.
Djudjaj S, Martin IV, Buhl EM, Nothofer NJ, Leng L, Piecychna M, et al. Macrophage migration inhibitory factor limits renal inflammation and fibrosis by counteracting tubular cell cycle arrest. J Am Soc Nephrol. 2017;28:3590–604.
Lv LL, Feng Y, Wen Y, Wu WJ, Ni HF, Li ZL, et al. Exosomal CCL2 from tubular epithelial cells is critical for albumin-induced tubulointerstitial inflammation. J Am Soc Nephrol. 2018;29:919–35.
Wang Y, Quan F, Cao Q, Lin Y, Yue C, Bi R, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. 2021;28:231–43.
Lu D, Xia Q, Yang Z, Gao S, Sun S, Luo X, et al. ENO3 promoted the progression of NASH by negatively regulating ferroptosis via elevation of GPX4 expression and lipid accumulation. Ann Transl Med. 2021;9:661.
Tsurusaki S, Tsuchiya Y, Koumura T, Nakasone M, Sakamoto T, Matsuoka M, et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019;10:449.
Li X, Wang TX, Huang X, Li Y, Sun T, Zang S, et al. Targeting ferroptosis alleviates methionine-choline deficient (MCD)-diet induced NASH by suppressing liver lipotoxicity. Liver Int. 2020;40:1378–94.
Qi J, Kim JW, Zhou Z, Lim CW, Kim B. Ferroptosis affects the progression of nonalcoholic steatohepatitis via the modulation of lipid peroxidation-mediated cell death in mice. Am J Pathol. 2020;190:68–81.
Liu B, Yi W, Mao X, Yang L, Rao C. Enoyl coenzyme A hydratase 1 alleviates nonalcoholic steatohepatitis in mice by suppressing hepatic ferroptosis. Am J Physiol Endocrinol Metab. 2021;320:E925–37.
Theurl M, Theurl I, Hochegger K, Obrist P, Subramaniam N, van Rooijen N, et al. Kupffer cells modulate iron homeostasis in mice via regulation of hepcidin expression. J Mol Med. 2008;86:825–35.
Bennett H, Troutman TD, Sakai M, Glass CK. Epigenetic regulation of Kupffer cell function in health and disease. Front Immunol. 2020;11:609618.
Asanuma T, Ono M, Kubota K, Hirose A, Hayashi Y, Saibara T, et al. Super paramagnetic iron oxide MRI shows defective Kupffer cell uptake function in non-alcoholic fatty liver disease. Gut. 2010;59:258–66.
Cheong H, Lee SS, Lee JS, Kim J, Kim SW, Lee WJ. Phagocytic function of Kupffer cells in mouse nonalcoholic fatty liver disease models: evaluation with superparamagnetic iron oxide. J Magn Reson Imaging. 2015;41:1218–27.
Wang H, Li L, Li Y, Li Y, Sha Y, Wen S, et al. Intravital imaging of interactions between iNKT and kupffer cells to clear free lipids during steatohepatitis. Theranostics. 2021;11:2149–69.
Yin Y, Wang Q, Qi M, Zhang C, Li Z, Zhang W. Ghrelin ameliorates nonalcoholic steatohepatitis induced by chronic low-grade inflammation via blockade of Kupffer cell M1 polarization. J Cell Physiol. 2021;236:5121–33.
Tran S, Baba I, Poupel L, Dussaud S, Moreau M, Gelineau A, et al. Impaired Kupffer cell self-renewal alters the liver response to lipid overload during non-alcoholic steatohepatitis. Immunity. 2020;53:627–40.
Riederer P, Monoranu C, Strobel S, Iordache T, Sian-Hulsmann J. Iron as the concert master in the pathogenic orchestra playing in sporadic Parkinson’s disease. J Neural Transm. 2021;128:1577–98.
Derry PJ, Hegde ML, Jackson GR, Kayed R, Tour JM, Tsai AL, et al. Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer’s disease from a ferroptosis perspective. Prog Neurobiol. 2020;184:101716.
Mi Y, Gao X, Xu H, Cui Y, Zhang Y, Gou X. The emerging roles of ferroptosis in Huntington’s disease. Neuromolecular Med. 2019;21:110–9.
Rui T, Li Q, Song S, Gao Y, Luo C. Ferroptosis-relevant mechanisms and biomarkers for therapeutic interventions in traumatic brain injury. Histol Histopathol. 2020;35:1105–13.
Huang L, He S, Cai Q, Li F, Wang S, Tao K, et al. Polydatin alleviates traumatic brain injury: role of inhibiting ferroptosis. Biochem Biophys Res Commun. 2021;556:149–55.
Cui Y, Zhang Z, Zhou X, Zhao Z, Zhao R, Xu X, et al. Microglia and macrophage exhibit attenuated inflammatory response and ferroptosis resistance after RSL3 stimulation via increasing Nrf2 expression. J Neuroinflammation. 2021;18:249.
Cao Y, Li Y, He C, Yan F, Li JR, Xu HZ, et al. Selective ferroptosis inhibitor liproxstatin-1 attenuates neurological deficits and neuroinflammation after subarachnoid hemorrhage. Neurosci Bull. 2021;37:535–49.
Keuters MH, Keksa-Goldsteine V, Dhungana H, Huuskonen MT, Pomeshchik Y, Savchenko E, et al. An arylthiazyne derivative is a potent inhibitor of lipid peroxidation and ferroptosis providing neuroprotection in vitro and in vivo. Sci Rep. 2021;11:3518.
Tang LJ, Zhou YJ, Xiong XM, Li NS, Zhang JJ, Luo XJ, et al. Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic Biol Med. 2021;162:339–52.
Heimerl M, Sieve I, Ricke-Hoch M, Erschow S, Battmer K, Scherr M, et al. Neuraminidase-1 promotes heart failure after ischemia/reperfusion injury by affecting cardiomyocytes and invading monocytes/macrophages. Basic Res Cardiol. 2020;115:62.
Chen X, Xu S, Zhao C, Liu B. Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem Biophys Res Commun. 2019;516:37–43.
Bu J, Huang S, Wang J, Xia T, Liu H, You Y, et al. The GABAA receptor influences pressure overload-induced heart failure by modulating macrophages in mice. Front Immunol. 2021;12:670153.
Hou K, Shen J, Yan J, Zhai C, Zhang J, Pan JA, et al. Loss of TRIM21 alleviates cardiotoxicity by suppressing ferroptosis induced by the chemotherapeutic agent doxorubicin. EBiomedicine. 2021;69:103456.
Zhang N, Shou B, Chen L, Lai X, Luo Y, Meng X, et al. Cardioprotective effects of latifolin against doxorubicin-induced cardiotoxicity by macrophage polarization in mice. J Cardiovasc Pharm. 2020;75:564–72.
Acknowledgements
Figures were created with Biorender.com.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81974532) and the Natural Science Foundation of Hunan Province, China (Grant No. 2020JJ4130).
Author information
Authors and Affiliations
Contributions
YY contributed to the conception and design of the review. YW drafted the work and revised it critically for the whole manuscript. LG and WG were responsible for manuscript structure and English grammar. T-L T gave feedback and suggestions for the article. MY gave final approval of the review to be published. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Y., Wang, Y., Guo, L. et al. Interaction between macrophages and ferroptosis. Cell Death Dis 13, 355 (2022). https://doi.org/10.1038/s41419-022-04775-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-022-04775-z
This article is cited by
-
Methionine enkephalin (MENK) protected macrophages from ferroptosis by downregulating HMOX1 and ferritin
Proteome Science (2024)
-
Unraveling the interplay of ferroptosis and immune dysregulation in diabetic kidney disease: a comprehensive molecular analysis
Diabetology & Metabolic Syndrome (2024)
-
Nrf2-mediated redox balance alleviates LPS-induced vascular endothelial cell inflammation by inhibiting endothelial cell ferroptosis
Scientific Reports (2024)
-
Pyruvate Kinase M2 Nuclear Translocation Regulate Ferroptosis-Associated Acute Lung Injury in Cytokine Storm
Inflammation (2024)
-
ACSL4 inhibition prevents macrophage ferroptosis and alleviates fibrosis in bleomycin-induced systemic sclerosis model
Arthritis Research & Therapy (2023)